Keywords
metagenome-assembled genome, MAG, genome quality, MIMAG, dereplication, completeness, contamination, nextflow, docker
This article is included in the Bioinformatics gateway.
metagenome-assembled genome, MAG, genome quality, MIMAG, dereplication, completeness, contamination, nextflow, docker
Genome-resolved metagenomics is one of the most promising approaches to identify and characterize novel microbial species. Large-scale environmental and host-associated studies demonstrated how metagenomics can expand our knowledge of uncultivated prokaryotes, recovering thousands of metagenome-assembled genomes (MAGs) of new archaeal and bacterial species.1,2 For this reason, automated and reproducible methods for assessing the quality of MAGs play a critical role.
To recover MAGs, metagenomic sequence reads are first assembled into contigs using specific algorithms.3 Contigs are then processed by tools like MetaBAT 24 or VAMB5 that use tetra-nucleotide frequency (TNF) profiles and abundance patterns to group sequences that are likely to belong to the same organism (binning). Binning improves the interpretability of metagenomic data, but at the same time represents (together with assembly) a significant source of error.6 Manual refinement7 can increase the quality of resulting MAGs, but undermines the reproducibility of the analysis and is unfeasible for large-scale studies.
The recently introduced Minimum Information about a Metagenome-Assembled Genome (MIMAG) standard8 recommends a set of measures for assessing the quality of MAGs. This comprises basic assembly statistics (e.g. N50), genome completeness, contamination and the presence of ribosomal RNA (rRNA) and transfer RNA (tRNA) genes.
Recovering this information involves computational pipelines composed of a series of specialized tools that are often difficult to use and install. Moreover, each task can require parameters and custom scripts that are often poorly documented, making reproducibility of results challenging. Tools and standards such as Galaxy,9 Nextflow10 and the Common Workflow Language,11 coupled with container technologies like Docker, allows researchers to circumvent these issues, providing a way to build, run and share reproducible computational workflows.12
We present metashot/prok-quality, a comprehensive and easy-to-use Nextflow pipeline for assessing the quality of draft prokaryotic genomes. Metashot/prok-quality reports the quality statistics and estimates recommended by the MIMAG standard. Basic assembly statistics, completeness, both redundant and non-redundant contamination, rRNA and tRNA genes are reported in a single, comprehensive table.
Metashot/prok-quality is written using the Nextflow domain-specific language. Nextflow is a framework for building scalable scientific workflows using containers, allowing implicit parallelism on a wide range of computing systems. Reproducibility is guaranteed by versioned Docker images, which enclose software applications together with their dependencies, allowing isolation from the host environment and portability across platforms. metashot/prok-quality v1.2.0 is composed of five main modules (Figure 1) and includes several custom scripts, designed to manipulate the output of the different tasks.
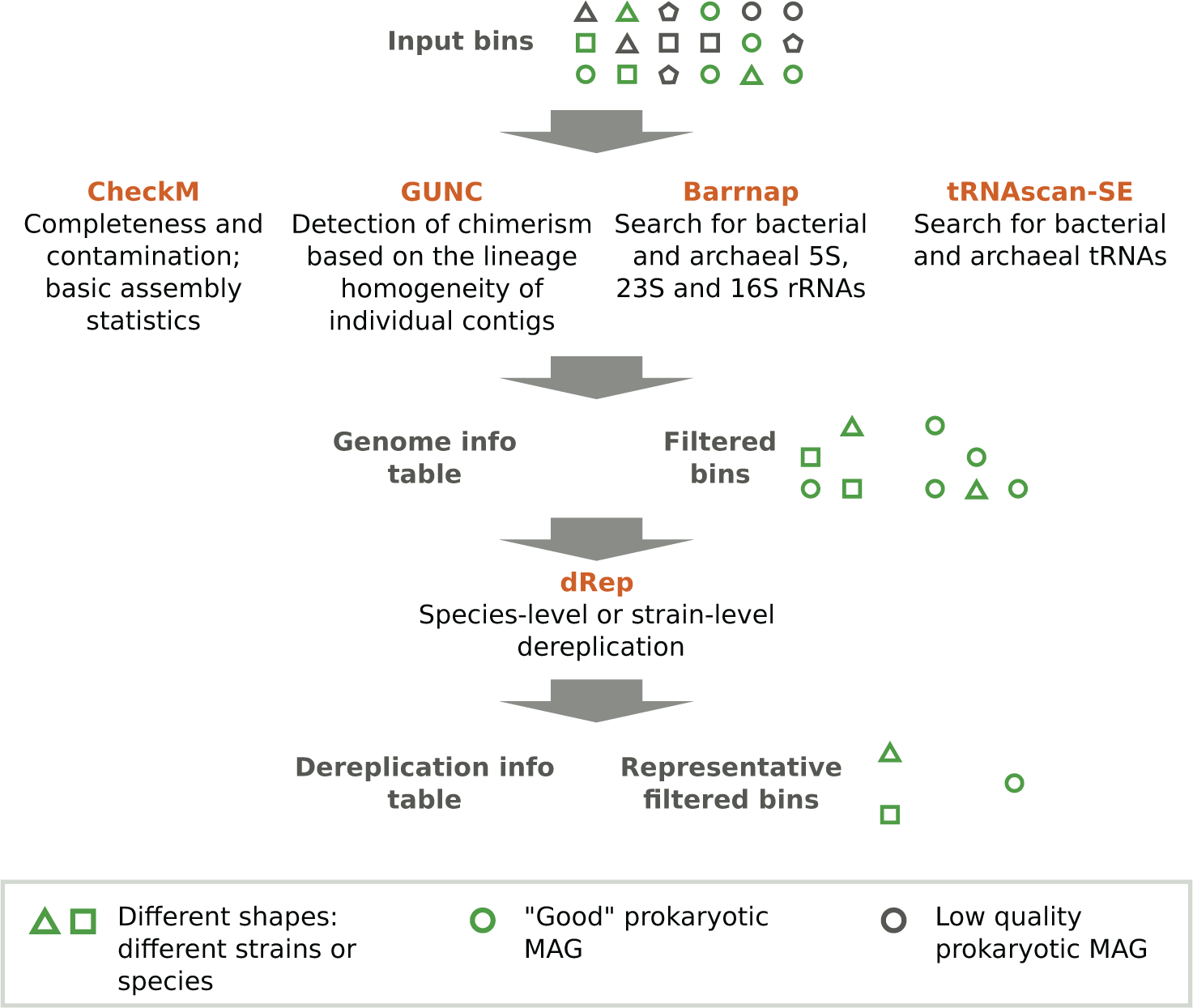
The workflow takes a series of genomes (input bins) in FASTA format and returns: i) a tab-separated values (TSV) file including, for each input genome, the quality information recommended by the MIMAG standard (genome info table); ii) a directory containing the bins filtered according the completeness and contamination thresholds; iii) a TSV file listing the cluster membership of each genome after the dereplication (optional) and iv) a directory containing the cluster representatives. The original outputs of each task (e.g. Barrnap’s GFF output) are also reported in dedicated folders.
Software included in version 1.2.0:
CheckM v1.1.2. Several tools have been developed for the assessment of completeness and contamination of MAGs. The proposed workflow includes the widely used CheckM13 which estimate these metrics using ubiquitous and lineage-specific, single-copy core genes (SCGs) catalogs. CheckM is also used to recover the basic assembly statistics.
GUNC v1.0.1. SCG-based tools like CheckM can have very low sensitivity towards contamination by fragments from unrelated organisms (non-redundant contamination).6 In order to circumvent this problem, the recent GUNC14 tool was added to the pipeline. GUNC quantifies the lineage homogeneity of contigs with respect to the full gene complement, accurately detecting chimerism induced by both redundant and non-redundant contamination.
Barrnap v0.9. The presence of 5S, 23S and 16S rRNA genes is predicted by the BAsic Rapid Ribosomal RNA Predictor (Barrnap) using Hidden Markov models (HMM). Both bacteria and archaea databases are used.
tRNAscan-SE v2.0.6. tRNA genes are searched using tRNAscan-SE,15 using bacteria and archaea covariance models. The number of tRNAs and tRNA isotypes found is reported.
dRep v2.6.2. Dereplication is a procedure that groups the input genomes according to their whole-genome similarity, using metrics such as the Average Nucleotide Identity16 (ANI). Dereplication dramatically simplifies downstream analysis when the input genomes come from different sources.17 In the proposed workflow, filtered genomes (genomes that pass completeness, contamination and GUNC filters) are optionally dereplicated using dRep.18 For each cluster, dRep reports, as the cluster representative, the best-scoring MAG using the CheckM’s quality estimates. The score is computed using the following formula:
score = completeness − 5 × contamination + 0.5 × log(N50)
Python3 custom scripts. The workflow includes three Python3 custom scripts, designed to manipulate the output of the different steps. The scripts make use of NumPy,17 Pandas and scikit-learn libraries.
metashot/prok-quality v1.2.0 requires Docker and Nextflow (tested on v20.07.1). Alternatively, the Singularity container engine can be used in place of Docker. At least 70 GB of RAM is required, a limit imposed by CheckM (v1.1.2). The workflow can run in a workstation with 16 GB of RAM using the options --reduced_tree and --max_memory 16.GB.
As mentioned above, metagenome assembly tools combine the sequence reads into larger regions called contigs. Recently, many metagenomic assembly tools have been proposed. Amongst these, metaSPAdes3 and MEGAHIT19 have been shown to be able to efficiently handle large-scale short read sequencing data, producing high-quality contigs. Metagenomics contigs are then processed by tools like MetaBAT 24 in order to group sequences that are likely to belong to the same organism (binning). After binning, it is essential to assess the quality of the resulting candidate draft genomes.
In this section, we will show how to assess the quality of draft prokaryotic genomes using metashot/prok-quality. Given a series of candidate genomes in FASTA format stored in the “bins” directory, the version 1.2.0 of the workflow can be run with the following command line:
nextflow run metashot/prok-quality -r 1.2.0 \--genomes 'bins/*.fa' \--outdir results
A series of files and directories are created in the output directory results. The main output file is “genome_info.tsv”. This TSV file contains, for each input genome, a set of quality statistics, including completeness, contamination, GUNC filter, N50, rRNA genes found, number of tRNA and tRNA types. The columns included in this file are:
• Genome: the genome filename;
• Completeness, Contamination, Strain heterogeneity: CheckM estimates;
• GUNC pass: if a genome does not pass GUNC analysis it means it is likely to be chimeric;
• Genome size (bp), ... , # predicted genes: basic genome statistics (see https://github.com/Ecogenomics/CheckM/wiki/Genome-Quality-Commands#qa);
• 5S rRNA, 23S rRNA, 16S rRNA: “Yes” if the rRNA gene was found;
• # tRNA, # tRNA types: the number of tRNA and tRNA types found, respectively.
The directory “filtered” contains the genomes (in FASTA format) filtered according to --min_completeness, --max_contamination and --gunc_filter options (see below). The TSV file “genome_info_filtered.tsv” includes the same information as “genome_info.tsv”, but for the filtered genomes only. Representative (dereplicated) genomes (default ANI threshold 0.95) are reported in the “filtered_repr” folder. The companion file “derep_info.tsv” contains the summary of the dereplication procedure, including the genome filename, the cluster ID and the representativeness. A set of secondary directories contains the original output of each tool included in the pipeline:
• checkm: contains the original CheckM's “qc” file;
• gunc: contains the original GUNC output file;
• barrnap: includes the predicted rRNA sequences for bacteria (.bac) and archaea (.arc) models in GFF and FASTA formats;
• trnascan_se: includes the predicted tRNA sequences for bacteria (.bac) and archaea (.arc) models in TSV and FASTA formats;
• drep: dRep original data tables, figures and log file.
The command options are:
Input and output
• --genomes: input genomes/bins in FASTA format (default “data/*.fa”);
• --ext: FASTA files extension, files with different extensions will be ignored (default “fa”);
• --outdir: output directory (default “results”);
• --gunc_db: the GUNC database. If “none” the database will be automatically downloaded and will be placed the output folder (gunc_db directory) (default “none”);
• --reduced_tree: reduce the memory requirements to approximately 14 GB, set --max_memory to 16.GB (default false);
• --checkm_batch_size: run CheckM on “checkm_batch_size” genomes at once in order to avoid memory issues, see https://github.com/Ecogenomics/CheckM/issues/118 (default 1000);
• --min_completeness: discard sequences with less than “min_completeness” % completeness (default 50);
• --max_contamination: discard sequences with more than “max_contamination” % contamination (default 10);
• --gunc_filter: if true, discard genomes that do not pass the GUNC filter (default false);
Source code available from: https://github.com/metashot/prok-quality
Archived source code at time of publication: http://doi.org/10.5281/zenodo.4475355.20
License: GPL-3.0
Docker image definitions available from: https://github.com/metashot/docker
Zenodo: metashot/prok-quality v1.2.0 with test data, v1.2.0, http://doi.org/10.5281/zenodo.4475355.3
This project contains test data and workflow documentation.
Data are available under the terms of GNU General Public License version 3 (GPL-3).
Docker Hub: metashot docker images, https://hub.docker.com/u/metashot
This registry contains the pre-built Docker images
GitHub: metashot/docker, https://github.com/metashot/docker
This project contains Docker image definitions.
The authors wish to thank Giuseppe Cossu and the Information Technology team of the Fondazione Edmund Mach for technical support.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the rationale for developing the new software tool clearly explained?
Yes
Is the description of the software tool technically sound?
Yes
Are sufficient details of the code, methods and analysis (if applicable) provided to allow replication of the software development and its use by others?
Yes
Is sufficient information provided to allow interpretation of the expected output datasets and any results generated using the tool?
Yes
Are the conclusions about the tool and its performance adequately supported by the findings presented in the article?
Yes
References
1. Lupo V, Van Vlierberghe M, Vanderschuren H, Kerff F, et al.: Contamination in Reference Sequence Databases: Time for Divide-and-Rule Tactics.Front Microbiol. 2021; 12: 755101 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: bioinformatics, including genomic contaminations
Is the rationale for developing the new software tool clearly explained?
Yes
Is the description of the software tool technically sound?
Yes
Are sufficient details of the code, methods and analysis (if applicable) provided to allow replication of the software development and its use by others?
Yes
Is sufficient information provided to allow interpretation of the expected output datasets and any results generated using the tool?
Yes
Are the conclusions about the tool and its performance adequately supported by the findings presented in the article?
Yes
References
1. Orakov A, Fullam A, Coelho L, Khedkar S, et al.: GUNC: detection of chimerism and contamination in prokaryotic genomes. Genome Biology. 2021; 22 (1). Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: metagenomics; microbiome
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 17 Aug 21 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)