Keywords
green synthesis, Rhizophora stylosa, silver nanoparticles, antibacterial activity
This article is included in the Nanoscience & Nanotechnology gateway.
green synthesis, Rhizophora stylosa, silver nanoparticles, antibacterial activity
In this new version we made some changes based on reviewers' suggestions. The theory has been paraphrased and well written to improve the readability of this article in the introduction. In the results and discussion section, the UV-Vis spectra of the synthesized nanoparticles have been added to support the statement according to Figure 1. We have also added supporting references for the statement. Explanations have been added in the text to support the relationship between the table and the statements in the text. We have added additional explanations to support the assertion that the addition of 3% leaf extract tends to have a smaller wavelength shift and have added references to support it as suggested by reviewers.
See the authors' detailed response to the review by Manoj Kumar Choudhary
Nanotechnology has become a new breakthrough in research, as it finds wide application, especially in biomedical fields.1 One of the most studied materials is silver nanoparticles, which have various methods of synthesis from physics, chemistry, and biology techniques. However, most processes have many disadvantages, especially chemical methods, which require the use of hazardous reagents that are not friendly to the environment. Furthermore, an alternative arises in the synthesis of nanoparticles with a biological approach by utilizing phytochemical compounds contained in plants.2 In general, these phytochemical compounds contain hydroxyl groups, which play a crucial role in the formation of silver nanoparticles through the reduction of Ag+ to Ag0.
The green synthesis of nanoparticles relies on the stability of silver nanoparticles of substances that are non-toxic, as well as solvents and reducing agents. The effectiveness of this product is based on the size and shape of the particles.3 To carry out applications, colloidal silver nanoparticles (AgNps) should have appropriate physical and chemical properties such as homogeneous shapes, small and narrow particle size distribution, and long-time stability. The control of nanoparticle growth from agglomeration is very important in the selection of stabilizers, donor metals, polymers, and surfactants since its potential application strongly depends on the stability. In addition, to reduce the hazardous effect on the environment, the use of natural reagents is being considered. The hydroxyl group in the natural polyphenol component acts as a reducing, as well as capping agent.4 By the double-function utilization of natural plants, the reagent used in the reaction could be diminished.
Currently, most of the natural plants used in the synthesis of silver nanoparticles are limited to terrestrial plants. Only a few studies report the use of marine plants. Indonesia as an archipelago has a wealth of marine resources such as mangroves. Its extracts are known to have antimicrobial abilities that have long been used by the community.5 One of them is Rhizophora stylosa (RS), which is a member of the Rhizophoraceae family in the Southeast Asian mangrove ecosystem. It is known to have several compounds that play an important role in biomedical applications. Some studies report that Rhizophora has several biological activities such as antibacterial, antioxidant and anti-cancer.6–9 Teraxerol, teraxerone (triterpenoids), sitosterol (steroids), protocatechuic acid and isovanillic acid (phenolic compounds), routine, astilbin, catechin (flavonoids) are the main compounds in RS mangrove plants. Those important compounds in plant extracts are hydroxyl groups which act as reducing agents as well as stabilizers. This component has good pharmacological ability.10,11 In this study, RS leaf extract was used as a reducing agent in the production of RS-AgNps as well as a stabilizer.
To the best of our knowledge, this is the first research on RS mediated synthesis of silver nanoparticles. This study aims to investigate the effects of reaction conditions, such as reaction time, the amount of silver solution, and amount of extract, on the properties of fabricated silver nanoparticles. The antibacterial activity of as-synthesized RS AgNps was evaluated against Escherichia coli and Staphylococcus aureus, which are the main microbes present in wounds.12
Fresh leaves of RS were collected from mangrove forest on Bintan Island, Riau Archipelago Indonesia. They were washed with double distilled water (DDW) to remove dust and other impurities. Clean leaves were shade-dried for 10–15 days and then ground to obtain leaf powder. The extraction of RS leaves was done by adding 20 grams of leaf powder into 100 ml of DDW (1:5) followed by heating at 65oC for 30 minutes with a hotplate stirrer. This mixture was then filtered using Whatman filter paper no. 1. The obtained extract was stored at 4°C for further experiments.
Synthesis of silver nanoparticles was carried out by stirring silver nitrate solution with RS leaf extract in a total volume of 50 mL at a constant speed of 500 rpm. The concentration of AgNO3 was varied to be 0.001 M, 0.005 M, and 0.01 M, while RS leaf extract was 1%, 3%, and 5% (v/v). The absorbance of the sample was periodically monitored after one, two, four, and six hours of stirring using UV-Vis spectroscopy. Therefore, all samples were stored in a sealed bottle to measure the absorbance after 24, 72, 168, and 720 hours. In this study, some synthesis variables, such as reaction time, extract, and silver precursor concentration were applied to determine the optimum conditions for fabricating stable colloidal silver nanoparticles.
The diameter and zeta potential of RS-AgNps were characterized using Dynamic Light Scattering (DLS) Zetasizer HORIBA (SZ-100) at 25 °C. Absorbance intensity and surface plasmon resonance (SPR) uptake were analyzed using UV-Vis spectroscopy (Shimadzu UV-1800 spectrophotometer) in wavelength range of 200-800 nm at 5 nm intervals. The device was fully controlled by the UV Probe version 2.42 software monitor package. The shape and particle size distribution of silver nanoparticles were calculated on the basis of TEM images (JEOL JEM 1400) processed using J software. X-ray diffraction (Shimadzu XRD-7000s, λ 1.5406 Å operated at a voltage of 30kV and current of 30mA) were used to investigate crystal structures of the samples.
Antibacterial activity of the as-synthesized RS-AgNps was tested against Escherichia coli (Gram negative bacteria) and Staphylococcus aureus (Gram positive bacteria) using the agar diffusion method. Firstly, the bacteria were planted in nutrient agar (NA) and then cultivated for 24 hours at 37 °C. The suspended bacteria were transferred into 100 mL NA media and then poured into a petri dish. Sterile cotton with a colloidal nanoparticle sample with a concentration of 100 mg/mL were deposited in the well. DDW and amoxicillin were adopted as negative and positive controls, respectively, and the area of inhibition was measured after 24 hours of precipitation and incubation, tests were carried out in duplicate and the results displayed were averaged.
The formation of silver nanoparticles in the samples were specifically recognized by color changes from colorless to light yellow right after mixing the silver precursor and leaf extract (Table 1). It was confirmed by UV-Vis spectrophotometry analysis where AgNps provide specific peaks at a wavelength of about 403–443 nm. This analysis was based on the SPR phenomenon of spherical metallic nanoparticles, which is strongly influenced by shape and size.13
Figure 1 shows the UV-Vis spectrum of as-synthesized RS-AgNps in a varied concentration of AgNO3 and RS leaf extract after a one-month reaction. The spectrum of silver nitrate solution (j) and RS leaf extract (k) provided peaks at wavelength 301 and 279 nm, respectively. In general, the peaks were then converted to a wavelength range of 419 to 446 nm, which were specific to spherical AgNps. The inset pictures show laser beam irradiation of the brown-colored samples based on the Tyndal effect. Those results strongly confirmed the formation of metallic silver nanoparticles through the reduction process of Ag+ to Ag0, which consisted of three stages of process i.e. reduction, nucleation, and growth.14 Similar studies on mangrove Rhizophora lamarckii plants have shown that spherical AgNps contribute to absorption bands of around 420 nm in the spectrum of particles.15
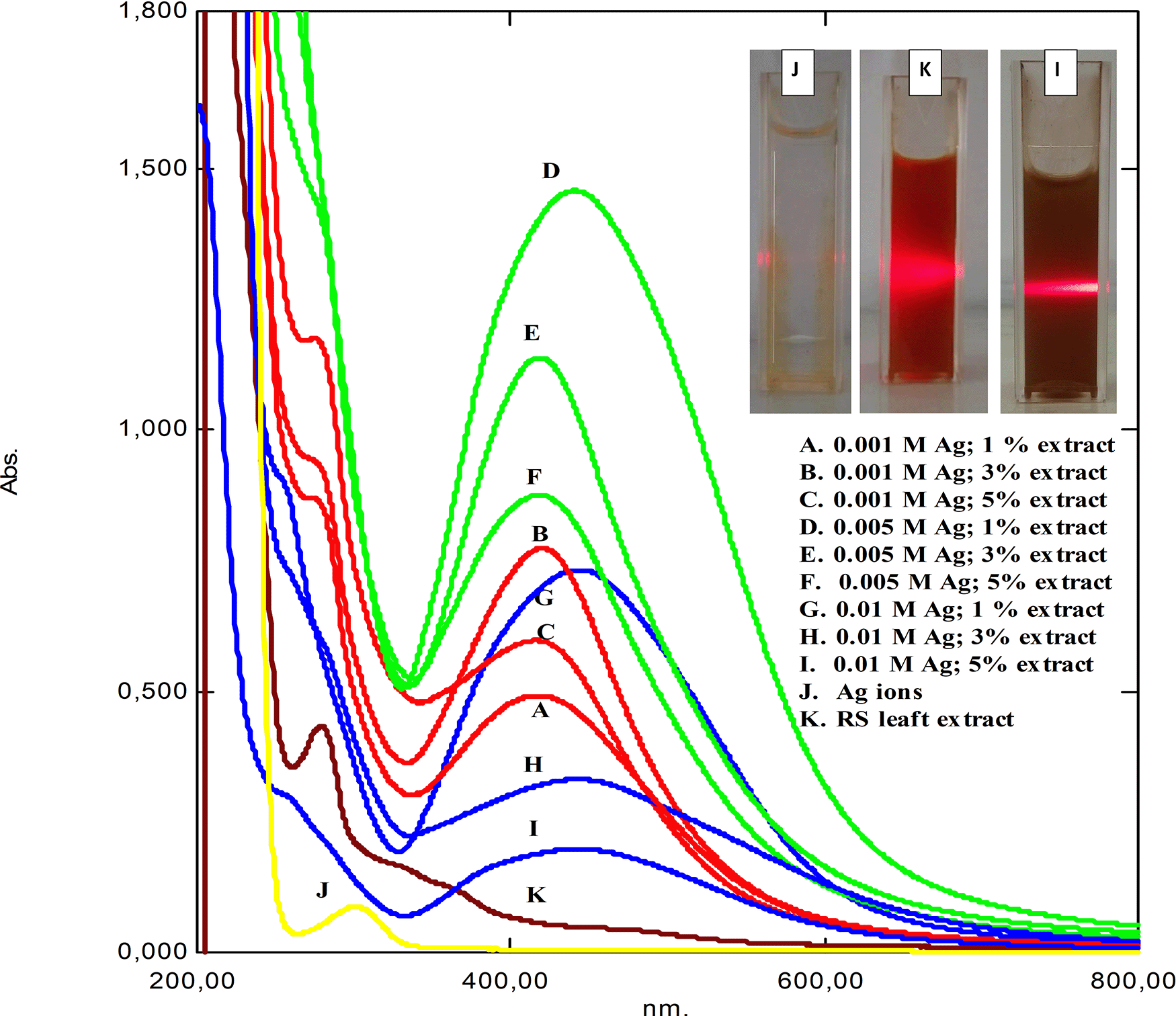
The inset shows the laser beam radiation.
From the UV-Vis spectrum above variations in the concentration of silver nitrate salt as a precursor (marked by the color difference in the image). Points A, D and G showed a high increase in precursor concentration compared to the amount of available extract, causing a high intensity of SPR to be formed with the formation of available silver, due to the increase in formation indicating a larger particle size. A narrow absorption peak indicates a smaller size.16,17 The higher the concentration of the extract, the more availability of polyphenolic compounds to carry out the reduction process.
In this study, RS-AgNps samples were prepared using a varied concentration of silver nitrate (0.001 M, 0.005 M, and 0.01 M) and RS leaf extract (1%, 3%, and 5% v/v) in order to determine the optimum reaction composition. The wavelength and absorbance value based on spectrophotometry analysis are shown in Table 1. It was shown that increasing the extract concentration (1% to 5%) significantly shortened the wavelength, indicating a smaller size of AgNPs were formed. According to the mie theory, particle size affects optical properties, when the particle diameter decreases, the maximum wavelength of the SPR band will shift to the left (blue shift).18–20 However, based on the length value waves, AgNps with the addition of 3% leaf extract tend to have a smaller wavelength shift than AgNps with 1% and 5%. This indicates that the stability and growth of AgNps was maintained with the addition of 3% extract. This condition is confirmed from the TEM results in the following discussion.
It was shown that an increase in extract concentration (1% to 5%) significantly shortened the wavelength, which indicates smaller-sized AgNps were formed. However, based on the wavelength values, AgNps with the addition of 3% leaf extract tended to have less wavelength shift than those of AgNps with 1% and 5%. An expansion of the SPR peak with a nominal redshift was observed for AgNPs, with an increase in the amount of extract above 3% (v/v), suggesting an increase in nanoparticle size.21 This kind of observation might be predicted at higher extract concentrations when additional biomolecules are available, thereby, leading to secondary interactions with cover molecules on the surface of the newly formed Ag0 core, giving rise to the formation of larger size particles.When extract less than 3%, the intensity of absorption peaks increases with increase in the concentration of the silver nitrate salt (points D, A, G in Figure 1).22 This suggested that the stability and growth of AgNps were maintained by the addition of 3% extract. This result strongly confirmed that RS leaf extract acted as a capping agent to stabilize and control the growth of AgNps. This was observed in all samples prepared using three different concentrations of silver nitrate.
In addition, an increase in silver nitrate concentration led to longer wavelength and showed the formation of bigger-sized AgNps. This phenomenon was related to the formation of aggregates due to the excessive amount of silver nitrate in the reaction, resulting in bigger sized AgNps.16 Hence, in order to fabricate stable small-sized AgNps, it is sufficient to apply a low concentration of silver nitrate i.e. 0.001 M and 0.005 M.
The leaf extract and silver ion concentration significantly affected the absorption intensity as well, as seen in Table 2. The AgNps samples prepared using 5% extract showed a higher absorption intensity than those prepared using 1% and 3%. This was observed both in the use of 0.001 M and 0.005 M silver nitrate. The absorption intensity reflects the number of metallic nanoparticles formed in the reaction. Hence, these results suggest that the more RS leaf extract used in the reaction, the greater the number of metallic nanoparticles formed. This strongly confirmed the role of leaf extract in reducing Ag+ to Ag0 and the formation of AgNps. This result is beneficial in industry because synthesis is carried out on a large scale.
In addition, the samples prepared using 0.005 M silver nitrate solution exhibited higher absorption intensity than those prepared using 0.001 M. It is assumed that the higher concentration of silver nitrate solution provided more reduction and led to a higher number of formed AgNps. However, the excessive silver nitrate solution in a concentration of 0.01 M resulted in a lower absorption intensity.
In general, there was no significant decrease in absorbance intensity during the reaction process up to a one-month reaction. This result suggested that the as-synthesized RS showed good stability. Furthermore, the AgNps size data and its correlation with the concentration of extract and silver solution is supported by the TEM data.
Figure 2 shows the TEM images of RS-AgNps, which are spherical in shape with a size range of 5–87 nm. The mean diameter of AgNps prepared using 0.001 M, 0.005 M, and 0.01 M silver nitrate and the addition of 3% extract were 25 nm, 22 nm, and 45 nm, respectively. These results show that the concentration of silver nitrate affects the size of the nanoparticles, where the use of 0.01 M silver nitrate resulted in a larger diameter of AgNps than those prepared using a lower concentration. It was confirmed that there is a strong correlation between the mean particle diameter with absorption wavelength of AgNps. The shift to longer wavelength (red shift) in UV-Vis spectrophotometry analysis indicates a larger mean diameter.17 Figure 2a and 2b show smaller particle size.
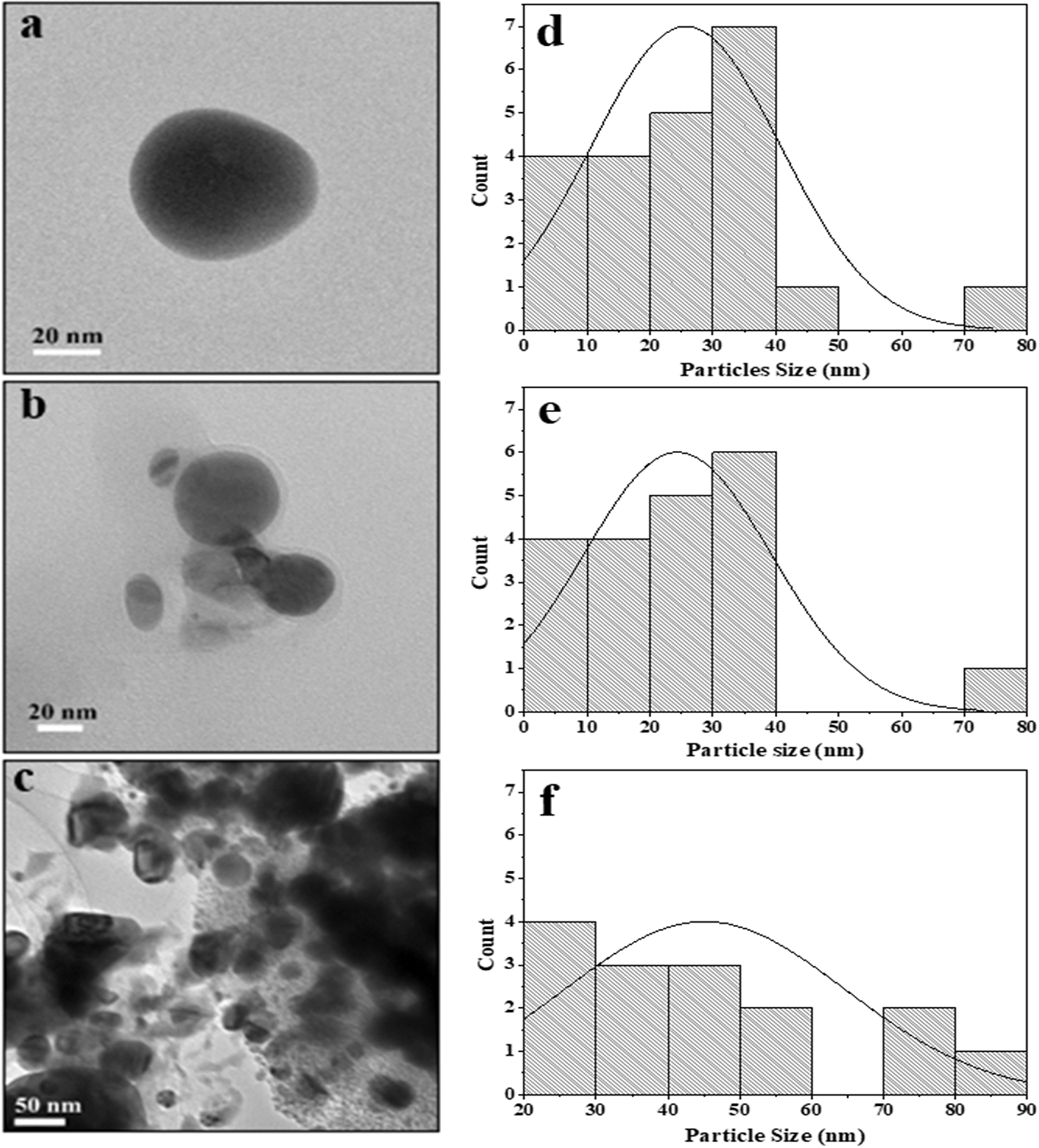
In Figure 2c, there was a slight agglomeration at 0.01 M. Particles in this study are ionic silver nanoparticles dispersed in aquades, permanent dipole and induced dipole between particles that are close together so that they undergo aggregation due to the van der Waals force.18,19 Particle aggregation causes the size to become large. Aggregation also indicates the small repulsion force between particles, which causes colloid instability. In previous studies, leaf synthesis for nanoparticles (NP) production in mangrove Rhizophora mucranata,20 Rhizophora apiculata21 and Rhizophora lamarckii17 had successive particle sizes of 60–95, 19–42, 12–28 nm through reduction of silver nitrate compounds. In this study the nanoparticles produced were much smaller in size by controlling the amount of extracts and precursors.
Based on the DLS analysis, the hydrodynamic particle size of colloidal RS-AgNps ranges from 98–125 nm, which is larger than TEM measurements. This is due to the different measurement principles between TEM, and DLS. The latter method measures the hydrodynamic size of nanoparticles in aqueous suspensions including the metal core, and any biological molecule attached to the surface of the particles.22 The zeta potential is an important parameter for assessing the stability of AgNps in aqueous suspensions. When the value is greater, either positive or negative, it suggests that molecules tend to repulse each other, hence it increases the suspension stability. The negative potential values shown by the bio-synthesized AgNps reflect the presence of bio-organic components in the extract as a capping agent.13,23
A zeta potential value of ± 30 mV is required for indication of a stable nanoparticle suspension.24,25 The zeta ability of RS-AgNps colloids range from (−24.9) mV up to (−27.7) mV. In addition, the extract concentration in colloidal AgNps influenced the polydispersity index as well. The polydispersity index showed the dimensions of the particle size distribution, while a polydispersity index value > 0.7 indicates a very wide dispersion.26 AgNps 0.001 M and 0.005 M with addition of 3% extract showed a narrow size distribution (Figure 2d and 2e). It was confirmed by a polydispersity index of 0.37. Similar research was carried out by Nasiriboroumand et al., who synthesized AgNps mediated by pomegranate rind.4 The zeta potential was found to be −37 mV to −32 mV and a PDI value of 0.25. It showed a narrow particle size distribution with good stability.
X-ray diffraction (XRD) analysis was carried out in order to investigate the crystallinity of as-synthesized RS-AgNps. The diffraction pattern (Figure 3) showed diffraction peaks that are well resolved at an angle of 2θ at 38.28, 44.45, 64.53 and 77.53, which correspond to (111), (200), (220), (311) of face-centered cubic structure of metallic silver (ICSD No. 4068). These results confirmed UV-Vis spectroscopy and the TEM analysis result, which showed that AgNps have been successfully obtained by the mediation of RS leaf extract. Some other peaks (marked by asterisks*) were observed in the diffraction pattern. It is assumed that these referred to the presence of reducing, and capping agent on the surface of the AgNps and the additional diffraction peaks in the XRD pattern were due to bio-organic crystallization in the plant extract.27 These results were similar to those found in AgNps synthesized through mediation of other marine plants i.e. Ecklonia cava algae extract.5 This happened in the use of other marine plants like Rhizophora stylosa as well.
Silver nanoparticles with sizes <100 nm have been of great concern to researchers due to their small particle size and high surface area properties.28 In this study, the antimicrobial activity of RS-AgNps was tested against two types of bacteria i.e. Escherichia coli and Staphylococcus aureus as representatives of Gram negative and Gram-positive bacteria, which were commonly used to evaluate the activity of nanoparticles in previous studies.29,30 The antibacterial testing method uses the agar diffusion method. Amoxcillin and distilled water were used as positive and negative controls, respectively. The test was run in duplicate, and all data presented were averaged.
The results showed that the three variations in the AgNp concentration significantly affected the inhibition zone. As shown in Table 3 and Figure 4, the area of restriction for a concentration of 0.001 M; 0.005 M and 0.01 M RS-AgNps respectively, are 5.5 mm, 7.2 mm, and 5.1 mm for E. coli and 4.3 mm for S. aureus bacteria. A higher zone of inhibition was found in the 0.005 M concentration of AgNps against E. coli and S. aureus.
Diameter of inhibition zone of synthesized silver nanoparticles (RS-AgNps) against Escherichia coli and Staphylococcus aureus.
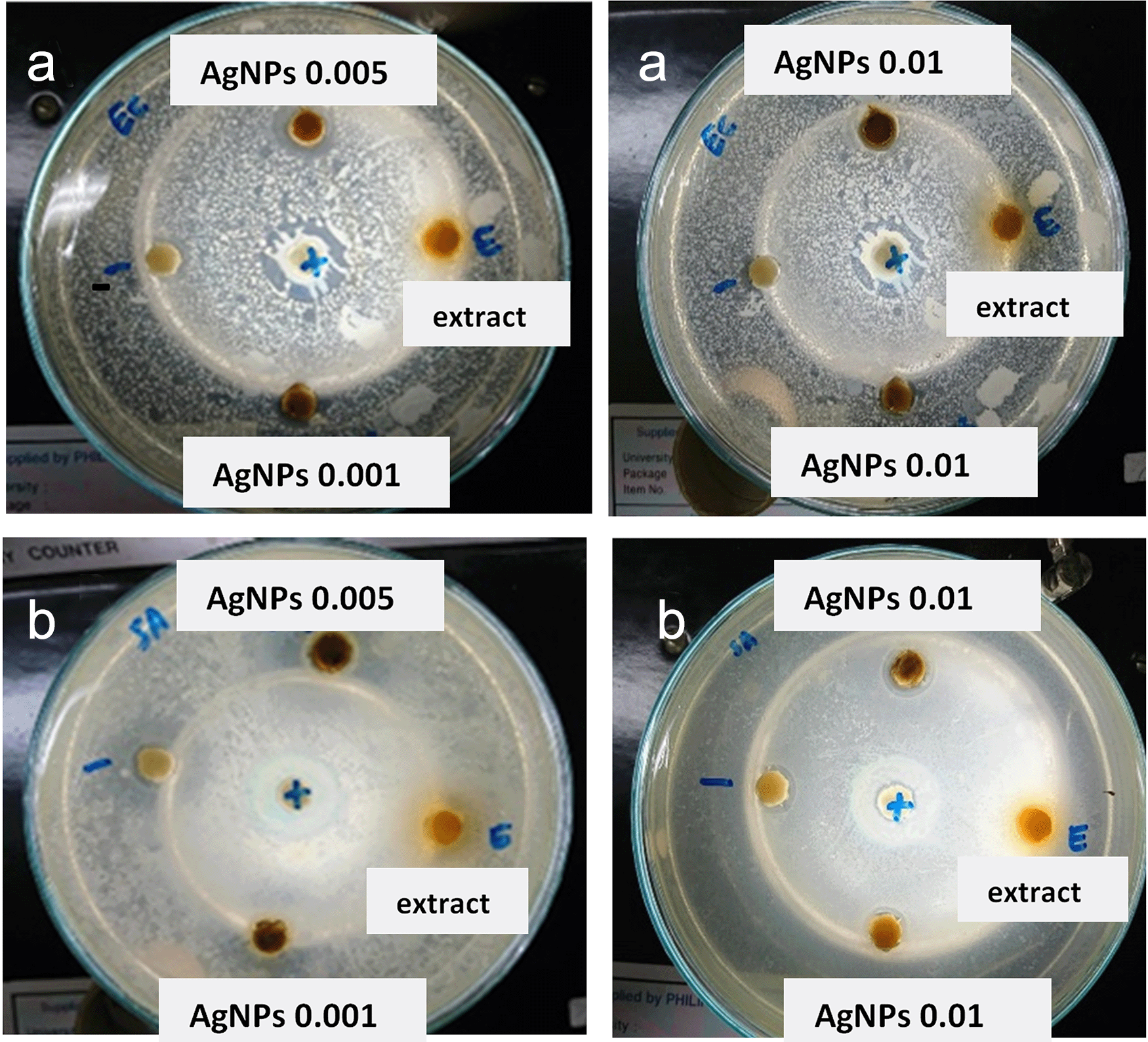
Generally, it was observed that as-synthesized AgNps showed a greater inhibitory zone diameter against E. coli. than S. aureus. These results suggest that RS-AgNps is more specific against Gram negative bacteria than against Gram positive bacteria. The Gram-positive bacteria have a thick layer of peptidoglycan (80 nm) in the cell wall, and this zone has covalent bonds with teichoic and teichuronic acids, whereas Gram negative bacteria have a thin peptidoglycan layer (~8 nm thick) with a lipopolysaccharide external membrane (1–3 μm thick). Another possible cause for their vulnerability to nanoparticles (NP) are these bacteria are coated with lipopolysaccharides, which are negatively charged. These negative loaded molecules are closer to positive ions, which are mostly released through NP leading to ion uptake and increased intracellular damage. NP exhibit their antibacterial activity by different pathways, which are summarized as a combination of ROS production, altered gene regulation, cell wall penetration, and binding metabolites, among other processes.31
In addition, the region of maximum inhibition of AgNps was observed based on the TEM results. As corresponding to the average diameter estimated by TEM analysis, there is a correlation between mean diameter of NP and its antibacterial activity. The resulting smaller-sized particles (22 nm) have a larger inhibitory zone, as well as stable colloidal AgNps. Choi and Hu (2008) reported that this phenomenon was due to the easiness of the small-sized NP to penetrate into the bacteria cell. In addition, smaller NP have a larger surface area, resulting in a larger interaction site of NP and bacteria.32
According to Aromal and Philip (2012), the mechanism of antibacterial activity of metallic NP is highly dependent on the interaction or bond between NP and compounds in the bacteria cell wall. Firstly, the metallic nanoparticles penetrate into the cell and then interfere with cell metabolism, especially with the organelle involved in protein synthesis.33 From this study, it can be concluded that RS-AgNps may be developed as an antimicrobial agent.
Other types of mangroves have also been used as reducing agents for the synthesis of AgNps against pathogenic bacteria including E. coli and S. aureus, such as Ceriops tagal,34 Exoecaria agallocha,35 Sonneratia apetala,32 and Rhizophora mucranata.33
Silver nanoparticles with a particle size range of 5–87 nm or an average of 22 nm have been successfully synthesized with a wavelength of 403–443 nm by bioreduction using leaf extracts of the Rhizophora stylosa mangrove plant. The results of UV-Vis spectroscopy and TEM analysis showed the formation of smaller sized nanoparticles. DLS, zeta potential and index polydispersion analysts have demonstrated the stability of nanoparticles within a month of the reaction. The influence of reaction time, silver nitrate dissolution concentration and effect of extract amount showed a joint relationship. A silver nitrate concentration of 0.001 M and 0.005 M was able to produce smaller nanoparticles and the optimal concentration of extract used was 3% (v/v). Antibacterial activity showed that silver nanoparticles mediated by RS mangroves have biomedical potential.
All data underlying the results are available as part of the article and no additional source data are required.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Synthesis and characterization of nanopartcles
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Nanomaterials
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Synthesis, Characterization and Environmental Applications of metal nanoparticles, carbonaceous materials and photocatalytic materials
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
References
1. Choudhary M, Kataria J, Sharma S: Evaluation of the kinetic and catalytic properties of biogenically synthesized silver nanoparticles. Journal of Cleaner Production. 2018; 198: 882-890 Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Synthesis, Characterization and Environmental Applications of metal nanoparticles, carbonaceous materials and photocatalytic materials
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 2 (revision) 16 May 22 |
read | read | read |
|
Version 1 06 Aug 21 |
read | ||
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)