Keywords
xylene, polycyclic aromatic hydrocarbon, cervical cancer, Pap test, pollution, Peru
xylene, polycyclic aromatic hydrocarbon, cervical cancer, Pap test, pollution, Peru
Details on xylene have been added to the methodology. We describe the characteristics of xylene (brand used) and the bioaccumulation process.
See the authors' detailed response to the review by Nasar Yousuf Alwahaibi
See the authors' detailed response to the review by Alexandre Rieger and Alessandra Koehler
Xylene (C6H4(CH3)2) is a polycyclic aromatic hydrocarbon (PAHs) part of the aromatic BTXs (benzene, toluene, and xylenes) obtained from petroleum, from the dry distillation of wood, and from coke gases. Xylene can have one of the three isomers of dimethylbenzene or a combination thereof, which are used as solvents (in fuel formulations, and glue in plastic model kits etc.).1 In medical practice, xylene is used in cellular clarification in the final phase of tissue processing.2,3
Since George Papanicolaou’s research began in 1914 at Weill Cornell University, these PAHs have been used as part of the Papanicolaou (Pap) stain to clear cells at the end (clearing) of the process.4 The preliminary Pap protocol as well as the modifications that he made in 1953 and 1959 indicates the use of three xylene baths (~350 ml each) during the process.5 These protocols, and several modifications6,7 suggest the use of one or three xylene baths, causing high exposure to workers (as several hospitals do not have adequate protection barriers), bioaccumulation, and environmental pollution (due to the incorrect handling of these wastes).8
Even though there are techniques for the biodegradation of xylene such as the use of iron and manganese from underground aquifers, the biofiltration of xylene by microorganisms adhered to a Nylon support,9,10 and modifications of the Pap stain that avoid the use of xylene (and other pollutants),8,11,12 laboratories in many countries continue using xylene as a cellular clarifier in two or three baths during the process. According to the standardized operating procedures of each cytology laboratory, these baths are replaced every thousand slides or once a week.12
In this study, we aimed to estimate the impact of xylene during the Pap test in terms of number of liters and costs for two baths of xylene, and also estimated the impact with three baths for the next five years (2020–2025) following the 2018 Peruvian Social Security cytology guideline.
This was a retrospective longitudinal study that used a model for estimating xylene prices and quantities through logistic regression and the Bayes test. This study was approved by the Institutional Review Board of the Universidad Norbert Wiener (Document UNW-N° 072-2020). The study was conducted in two stages with data on costs and quantities (in liters) of xylene used in cytology over a five-year period (2015–2019) in four hospitals (Figure 1) of Social Security (EsSalud) of Peru.13

Hospital Nacional Guillermo Almenara Irigoyen, Hospital Nacional Edgardo Rebagliati Martins, and Hospital Nacional de Emergencias Grau in Lima, and the Hospital Nacional Alberto Sabogal Sologuren in Callao province. Maps by ©Jeel Moya-Salazar.
o Hospital Nacional Guillermo Almenara Irigoyen (HNGAI)
o Hospital Nacional Edgardo Rebagliati Martins (HNERM)
o Hospital Nacional Alberto Sabogal Sologuren (HNASS)
o Hospital Nacional de Emergencias Grau (HNEG)
The EsSalud cytology guide, Chapter 9. Cervical cytology procedures in the laboratory: Section 9.5.1. Manual staining describes the process, which includes three xylene baths (10 minutes each) at the end of the staining procedure (https://ww1.essalud.gob.pe/compendio/pdf/0000003706_pdf.pdf).14,15 This new EsSalud cytology guideline aims to improve the Pap test procedure, seeking to standardize the process to reduce the incidence of cervical cancer. The report does not present a cost-effectiveness analysis and explains the necessary materials and equipment, but does not clearly describe the importance of changing each cytological sub-process. A brief English summary of the Pap stain process is available as supplemental material.14
The inclusion criteria for this study were: i) EsSalud Hospital, ii) a hospital that performs a large number of Pap tests per year (approximately 25,000 Pap tests each), iii) a hospital that uses manual exfoliative cytology procedures. Hospitals belonging to the Ministry of Health, Armed Forces and Private Care Centers were excluded. These four tertiary hospitals are EsSalud's main reference hospitals for cytology and cancer screening in Lima and Peru.
In the first stage we collected the data on xylene use for the period 2015–2019 in the four hospitals.13 The amount of xylene used for exfoliative cytology was ~10 liters per bath/month (with two xylene-baths),14,15 and the global cost (EsSalud) per liter of xylene was 8.13 US dollars (USD) per liter (27 soles). We estimated the number of cytology tests and the amount of xylene per year used as part of the routine Pap test, which follows the second protocol of Papanicolaou.4
For the second stage, we followed the recommendations of the 2018 EsSalud cytology guidelines14,15 that establishes the addition of a third xylene bath during Papanicolaou staining. We considered the use of three xylene baths for each cervical smear, estimating the monthly usage in each hospital. As in the previous stage, the cost of xylene was estimated.
We used the direct costs of purchasing xylene without considering the indirect costs of processing, logistics management and usage (man-hours) during the Pap test. The cost of one liter of xylene was determined according to the contract between the manufacturer and EsSalud, which is a standardized contract for all hospitals in Lima. EsSalud hospitals mostly use Neo-Mont or DPX from Merck (Darmstadt, Germany).
Hence, we estimate the impact of pollution of xylene, initially by the amount of xylene used by the workers who were exposed daily to xylene under the biosafety conditions available in each hospital. Direct exposure to xylene in the daily workflow puts laboratory personnel at high risk, as the EsSalud laboratory assessed does not have a biological safety cabinet (Figure 2). Then, we estimated the pollution load based on the bioaccumulation of xylene during use, because the waste collection cycle is once every two months (hospital accumulation process). The used xylene is poured back onto the initial vials, recording the start and end days of the cycle of use. The quantity of liters and the costs (in USD) for xylene were estimated only for the Pap tests (we exclude the amount of xylene used in histology).

(A) Guillermo Almenara Irigoyen National Hospital, (B) Hospital Nacional de Emergencias Grau, (C) Edgardo Rebagliati Martins National Hospital. Note that all staining centers are manual and are exempt from basic protection barriers such as the laminar flow chamber and air filtration systems.
In this sense, the monthly amount of xylene used in each hospital was estimated to establish the annual and the total cost (in liters). In the second stage, these costs and contamination estimates were made for the next five years (2020-2025) under the same parameters. The maximum and minimum amounts of xylene per year were used to estimate the costs and future quantities of xylene.
Statistical analysis was performed with descriptive statistics and frequency measures. To estimate the cost, directly purchased xylene was used, with an estimated price of USD 8.13 (27 soles) per liter. In order to assess the impact of contamination (two baths for cytological clearance), the annual reagent usage and the annual xylene bioaccumulation rate of four hospitals in Lima were considered. To estimate the impact of pollution and direct costs (between 2020–2025), of using three xylene baths, multiple logistic regression with Bayesian analysis was used, with a p-value <0.05 and a 95% confidence interval (CI) as significant. We used IBM Statistical Package for the Social Sciences (SPSS) v25.0 (Armork, US) for Linux for all data analysis.
We included 131,456 cytology tests in the HNGAI, 254,106 in the HNERM, 110,858 in the HNASS, and 98,478 in the HNEG. The use of xylene during for the HNGAI, HNERM, HNASS and HNEG was 2,369, 2,299, 1,637, and 1,179 liters, respectively. Overall, a total of 7,484 liters of xylene were used for Pap stains in four EsSalud hospitals during a five-year period in Lima, Peru (Table 1). The mean rate of xylene use was 473.8 ± 88.1 liters (95%CI 396.5 to 551.1) for HNGAI, 459.8 ± 63.5 liters (95%CI 404.1 to 515.5) for HNERM, 327.4 ± 136.3 liters (95%CI 207.9 to 446.9) for the HNASS, and 235.8 ± 111.9 liters (95% CI 137.7 to 333.9) for the HNEG (Figure 3). Using this amount of xylene in four hospitals resulted in a cost of USD 60,853 (202,068 soles).
These data were estimated according to the amount of annual Pap tests during 2015–2019.
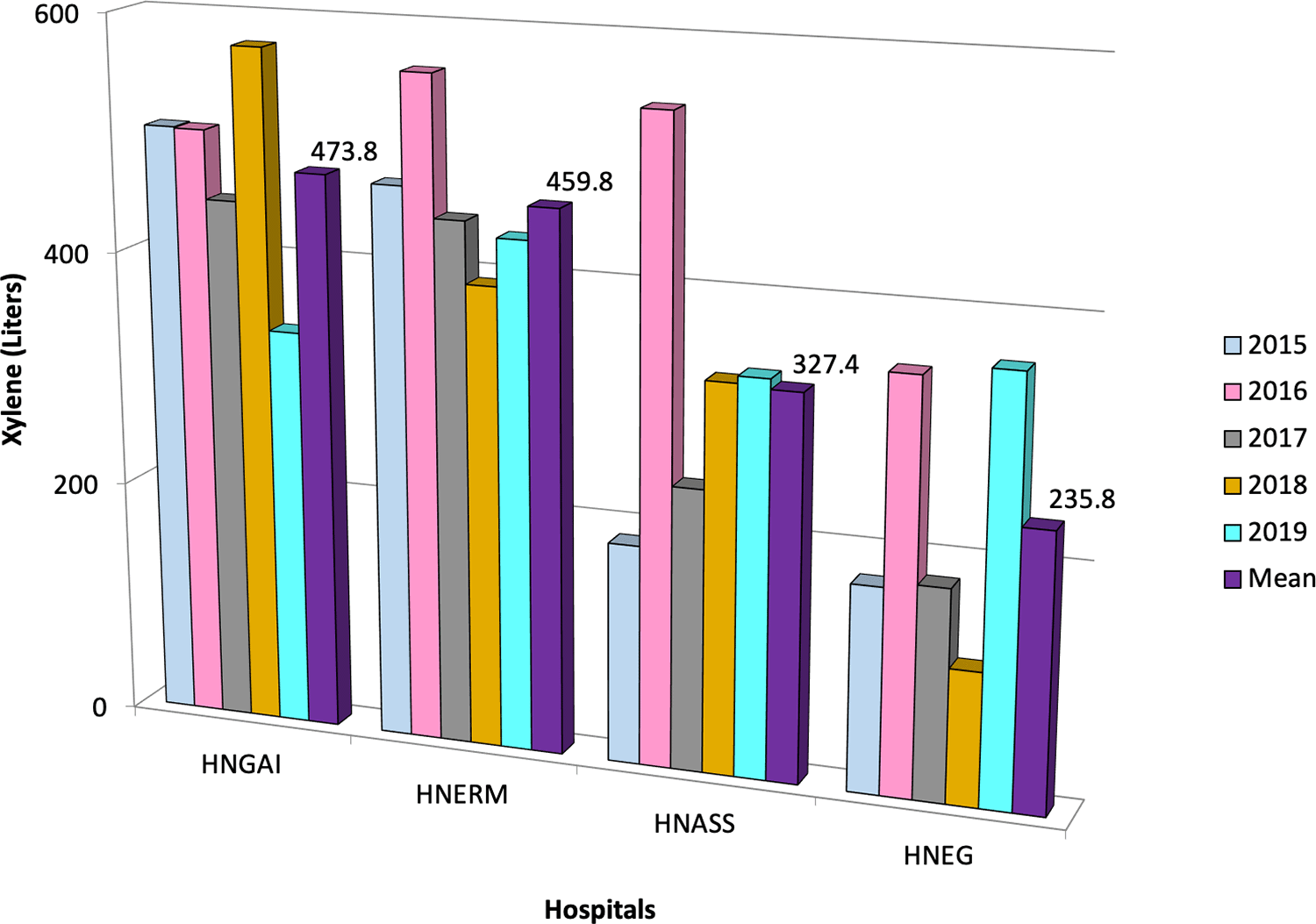
Within the study period, the mean xylene used is shown for each year according to color. HNGAI: Hospital Nacional Guillermo Almenara Irigoyen, HNERM: Hospital Nacional Edgardo Rebagliati Martins, HNEG: Hospital Nacional de Emergencias Grau, HNASS: Hospital Nacional Alberto Sabogal Sologuren in Callao province.
When estimating future costs for the five-year period from 2020 to 2025, the biggest assumption is that the use of three xylene baths in four hospitals is estimated at 9,483 liters and 77,110 USD (256040 soles). The lowest assumption for the use of three xylene baths in the next five years is estimated to be 5,485 liters and 44,590 USD (148,080 soles) (p = 0.0025) (Table 2). For HNGAI, HNERM, HNASS and HNEG, the maximum volume of the three xylene baths used is 561.9, 523.3, 463.7 and 347.7 liters, respectively. For HNGAI, HNERM, HNASS and HNEG, the minimum volume of the three xylene baths used is 385.7, 396.3, 191.1, 123.9 liters, respectively.
We describe the quantities and, maximum and minimum costs estimated in this study at four EsSalud hospitals during 2015–2019 in Lima, Peru.
These estimates of future costs showed increases and decreases according to the hospitals (Figure 4). In the four hospitals, the liter and cost of xylene increased by 30.4% and 30.3% USD on average, respectively. The average reduction in the cost and liters of xylene was 25.3% liters and 24.3% USD, respectively. A difference was determined between the increases in xylene use among EsSalud hospitals (p = 0.0001).

A. Hospital Nacional Guillermo Almenara Irigoyen, B. Hospital Nacional Edgardo Rebagliati Martins, C. Hospital Nacional Alberto Sabogal Sologuren, D. Hospital Nacional de Emergencias Grau. The increase indicates the maximum percentage of increase in the 2020–2025 periods in liters and costs of xylene. The reduction indicates the minimum percentage of reduction in the five-year period 2020–2025 in liters and costs of xylene.
We determined that xylene had a high impact during the Pap test with two baths in four EsSalud hospitals, and a dramatic increase in costs and xylene contamination in the next five years (2020–2025) with the use of three xylene baths on the Pap smear according to the recommendations of the 2018 EsSalud cytology guidelines.
In the environment, xylene can cause air pollution due to the production of toxic gases (when it is thermally decomposed), polluting water, soil, and subsoil.16 Inhalation of xylene can irritate the mucous membranes of the nose and throat, and high concentrations can cause nausea, vomiting, headache, respiratory failure, cough, heart abnormalities, proteinuria, and hematuria.17 Cancer, leukemia, brain tumors and tissue changes (pulmonary long-term exposure and long-term high concentration) are also related to xylene contamination.18,19 Other effects include fetal kidney disease, infertility and miscarriage in children whose mothers have been in contact with xylene for a long time.20
Our estimates of xylene use in hospitals indicate that 594,898 cervical smears analyzed by Pap staining use >7,000 liters of xylene every five years. The same estimation of the use and contamination by xylene can be applied in other realities such as the 243,374 smears of the Hospital Nacional Docente Madre Niño San Bartolomé in Lima during two periods [118,016 for the conventional Pap staining (2011–2013 years) and 125,358 for the prolonged Pap staining (2013–2014 years)],11,21 to the 3,276,045 cervical smears of the Instituto Mexicano del Seguro Social in Mexico,22 and in the 60 million cervical smears performed annually in the United States.23 We can infer that in every country, cervical cancer screening will involve a large amount of xylene, which will threaten the health of laboratory workers and global environmental health.
Implementing a third xylene bath to the Pap test would undoubtedly have even more counterproductive results. As our findings indicate, there will be an increase in the overall costs of the tests (77,110 USD), the time to stain each sample, and the daily exposure of workers to higher amounts of xylene (9,483 liters). A previous study demonstrated that 37 national guidelines on prevention measures for the use of xylene in South American cytology laboratories were of little importance.24 In Peru, xylene is usually stored after use until disposal. It is not reused due to the regulation of controlled reagents, therefore it bioaccumulates until its elimination as a whole. However, there is no global regulation that controls all health centers, so many hospitals eliminate xylene through the drain as well as other hospitals that massively bioaccumulate xylene until they are finally disposed of without environmental protection due to the inefficient waste management system. In this sense, under the actual conditions of cytological screening in low-and-middle-income countries, adding an additional xylene bath to the Pap stain is unsustainable and therefore should not be considered.
Currently, there are xylene substitutes that allow diagnostic activities to be carried out in the pathology laboratory in a less toxic and eco-friendly environment. These substitutes are mainly the solution of Neo-Clear (Merck, Darmstadt, Germany), Pathoclear (Biopack, Buenos Aires, Argentina), Master clear (American MasterTech Scientific, CA, USA), UltraClear™ (Avantor’s JT Baker, Deventer, Netherlands), Shandon™ (Thermo Scientific, Walthman, MA, USA), and Ottix Plus (DiapathS.pA, Martinengo, Italy), which have shown different degrees of performance and health risks.25–27 Regardless of the opportunities these surrogates offer, they come with costs for Pap processing that many cytology labs cannot afford. In view of this, Eco-Pap (patent application pending) brings an alternative to ecological staining, which can reduce the number of reagents, staining time and cost without reducing test performance.8,12
On the other hand, our estimated analysis model for three xylene baths shows that the cost of the Pap stain and the liters of xylene solvent are reduced. However, this assumption could not be further from reality, given that exfoliative cytology has a new stage.28 This fact is partly due to the co-testing in which the Human Papilomavirus test and cytology are used as a strategy with better performance and diagnostic accuracy.29 It is also because many countries, mainly low- and middle-income countries, have strategies based on exfoliative cytology due to the benefits that this technique offers and for the economic limitations that these countries face.30,31
Another component that distances this assumption of reducing quantities and costs of xylene from reality is undoubtedly the alarming increase in the incidence and mortality from cervical cancer between 2012 and 2018 according to the International Agency for Research on Cancer (IARC) from 527,624 and 569,847 cases for the former, and from 265,672 to 311,365 cases for the second.32
In this sense, the use of cytology as a cervical cancer strategy will continue to develop and grow around the plausibility of other techniques such as molecular biology. Therefore, if a xylene bath is added to the Pap test, the amount and cost of xylene in Peru’s EsSalud Hospitals will increase by an average of 16.6% in the next five years.
This study had limitations. 1) The analysis was conducted in four EsSalud hospitals in Lima, however, there are other hospitals that have not been included. Further studies that include other Social Security hospitals are needed; 2) This is the first study in Peru that evaluates the cost of using xylene in cervical cytology. However, due to restricted access and the limited availability of data from each hospital, the cost found has not included the time of use, the need for skilled labor, process automation, xylene bioaccumulation, waste management, and the use of xylene substitutes. These characteristics should be included in future studies; and 3) The analysis evaluated the xylene used in the Pap tests; however, it is necessary to evaluate its impact on histopathology.
We demonstrated a high economic impact of xylene during the Pap test with two xylene baths in four EsSalud hospitals, in Lima, Peru. Our three-bath estimates for 2020–2025 indicate that the economic growth of xylene use is significant, which is why the 2018 EsSalud cytology guidelines are not applicable.
Nowadays, there are some alternative methods that can make Pap smears free of xylene during the clearing process, thereby becoming ecofriendly, which is beneficial to occupational health and global environmental health.
Figshare: ‘Cost and pollution by the use of xylene in cervical cytology in four Peruvian hospitals’,
https://doi.org/10.6084/m9.figshare.14615508.v113
This project contains the following underlying data:
• Table 1. Xylene database used in EsSalud hospitals 2015–19
Figshare: ‘Cost and pollution by the use of xylene in cervical cytology in four Peruvian hospitals’, https://doi.org/10.6084/m9.figshare.15048903.v1.14
This project contains the following underlying data:
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY-4.0)
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Histopathology
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Molecular biology; Genetics; Genotoxicity
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Histopathology
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Molecular biology; Genetics; Genotoxicity
Is the work clearly and accurately presented and does it cite the current literature?
No
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Molecular biology; Genetics; Genotoxicity
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Histopathology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 3 (revision) 08 Jun 22 |
read | read |
|
Version 2 (revision) 21 Apr 22 |
read | read |
|
Version 1 25 Aug 21 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)