Keywords
Ageing, antioxidant, sea grapes, tempe, functional food
This article is included in the Agriculture, Food and Nutrition gateway.
This article is included in the The Role of Nutrition in Healing and Improving Health collection.
Ageing, antioxidant, sea grapes, tempe, functional food
Unhealth diet and excessive exposure to UV (ultra-violate) light can cause premature skin aging, leading to excess melanin production (hyperpigmentation), and darker patches (depigmentation) (Saeedi et al., 2019). Excessive UV light exposure can trigger oxidative stress, causing damage and apoptosis in skin cells. Oxidative stress occurs due to the increased intercellular levels of reactive oxygen species (ROS), which play an important role in pathogenesis of aging and chronic disorders (Peñalver et al., 2020; Park, 2013; Park et al., 2004). Consumption of high antioxidant functional foods in recent years has become popular as they can reduce oxidative stress damage. The presence of hydroxyl groups in antioxidant compounds acts as hydrogen donors to stabilize and prevent the formation of new ROS (Pereira et al., 2009).
In some Asian countries, such as Malaysia, Indonesia, and the Philippines, sea grapes or Caulerpa racemosa, which are edible marine macroalgae, are believed to be functional foods or nutraceuticals packed with antioxidant properties that can delay or prevent premature skin aging (Eren et al., 2019; Schumacker, 2015; Peñalver et al., 2020; Tanna et al., 2020; Yep et al., 2019; Pakki et al., 2020). Studies have explored several bioactive components in sea grapes, such as bioactive peptides, fibers (polysaccharides), polyphenols, flavonoids, antioxidants, and their distinctive compounds caulerpin (Cao et al., 2021; Yang et al., 2015; Yep et al., 2019). In line with this, sea grapes extract tested in diabetic rats indicated a lowering effect on glucose levels, reduced aspartate aminotransferase, and alanine aminotransferase activities, and a had a hepatoprotective effect (Qudus et al., 2020).
Similar to sea grapes, tempe (Fermented soyabeans), which is a local Indonesian food and known worldwide as a functional food, also has a high antioxidant activity (Kadar et al., 2020; Mani & Ming, 2017).
Premature aging can be exacerbated by an unhealthy diet as well. High glucose levels at the presence of limited insulin can trigger the glycation process, whereby glucose is attached to the proteins, lipids, and DNA of the skin, producing Advanced Glycation End-products (AGEs) (Hantzidiamantis & Lappin, 2019; Kim et al., 2017). Consequently, AGEs can deactivate the antioxidants, attack collagen, and elastyn, leaving the skin to lose moisture, become wrinkled, dull, and prone to damage and premature aging (Gill et al., 2019). Consumption of antioxidants and collagen, such as those found in sea grapes and tempeh, can inhibit AGEs (Aubry et al., 2020; Kadar et al., 2020; Yang et al., 2015).
Tyrosinase inhibition is another useful way of avoiding depigmentation. Tyrosinase transforms tyrosin to 3,4-dihydroxyphenylalanine (DOPA), then transforms DOPA to dopakuinone; which results in melanin at the end of the process (Pillaiyar et al., 2017). As such this study aimed to determine the anti-aging potential effect of sea grapes and tempe collagen powder, by analyzing the activities of anti-glycation, antioxidant and tyrosinase inhibitors.
Sea grapes (Caulerpa racemosa) were rinsed and cleaned with the use of CO2 free water. The soybean-based tempe is mixed with sea grapes (0.25:1) with a blender, and frozen at the −22°C for 12 hours. Samples were dried with the use of freezer dryer (LyovaporTM L-200) for 24 hours, which resulted in 0.3-0.5 mm powder.
The determination of water content was based on the Association of Official Analytical Chemists (AOAC) drying method (Thermogravimetry) (Latimer, 2019) (Table 1), and the content was calculated by using the following formula:
W0 = Weight of empty cup
W1 = Weight of the cup + initial sample (before heating in the oven)
W2 = Weight of cup + initial sample (after cooling in desiccator)
The procedure for determining the ash content was also with the use of the AOAC method (Latimer, 2019), and the content was calculated by using the following formula:
Collagen from sea grapes and tempe powder is isolated by treating the samples (ready-to-eat dry products) with three variations of NaOH concentrations (0.10 M; 0.20 M; 0.30 M) with a ratio of 1:10 (w/v), for 48 hours. The samples were then dried with the use of a freeze dryer (Lyovapor ™ L-200) and treated with 1 M CH3COOH solution at a ratio of 1:10 (w/v), for 24 hours. Whatman filter paper (Grade 1) was used to obtain the filtrate. Lastly, the collagen obtained was once again dried with a freeze dryer.
The optimal NaOH treated collagen is dissolved with distilled water (1:2 (v/v)) and spun for 5 and 10 hours with a magnetic stirrer (1000 rpm) to establish size transformation. The size of the particles was measured by using the Particle Size Analyzer (PSA), and the antioxidant activity was tested with DPPH Assay (2,2-diphenyl-1-picrylhydrazyl), antiglycation, and tyrosinase inhibitors.
The enzyme-linked immunosorbent assay (ELISA, Sigma #CS0790) was used to determine the antioxidant activity of DPPH (Batubara et al., 2015). 100 μL of each sample along with 100 μL of DPPH (0.3 mM) was added to the 96-well microplate and incubated for 30 minutes in a dark room. The absorbance was measured by using an ELISA reader at a wavelength of 517 nm (Underlying data) (Nurkolis, 2021). The antioxidant activity is calculated as follows:
A0 = Absorbance of blank
A1 = Absorbance of standard or sample
The anti-glycation measurement (Table 2) was carried out as previously described (Povichit et al., 2010) (Underlying data) (Nurkolis, 2021). All the test solutions were incubated at 60°C for 40 hours. After incubation, the aliquots (100 μL) were pipette into a 96-well plate. The relative amount of glycated Bovine Serum Albumin (BSA) was measured using a fluorometer at an excitation wavelength of 370 nm, and emission of 440 nm.
The tyrosinase enzyme inhibitory activity was measured as previously described (Batubara et al., 2015). L-tyrosine and L-DOPA (l-3,4-dihydroxyphenylalanine) were used as substrates (MyBioSource #MBS9301852), and kojic acid as positive controls (Table 5) (Underlying data) (Nurkolis, 2021). Samples were dissolved with dimethyl sulfoxide (DMSO) as stock solution. The concentration variant was prepared by dissolving collagen with a phosphate buffer (pH of 6.5). A total of 70 μL of solution along with 30 μL of tyrosinase enzyme (Sigma, 333 units mL-1 in phosphate buffer solution was added) was pippeted into the 96-well plate, and the mixture was incubated for 5 minutes. To this mixture, 110 μL of substrate (L-tyrosine 2 mM) was added and incubated at 37°C for 30 minutes. The absorbance was measured at a wavelength of 492 nm, by using the microplate reader (Spectrophotometer).
Statistical analyses were performed by using SPPS 26.0 for the Windows version. The differences between samples are analysed based on the antioxidant activity, anti-glycation activity, and tyrosinase inhibition activity tests. The data obtained from three replications (triples) were analyzed by ANOVA at 95% CI (p < 0.05). The result is defined as significant if the p-value is < 0.05.
Table 1 shows the triplicate process resulted in 3.42 (± 1.05%) water content and 2.65 (± 0.50%) ash content.
Collagen yield obtained by each concentration is shown in Table 3. The isolation with NaOH 0.10 M produced the highest collagen yield (p < 0.05), this showed that there was a significant difference in the yield of the three variations of NaOH and CH3COOH treatment. Levenes’ test of homogeneity of variants was p = 0.397 (p > 0.05).
Particle Size Analyzer (PSA) was used to determine the collagen particles size. The collagen yields ranged from 1012 nm to 2455 nm, with the highest DPPH and glycation inhibitions at 2455 nm (11.74% and 62.76%, respectively) (Table 4). In addition to producing significantly different yields, different treatments across the three samples were also significantly different in the particles size (p = 0.000), with p > 0.05 homogeneity. The collagen with the largest particle size of 2455 nm was obtained from 0.10 M NaOH treatment for 5 hours (Table 4).
| Collagen treatment | Particle size (nm) | DPPH inhibition (%) | Glycation inhibition (%) |
|---|---|---|---|
| NaOH 0.10 M | 2455 | 11.74* | 62.76* |
| NaOH 0.20 M | 1012 | 8.13* | 42.50* |
| NaOH 0.30 M | 1922 | 12.39* | 57.43* |
| NaOH 0.10 M (5 Hours) | 1482 | 24.70* | 62.60* |
| NaOH 0.10 M (10 Hours) | 1568 | 9.98* | 41.48* |
The 0.10 M NaOH treatment for 5 hours, resulted in 24.70% and 62.60% antioxidant and anti-glycation activities, respectively (Table 4). However, treatment with 0.10 M NaOH for 10 hours resulted in 9.98% antioxidant and 41.48% anti-glycation activities. Additionally, treatment with 0.10 M NaOH for 5 hours inhibited 8.97% of L-tyrosine and 26.77% of L-Dopa activities (Table 5).
Based on the ash and water content analysis, the powder made from sea grapes and tempe is considered safe to consume, based on the Indonesia National Standard (SNI) No. 01-4320-1996 regulations for food in powder form or powder extract (3% maximum water content). Moreover, pre-treatment was done to remove the non-collagen proteins, as well as assessing the amount of pure collagen proteins in the final product. Collagen is usually insoluble in alkaline solutions, however, NaOH treatment is commonly used in the collagen extraction process as it can significantly minimize collagen loss, compared to other alkaline solutions (Liu et al., 2015). In this study collagen from sea grapes and tempe powder treated with 0.10 M NaOH produced the highest yield, which showed the effectiveness of the extraction process. As indicated by Potaros and colleagues, the difference in yield can be caused by the extraction method, such as the concentration of a solution in the non-collagen protein separation process, and the type of material used (Potaros et al., 2009). Therefore, treatment with variations of NaOH concentration could affect the collagen yields (%), particle size, DPPH inhibition (%) and anti-glycation produced (%).
The collagen particle measurements in this study ranged from 1012 to 2455 nm (Table 4), which was too large to be considered as nanoparticles (10-1000nm) (Mohanraj & Chen, 2007). Therefore, further optimization was carried out in order to reduce the collagen particle size of the 0.10 M NaOH treatment, through stirring for 5 or 10 hours. It is necessary to reduce the particle size in order to increase its absorption by the digestive system (Mohanraj & Chen, 2007). In a study by Mohanrja et al., reducing the particle size should be through the hydrolysis process, and not by a mechanical process such as stirring, as it can re-solidify or coagulate the collagen (Mohanraj & Chen, 2007). However, the hydrolysis process was avoided in this study, as it might have broken down other important compounds, such as antioxidants. Mechanical stirring for 5 hours, resulted in almost 2-fold reduction in the size of the collagen particles. However, stirring for 10 hours did not reduce the particle size due to the reasons described by Mohanraj et al. (2007).
The treatment with 0.10 M NaOH (Table 4), produced the largest particle size with the highest anti-glycation activity compared to other concentrations, however, its antioxidant activity was lower compared to 0.30 M NaOH. The percentage of antioxidants produced is similar to commercial collagen (IC50), which is greater than the result in the study by Fauzi (2018). At 0.10 M NaOH treatment with 5 hours had a better anti-glycation activity than at 10 hours. The resulting anti-glycation activity was higher when compared to the 17.74% activity of the collagen produced in Fauzi dissertation research (Fauzi, 2018).
Excessive melanin production or hyperpigmentation caused by exposure to excessive UV rays can lead to dark skin or depigmentation (Saeedi et al., 2019). Tyrosine inhibition can reduce excessive melanin production, which can prevent skin damage. The results of this study showed that treating L-tyrosine and L-DOPA substrates for 5 hours had a greater tyrosinase enzyme inhibitory activity, compared with treatment for a longer period (Table 5) (Figure 1). In the Fauzi study, commercial collagen did not show tyrosinase enzyme inhibitory activity at 1000 mg/L and exhibited lower activity than the collagen obtained in the present study (Fauzi, 2018).
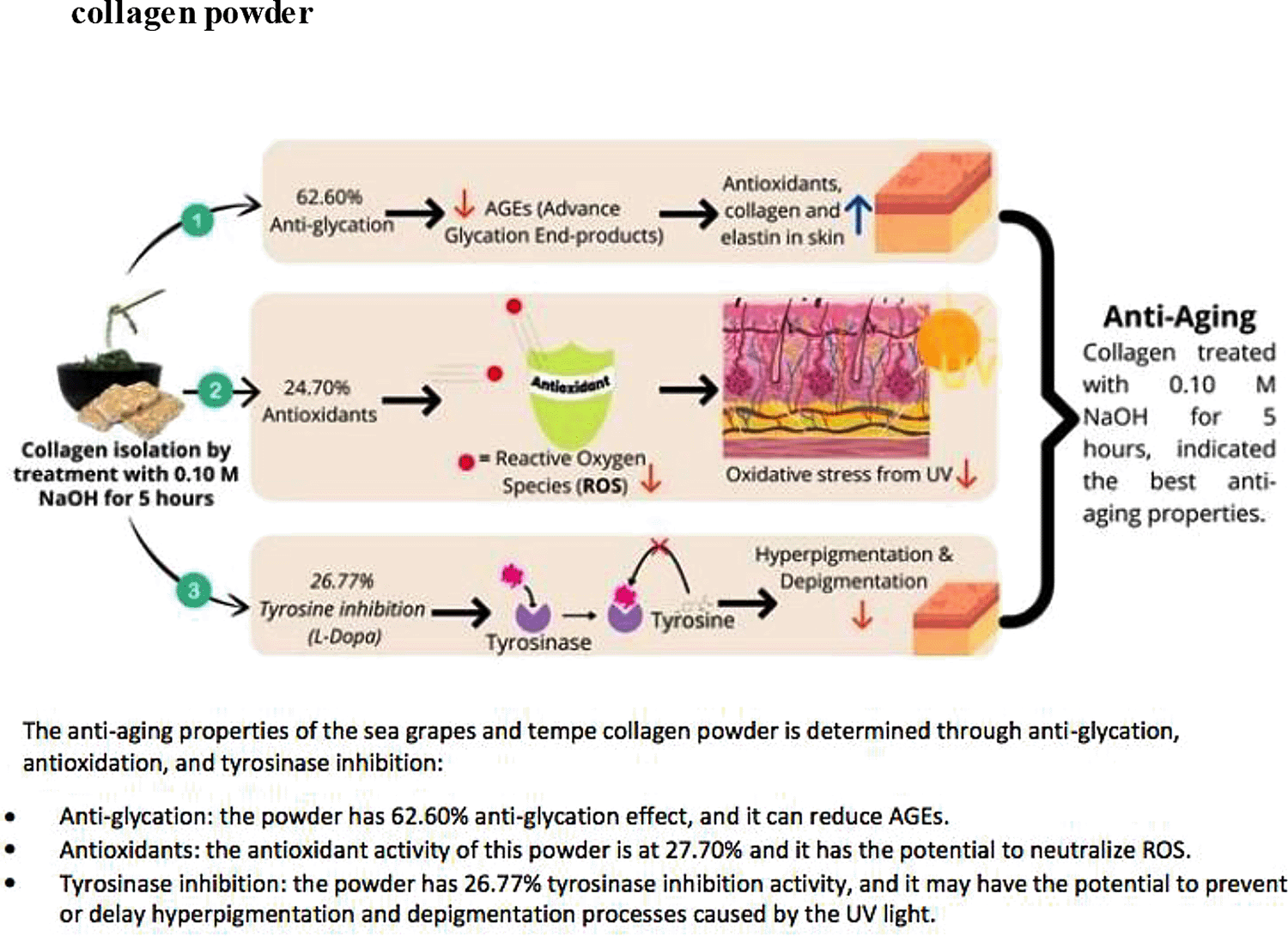
Sea grapes and tempe powder combined with a variety of food additives can be used by manufacturing companies as functional foods or anti-aging nutraceuticals, by NaOH (0.10 M) and CH3COOH (1 M) treatment at 1000 rpm for 5 hours (Figure 1). However, this in vitro pilot study has the potential to be a basic reference for pre-clinical research. Further trials are needed to determine the continued efficacy of this study.
Sea grapes and tempe collagen powder as functional foods or nutraceuticals have anti-aging properties. Based on the anti-glycation, anti-tyrosinase and antioxidant activities, the collagen of this powder treated with 0.10 M NaOH for 5 hours, has the most optimal anti-aging effect. Manufacturers seeking to produce anti-aging food products rich in collagen can use this method for determining the optimal powder formulation, however extensive trials are still needed to further analyze its clinical effects.
Figshare: Sea grapes powder with addition of tempe rich in collagen: An anti-aging functional food.
DOI: https://doi.org/10.6084/m9.figshare.15072597.v3 (Nurkolis, 2021).
The project contains the following underlying data:
• Raw data: Water and ash content, antioxidant activity, glycation inhibition activity, particle size, anti-tyrosinase activity of the collagen. The chemical composition of the solution in the anti-glycation activity test.
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
All authors contributed to the writing and revision of this article; and all authors have read and approved the final manuscript. H. K. P. and F. N. gathered study ideas, designed the experiments, analyzed data, and compiled manuscripts. N. A. T., H. H., N. S., M. K., S. R., R. R. and N. M. analyzed and interpreted data and critically revised the manuscript. The F. N., S. L. N., D. S. W. and H. K. P. conducted experiments, analyzed biochemistry, and critically revised the manuscript. N. M., S. C. B., W. B. G., and C.D.V., implemented experimental protocols, assisted in statistical analysis, interpreted data, and critically revised manuscripts. All writers read and approve the final manuscript.
We thank State Islamic University of Sunan Kalijaga; Faculty of Medicine-Brawijaya University and all of contributors for their outstanding help in formatting the paper. I would also like to express my gratitude to Prof. Ir. Hardinsyah, MS., Ph.D. (as President of the Federations of Asian Nutrition Societies; President of the Food and Nutrition Society of Indonesia; and Chair of Southeast Asia Probiotics Scientific and Regulatory Experts Network), and Prof. Dr. Nurpudji A Taslim, MD., MPH.,Sp.GK(K) (Chair of Indonesian Clinical Nutrition Physician Association), and also to Nindy Sabrina, S.Gz., M. Sc. who has provided comments, suggestions, and input in the research and writing of this paper, as well as the motivation he has given the writers to keep the passion for research during the pandemic.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: I collaborated with Professor Taslim on an article titled, Protein-Energy Nutritional Status of Moderately Low Protein Intake-Sago Diets Compared to Sufficiently Protein Intake-Rice Diets in Well-Nourished Lowlanders in Papua, Indonesia (https://f1000research.com/articles/11-138/v1). I am convinced that I have been fair in my peer review. I have not heard anything about the article concerned from my collaborator, Professor Taslim. Professor Taslim is neither the first nor the lead author of the paper. Therefore, I have no involvement in the planning or execution of this research. Professor Taslim has made significant contributions to our "Papua Study," particularly in the conduct of field research. However, the collaborative research relationship in this "Papua Study" is unrelated to the collaboration between Professor Taslim and the sea grape researchers, and I am neither a potential competitor nor a potential collaborator of theirs. I declare that I have had no contact with Professor Taslim between the time I was asked to review the manuscript and the time I submitted the report.
Reviewer Expertise: Pharmacology and Nutritional Science
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 3 (revision) 27 Jul 22 |
read | read |
|
Version 2 (revision) 19 Apr 22 |
read | read |
|
Version 1 11 Aug 21 |
read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)