Keywords
Voriconazole, Hyperkalemia. Adrenal Insufficiency, Fungal infection, Adverse effects.
Voriconazole, Hyperkalemia. Adrenal Insufficiency, Fungal infection, Adverse effects.
Voriconazole is a second-generation azole anti-fungal drug. One side effect of Voriconazole is interference with CYP 450 as it is a potent inhibitor of CYP 450.This can cause interactions with other drugs. Other adverse effects documented, include hormonal, cardiac, neurotoxic effects, and electrolyte imbalance1,2. In our literature review we found a case report of adrenal insufficiency due to concomitant use of voriconazole and glucocorticoids3, a case series of hyponatremia associated with voriconazole use2 and a case of iatrogenic Cushing syndrome associated with concomitant use of voriconazole and budesonide4. Hyperkalemia, in the light of the literature review is a rare side effect associated with voriconazole. Our case is noteworthy as in our patient the adrenal insufficiency and hyperkalemia manifested in absence of use of any other accompanying drug that could cause hyperkalemia. We present a case of hyperkalemia caused by voriconazole in a hospitalized patient. The likely cause of hyperkalemia in our patient is adrenal deficiency due to inhibition of adrenal enzymes.
A 47-year-old white male, retired trailer driver by occupation, with known Crohn’s disease and hypertension presented with productive cough and worsening shortness of breath over three weeks in August 2020. On physical exam, patient was febrile with a temperature of 100-degree F, slightly tachypneic, with a respiratory rate of 24 per minute; blood pressure was 96/89 and he had right lower zone bronchial breathing on auscultation. The patient was admitted and initially started on piperacillin-tazobactam 4.5 gm. IV q6 hr, azithromycin 500 mg IV daily, vancomycin 1gm IV BID, and oseltamivir 75 mg BID. However, his respiratory status worsened over the first 48 hours and required transfer to the intensive care unit on the second day of hospitalization with septic shock and acute respiratory distress syndrome. The patient was intubated and mechanically ventilated and therapeutic paralysis induced. The patient was administered rocuronium IV at the dose of 8mcg/kg/min. Patient was supported by ventilator with volume assisted control. He required two vasopressors, epinephrine (0.3 mcg/kg/min) and nor-epinephrine (12 mcg/kg/min) as IV infusion to maintain his blood pressure. The patient failed to show improvement, therefore, voriconazole 240 mg IV BID was added on the fifth day of hospitalization. Following this, the patient started developing electrolyte abnormalities with rising potassium (Figure 1 and Table 1),and falling sodium (Figure 2 and Table 2). While the patient’s creatinine remaining stable (Figure 3 and Table 3).
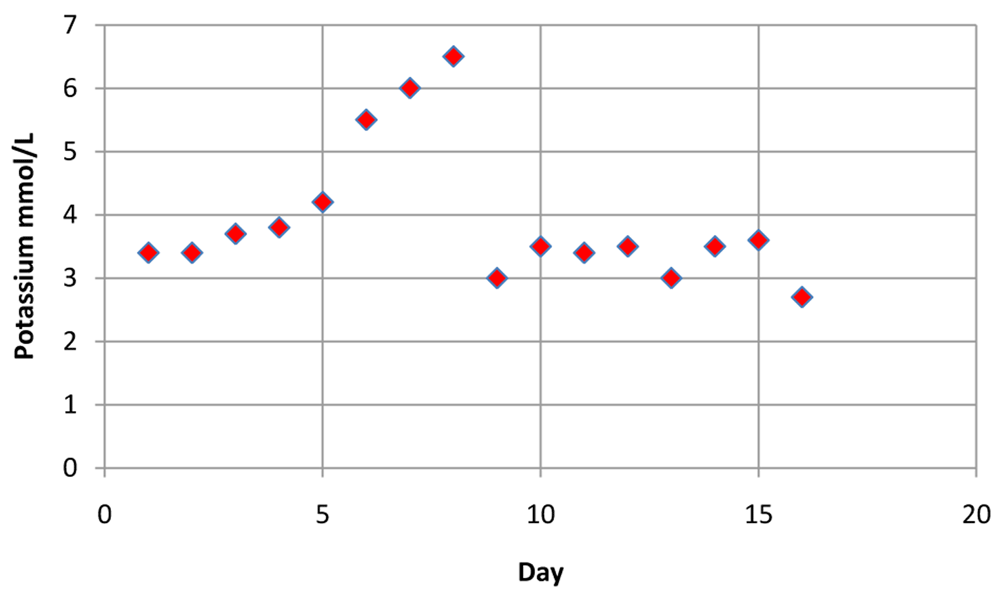
The patient serum potassium level was noted to increase following voriconazole addition to the drug regimen. Discontinuing voriconazole shows normalization of potassium levels, establishing that voriconazole was the offending agent.
| DAYS | POTASSIUM mmol/L |
|---|---|
| 1 | 3.4 |
| 2 | 3.4 |
| 3 | 3.7 |
| 4 | 3.8 |
| 5 | 4.2 |
| 6 | 5.5 |
| 7 | 6 |
| 8 | 6.5 |
| 9 | 3 |
| 10 | 3.5 |
| 11 | 3.4 |
| 12 | 3.5 |
| 13 | 3 |
| 14 | 3.5 |
| 15 | 3.6 |
| 16 | 2.7 |
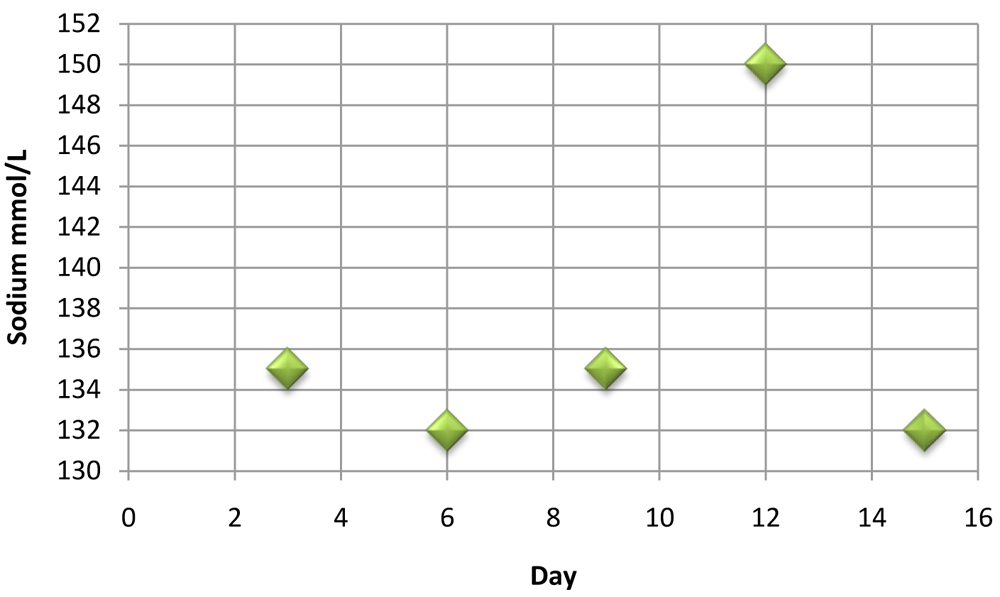
Hyponatremia trend shown after voriconazole administration; along with finding of hyperkalemia suggests voriconazole induced adrenal insufficiency.
| DAYS | SODIUM mmol/L |
|---|---|
| 3 | 135 |
| 6 | 132 |
| 9 | 135 |
| 12 | 150 |
| 15 | 132 |
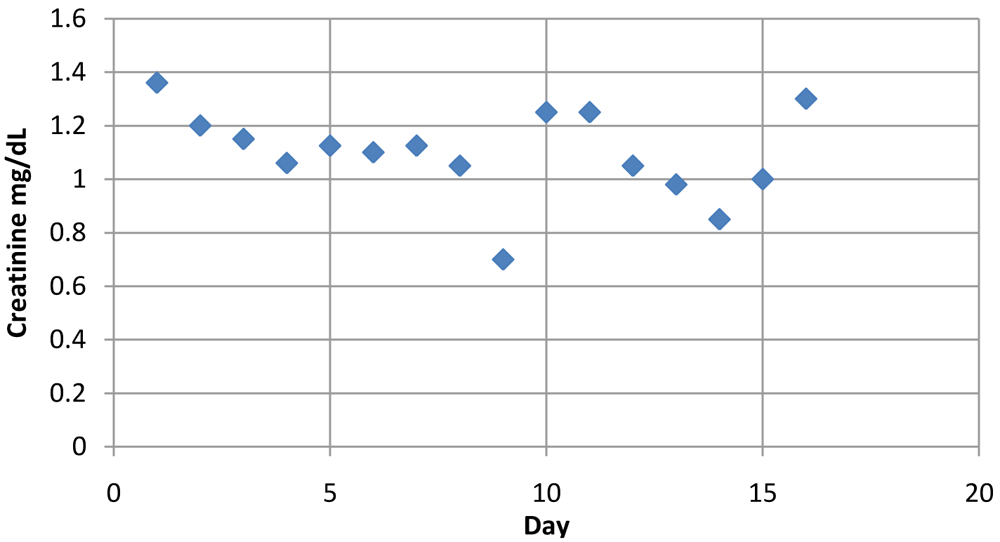
The trends in creatinine levels were seen to be within normal range, reflecting adequate renal function.
| DAYS | CREATININE mg/dL |
|---|---|
| 1 | 1.36 |
| 2 | 1.2 |
| 3 | 1.15 |
| 4 | 1.06 |
| 5 | 1.125 |
| 6 | 1.1 |
| 7 | 1.125 |
| 8 | 1.05 |
| 9 | 0.7 |
| 10 | 1.25 |
| 11 | 1.25 |
| 12 | 1.05 |
| 13 | 0.98 |
| 14 | 0.85 |
| 15 | 1 |
| 16 | 1.3 |
For the rising potassium, immediate medical management with intravenous furosemide 80mg, rectal sodium polystyrene sulfonate 60 gm., and intravenous regular insulin 7 units were given, with 50 ml of 50% dextrose, continuous albuterol 2.5 mcg nebulization, and intravenous sodium bicarbonate 50 mEq intravenous push .However, this failed to improve the hyperkalemia. His urine output during this period was within normal range. Due to the intractable hyperkalemia, continuous renal replacement therapy (CRRT) was initiated. The patient was also started on intravenous hydrocortisone 60 mg every six hours and fludrocortisone 0.2 mg. The voriconazole was discontinued, the patient’s electrolyte abnormalities normalized and the CRRT was stopped in less than 48 hours. Fludrocortisone was discontinued in 48 hours as well. The patient continued to improve and was extubated on day 11 of hospitalization. He was discharged to a specialist nursing facility on day 14. His follow up chemistry showed Na+ levels of 139 mEq/L (Normal range: 135 mEq/L– 145 mEq/ L), K+ levels of 4 mEq/L (Normal range:3.5mEq/L–5.5mEq/L), HCO3 levels of 20 mEq/L ( Normal range: 23mEq/L – 30mEq/L) , Creatinine level of 1.3 mg/dl (Normal range: 0.7– 1.5 mg/dL).
Amphotericin B had been the drug of choice for mycosis up to the development of azole drugs. Azoles have the convenience of being administered orally and have a safer side effect profile compared to Amphotericin B5. Azoles are synthetic compounds and are further classified into two groups. The imidazole sub-group includes miconazole and ketoconazole. The triazole sub-group includes itraconazole, fluconazole, and voriconazole5.
Voriconazole has a potent antifungal effect. Voriconazole inhibits ergosterol synthesis in the fungal cell wall by inhibiting the 14-alpha demethylase enzymes hence causing eradication of fungal infection. Voriconazole leads to many drug-drug interactions because it causes inhibition of CYP 450 enzymes. Many drugs are metabolized by CYP450 enzymes hence voriconazole causes their level to increase in blood if used concomitantly.
Voriconazole is a relatively new, second-generation addition to the triazole anti-fungal family. It is both a substrate and an inhibitor of the enzymes of cytochrome P450 family, including CYPC19, CYPC9, and CYP3A4, thus leading to a number of adverse effects4.
Side effects in human, caused by inhibition of CYP 450 include decreased synthesis of many adrenal and gonadal enzymes. This can lead to adverse effects including gynecomastia and adrenal insufficiency5. Voriconazole also decreases enzyme function in the kidney and liver6.
Other adverse effects include liver toxicity; hormonal effects like alopecia, decreased libido, and impotence2. Electrolyte imbalances like hyponatremia2, hypokalemia, and adrenal insufficiency have been previously reported1. Certain side effects associated with voriconazole itself include neurotoxicity, cardiac arrhythmias, pancreatitis phototoxic reactions, and periostitis1.
Severe adverse effects due to drug interaction of steroids2, and tacrolimus7 are noted with concomitant use of azole drugs. The azole potentiates the drug effect by CYP 450 inhibition. There have been cases of increased steroid effect causing Cushing disease due to potentiated effect and later adrenal insufficiency due to hypothalamic-pituitary-adrenal (HPA) axis suppression3,4,8. Due to inhibition of CYP 450 as a side effect of voriconazole, the metabolism of tacrolimus may be decreased, causing high levels of tacrolimus in blood which causes hyperkalemia8.
Our patient presented with shortness of breath, was diagnosed with acute respiratory distress syndrome and was treated with broad-spectrum antibiotics; he was also prescribed voriconazole for antifungal coverage. As voriconazole was initiated, the patient developed hyperkalemia and hyponatremia. Creatinine was noted to be within normal limits during hospitalization. The patient was treated on the lines of adrenal insufficiency. Voriconazole, when removed, resulted in clinical improvement. Sodium and potassium levels improved, establishing that the adrenal insufficiency had been due to an adverse reaction to voriconazole. In our case, adrenal insufficiency was treated on clinical suspicion. Voriconazole was confirmed as the causative agent because removing voriconazole relieved the patient’s symptoms.
Voriconazole can cause adrenal insufficiency by directly inhibiting the adrenal enzymes. In case of concomitant use of steroids, HPA suppression can be the reason for adrenal insufficiency. The diagnosis of adrenal insufficiency is made by measuring ACTH level. The cortisone challenge test helps differentiate between primary and secondary adrenal insufficiency8.
Our case, has limitations as the diagnosis was made on clinical suspicion. However, in the absence of a history of steroid use, direct adrenal enzyme inhibition due to voriconazole is the most likely cause. The diagnosis is supported by the fact that removal of voriconazole resulted in an improvement in the patient’s condition.
Hyperkalemia resulting from voriconazole use is an uncommon adverse effect occurring in less than 2% of cases but it is important because of the serious and potentially life-threatening consequences. It should be kept in mind whenever initiating treatment and an alternative treatment sought if deemed necessary. Moreover, electrolyte levels should be monitored when initiating voriconazole. Our case report underscores the need for case control studies to establish this association.
Written informed consent for publication of their clinical details was obtained from the patient.
The case has been previously reported as conference abstract at American Society of Nephrology (ASN), Kidney Week, San Diego, 2018. https://www.asnonline.org/education/kidneyweek/archives/
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the background of the case’s history and progression described in sufficient detail?
Partly
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Partly
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Partly
Is the case presented with sufficient detail to be useful for other practitioners?
Partly
References
1. Choi J, Cho S, Jang K, Kim G: Voriconazole-induced Severe Hyperkalemia Precipitated by Multiple Drug Interactions. Electrolytes & Blood Pressure. 2020; 18 (1). Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Nephrology, onco-nephrology, critical nephrology, acute kidney injury, electrolytes
Is the background of the case’s history and progression described in sufficient detail?
Yes
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Yes
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Yes
Is the case presented with sufficient detail to be useful for other practitioners?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Gastroenterology and Transplant Hepatology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 02 Feb 21 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)