Keywords
Aeromonas hydrophila, bivalent vaccine, monovalent vaccine, Pseudomonas fluorescens, Streptococcus agalactiae.
This article is included in the Agriculture, Food and Nutrition gateway.
Aeromonas hydrophila, bivalent vaccine, monovalent vaccine, Pseudomonas fluorescens, Streptococcus agalactiae.
This new version of the article has been improved according to the reviewers' comments and suggestions. The improvements in the introduction part, include more references on vaccination in tilapia, an explanation of the stage of offspring is the immune system not ready for immune response, and an explanation of the types of Ig that are transferable through eggs. The improvement in the method such as the reference for the two formalin concentrations used for the inactivation of bacteria, the site of IM injection, and provide the reference, the final bacterial concentration (cfu/mL), and the antigen preparation for the direct agglutination test. The author has discussed low survival and how to improve them, the negative control.
To read any peer review reports and author responses for this article, follow the "read" links in the Open Peer Review table.
Tilapia was originally considered to be more resistant to bacterial, parasitic, mycological, and viral diseases than other species of cultivated fish. However, they are found to be susceptible to bacterial and parasitic diseases1–3, particularly during the offspring phase4. Globally, the control of bacterial disease mostly uses antibiotics that are proven not environmentally friendly5–7. Some common diseases of tilapia found in several Southeast Asian countries including Indonesia are Streptococcus agalactiae, Aeromonas hydrophila, Edwardsiella ictaluri, Flavobacterium columnaris, and Pseudomonas fluorescens8–10. In addition to the bacterial disease, a new disease has emerged called Tilapia Lake Virus (TiLV) whose specific host is tilapia, causing disease outbreaks with high mortality rates in several Southeast Asian countries such as Thailand11 and Malaysia12.
Among the various methods of disease control, vaccination is one of the most effective ways, which is commonly used5,13–16. The administration of vaccines is meant to produce antibodies that could improve the immunity of tilapia3,5. Unfortunately, they could not be administered to their offspring because the organs that form the immune response are not yet fully developed, therefore they are unable to produce antibodies7,13–17. Tilapia fry was not able to produce their own immune system at the age of less than 21 days18, Immune systems of Xenopus laevis develop within 2 weeks of age19, while Indian major carp develop within 3 weeks of age20.
An effective solution to the aforementioned issue is the application of maternal immunity transfer. This is the transfer of immunity from broodstock to offspring, by which immunoglobulin (IgM type) are transferred through eggs19,21,22. Maternal immunity has been shown to improve the fish offspring’s immunity against pathogens in the early phases of their life23–26.
This process is usually carried out using monovalent vaccines27–30. However, a polyvalent vaccine would be more effective because it could control multiple diseases3,31,32 especially using a formalin-killed vaccine with low production cost compared to other types of vaccines3. Though the effectiveness has been known, the application of polyvalent vaccines through maternal immunity has not been extensively investigated, particularly in Nile tilapia (O. niloticus).
The transfer of maternal immunity using polyvalent vaccine for S. agalactiae, Lactococcus garvieae, and Enterococcus faecalis has been studied by Abu-elala et al.,33 and three vaccine strains for S. agalactiae by Nurani et al.34. The types of bacterial diseases studied in the aforementioned studies are very limited even though Nile tilapia often suffer from them in fish farms and hatcheries35. Besides being infected by S. agalactiae29,34–36, Nile tilapia are often infected by A. hydrophila9,35,37 and P. fluorescens37,38 leading to high mortality, including in Indonesia. Therefore, this study aimed to examine maternal immunity transfer using the polyvalent vaccine for S. agalactiae, A. hydrophila, and P. fluorescens (PAPS). It was expected that the broodstock could pass their immunity to their offspring, making them resistant to the three types of diseases (A. hydrophila, S. agalactiae, and P. fluorescens bacteria), and also the production of tilapia offspring could also be increased. Furthermore, this study aimed to determine the effectiveness of the transfer of immunity induced by PAPS against A. hydrophila, S. agalactiae, and P. fluorescens from the Nile tilapia (O. niloticus) broodstock to their offspring and the protection against S. agalactiae, A. hydrophila, and P. fluorescens bacterial infections.
Nile tilapia broodstock, obtained from the Ompo Inland Hatchery, Soppeng, Indonesia, with an average weight of 203g (±SD 23 g) was used as experimental animal. They were kept in spawning ponds and fed with pellets that have a protein content of 30% ad libitum in the mornings and afternoons. Also, 25% of the water was replaced daily. One week after the fish spawned, they were harvested and a large number of Nile tilapia broodstock at gonad developmental stage 2 were obtained.
Pure isolates of the A. hydrophila, S. agalactiae, and P. fluorescens bacteria were obtained from the Research and Development of Fish Disease Control Installation, Ministry of Marine Affairs and Fisheries, Depok, Indonesia. The vaccine tested was formalin-killed, whereby S. agalactiae and P. fluorescens were inactivated with 1% formalin while A. hydrophila was inactivated using 0.6% formalin39.
The vaccine treatments consist of (1) a monovalent vaccine against A. hydrophila (MA) , (2) a monovalent vaccine against P. fluorescens (MP), (3) a monovalent vaccine against S. agalactiae (MS) , (4) a bivalent vaccine against A. hydrophila, P. fluorescens and (BAP), (5) a bivalent vaccine against A. hydrophila and S. agalactiae (BAS), (6) a bivalent vaccine against P. fluorescens and S. agalactiae (BPS), (7) a polyvalent vaccine against A. hydrophila, P. fluorescens and S. agalactiae (PAPS), and (8) the control, fish injected with PBS solution.
The vaccination method used was intramuscular (i.m.)40,41 by injecting between the first and second scales of the dorsal fin and was administered at a dose of 0.4 mL/kg of fish (±0.08 mL/fish). After the fish were vaccinated, a booster with the same dose as the initial vaccination was later administered on the 7th day. However, before being injected with the vaccines, they were first anesthetized using MS-222, Sigma.
The gonad developmental stage 2 fish post-vaccination were reared using 3×3 m cages and installed in dirt ponds 25×30×1.2 (L×W×H). Furthermore, 20 broodstock were reared per cage, consisting of 15 females and 5 males. The fish were fed with pellets at a dose of 4%/day in the morning, at midday, and in the afternoon. The water was replaced daily at a rate of 20%/day. The fish would spawn after being reared for approximately 4 weeks.
Following vaccinations, the fish’s immune response was observed on the 7th, 14th, 21st, and 28th day by collecting intramuscular blood samples. The immune response parameters were the antibody titer using the direct agglutination method42, total leukocyte9,34,43, phagocytic44,45 and lysozyme activities27,34,45,46.
Random blood sampling from the offspring was conducted on each treatment group on the 10th, 20th, 30th, and 40th day post-spawning period. Serum was collected by grinding the offspring in a tube with PBS-tween at a ratio of 4:1. It was then centrifuged at 6000 rpm for 5–10 minutes. Furthermore, the serum in the second layer of the centrifugation result was harvested and stored at 47°C for 30 minutes to inactivate the complements47. It was then stored for agglutination titer and lysozyme activity. The direct agglutination test on both broodstocks and offspring was carried out by adding 25 µL of antigen48 of A. hydrophila, P. fluorescens, and S. agalactiae (107 cfu/mL) bacteria into the well, starting from the 1st well to the 12th well. It was found that the last well showed an agglutination reaction.
The offspring challenge test was conducted on the 10, 20, 30, and 40 days old during the post-hatching period. It was carried out by dividing the fish into 7 groups based on the type of vaccine administered plus one unvaccinated. Challenge tests on all treatments were carried out using three types of pathogenic bacteria; A. hydrophila, S. agalactiae, and P. fluorescens. This test was carried out by placing 20 offsprings into containers containing 4 liters of water and then they were immersed in water containing pathogenic bacteria at a dose of 2.1×108 cfu/mL according to their relative treatments, each conducted triplicate. To observe the effectiveness of the vaccine, the relative percentage survival (RPS) was calculated49,50 on the 14th day post-challenge test.
In general, the different types of vaccines at each period of post-vaccination had a significant effect (P<0.05) on the broodstock's total leukocyte (Figure 1), and phagocytic activity (Figure 2). The follow-up test showed that the fish vaccinated with PAPS had the highest total leukocyte (7.56–10.70×106 cell/mm3) and phagocytic activity (8.33–19.33%), followed by those vaccinated with bivalent and monovalent vaccines, while the lowest was found in control (total leukocyte was 7.40–7.86×106 cell/mm3, phagocytic activity was 9.00–9.33%).
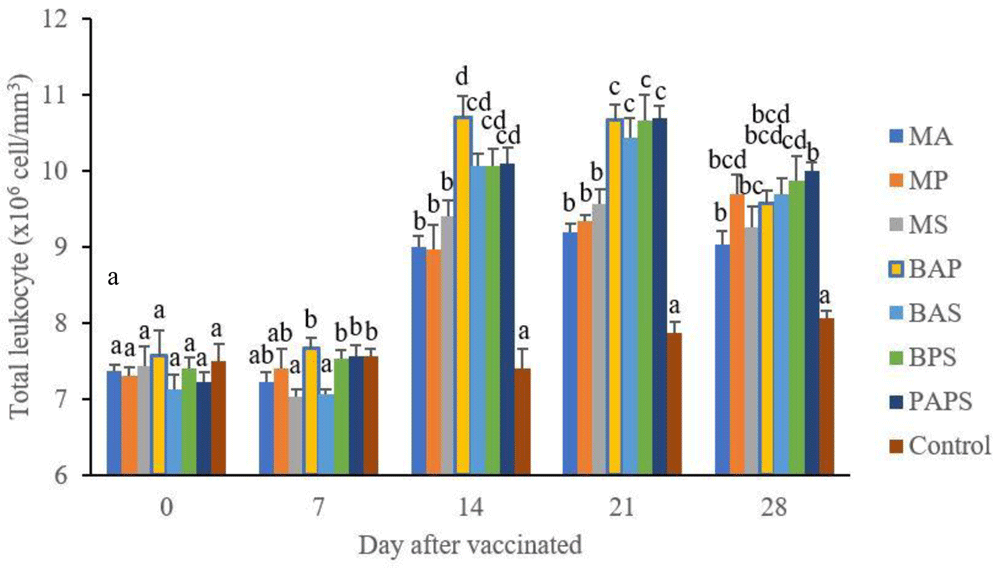
M: monovalent, B: Bivalent, P: Polyvalent vaccine, A: A. hydrophila, S: S. agalactiae, P: P. fluorescens. Values with different superscripts a,b indicate that their corresponding means are significantly different (P<0.05) according to one-way ANOVA followed by Duncan’s test.
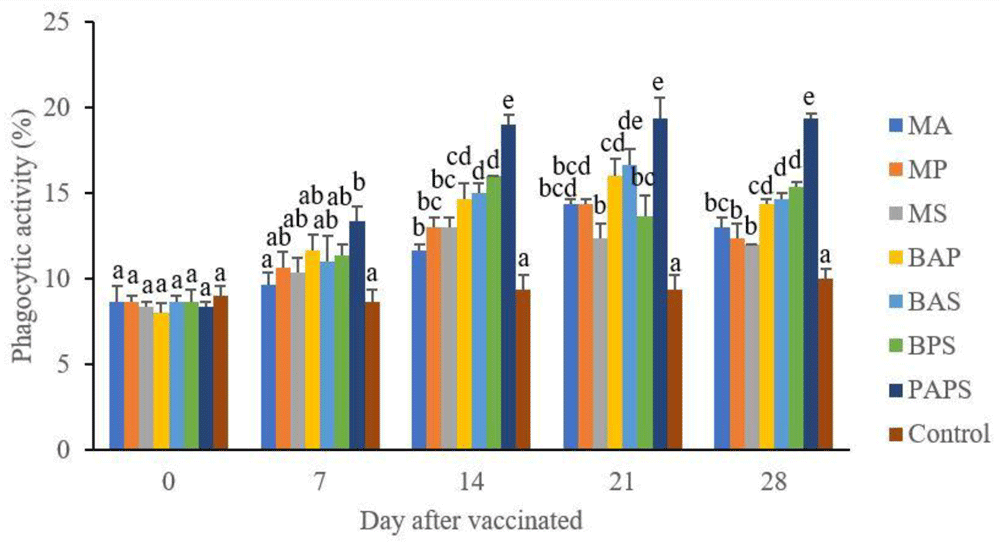
M: monovalent, B: Bivalent, P: Polyvalent vaccine, A: A. hydrophila, S: S. agalactiae, P: P. fluorescens. Values with different superscripts a,b indicate that their corresponding means are significantly different (P<0.05) according to one-way ANOVA followed by Duncan’s test.
The broodstock’s antibody (Table 1) increased, especially after the booster, except in the unvaccinated fish. After the peak, the broodstock’s immune response remained high up to day 28 even though there was a tendency for it to decrease. All the types of vaccines at each point in time had a significant effect (P<0.05) on the agglutination titer in the broodstock. The Duncan’s follow-up test showed that the vaccinated broodstock had a higher agglutination titer than the unvaccinated fishes. Also, the highest significant value was found in the vaccinated fishes with PAPS (1.67–6.67), followed by those vaccinated with the bivalent and monovalent vaccines, while the lowest was in the control (1.33–1.67)
M: monovalent, B: Bivalent, P: Polyvalent vaccine, A: A. hydrophila, S: S. agalactiae, P: P. fluorescens. Values with different superscripts a,b indicate that their corresponding means are significantly different (P<0.05) according to one-way ANOVA followed by Duncan’s test.
| Type of vaccine | Day after vaccinated (day) | ||||
|---|---|---|---|---|---|
| 0 | 7 | 14 | 21 | 28 | |
| MA | 1.67±0.33a | 2.00±0.00a | 3.33±0.33a | 3.67±0.3bc | 3.67±0.33bc |
| MP | 1.67±0.33a | 2.67±0.33a | 3.67±0.33a | 3.33±0.33bc | 3.33±0.33b |
| MS | 1.33±0.33a | 2.33±0.33a | 3.33±0.33a | 3.00±0.00b | 3.33±0.33b |
| BAP | 2.00±0.58a | 2.33±0.33a | 4.33±0.33ab | 4.33±0.33c | 4.67±0.33bc |
| BAS | 1.67±0.33a | 2.33±0.33a | 4.33±0.33ab | 4.33±0.33c | 4.33±0.88bc |
| BPS | 1.67±0.67a | 2.33±0.33a | 4.33±0.33ab | 4.33±0.33c | 5.00±0.58c |
| PAPS | 1.67±0.33a | 3.67±0.33b | 5.33±0.33b | 6.67±0.33d | 6.67±0.33d |
| Control | 1.67±0.33a | 1.67±0.33a | 1.33±0.33a | 1.33±0.33a | 1.67±0.33a |
Based on the effect of the vaccine on the broodstock’s immune response, the agglutination titer in the offspring from the vaccinated broodstock at ages 10, 20, 30, and 40 days was higher than unvaccinated (P<0.05). The follow-up test showed that PAPS was more effective in increasing the agglutination titer in the offspring (6.33–3.00) than the bivalent and monovalent vaccines. The results showed that the administration of vaccines in tilapia broodstock had a significant effect on the maternal immunity transfer to the offsprings that were up to 30 days old (Table 2).
M: monovalent, B: Bivalent, P: Polyvalent vaccine, A: A. hydrophila, S: S. agalactiae, P: P. fluorescens. Values with different superscripts a,b indicate that their corresponding means are significantly different (P<0.05) according to one-way ANOVA followed by Duncan’s test.
| Type of vaccine | Day post-hatching (day) | |||
|---|---|---|---|---|
| 10 | 20 | 30 | 40 | |
| MA | 4.00±0.58ab | 3.67±0.33bc | 1.67±0.33a | 1.33±0.33a |
| MP | 4.00±0.00ab | 3.67±0.33bc | 1.67±0.33a | 1.33±0.33a |
| MS | 3.67±0.33b | 3.33±0.33b | 2.33±0.33ab | 1.33±0.33a |
| BAP | 4.67±0.33ab | 4.67±0.33c | 2.33±0.33ab | 1.67±0.33a |
| BAS | 5.00±0.58c | 4.33±0.33bc | 2.33±0.33ab | 1.67±0.33a |
| BPS | 4.33±0.33ab | 4.33±0.33bc | 2.33±0.33ab | 1.33±0.33a |
| PAPS | 6.33±0.33d | 5.67±0.33d | 3.00±0.33b | 1.67±0.33a |
| Control | 1.67±0.33a | 1.67±0.33a | 1.67±0.33a | 1.33±0.33a |
The lysozyme activity. of broodstock vaccinated with PAPS (29.87–103.08 U/mL) was higher than other vaccines, and the lowest was in broodstock that was not vaccinated (27.65–33.89 U/mL) (P<0.05) (Figure 3). Generally, the offspring from the broodstock vaccinated with PAPS had a higher lysozyme activity (77.81–43.11 U/mL) than those of other treatments (P<0.05) up to the 30th day, the lowest was in the control (20.29–20.24 U/mL) The results showed that the application of PAPS in tilapia broodstock could increase lysozyme activity transferred to the offsprings (Figure 4).
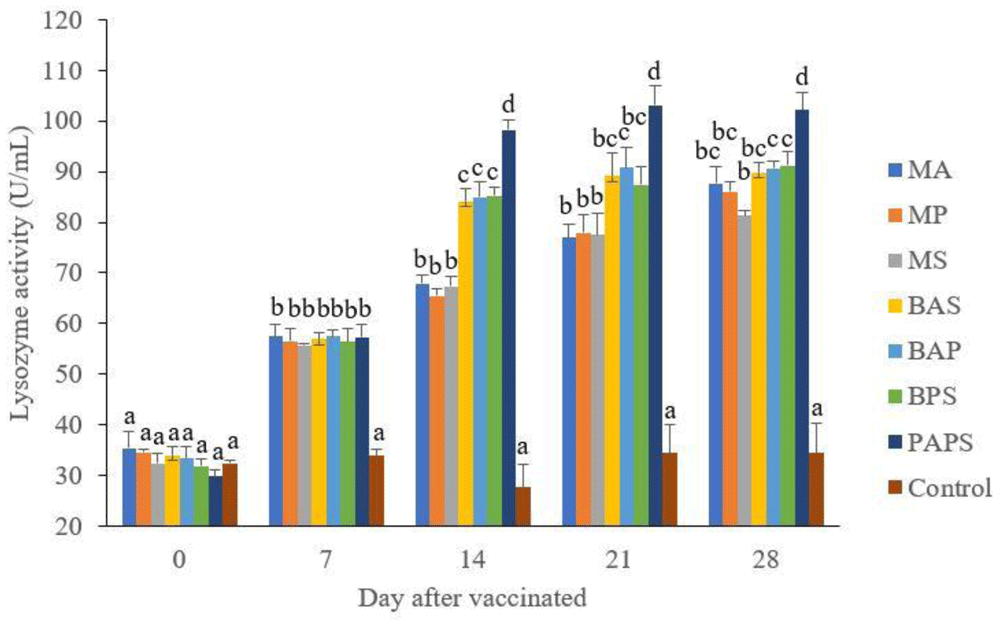
M: monovalent, B: Bivalent, P: Polyvalent vaccine, A: A. hydrophila, S: S. agalactiae, P: P. fluorescens. Values with different superscripts a,b indicate that their corresponding means are significantly different (P<0.05) according to one-way ANOVA followed by Duncan’s test.
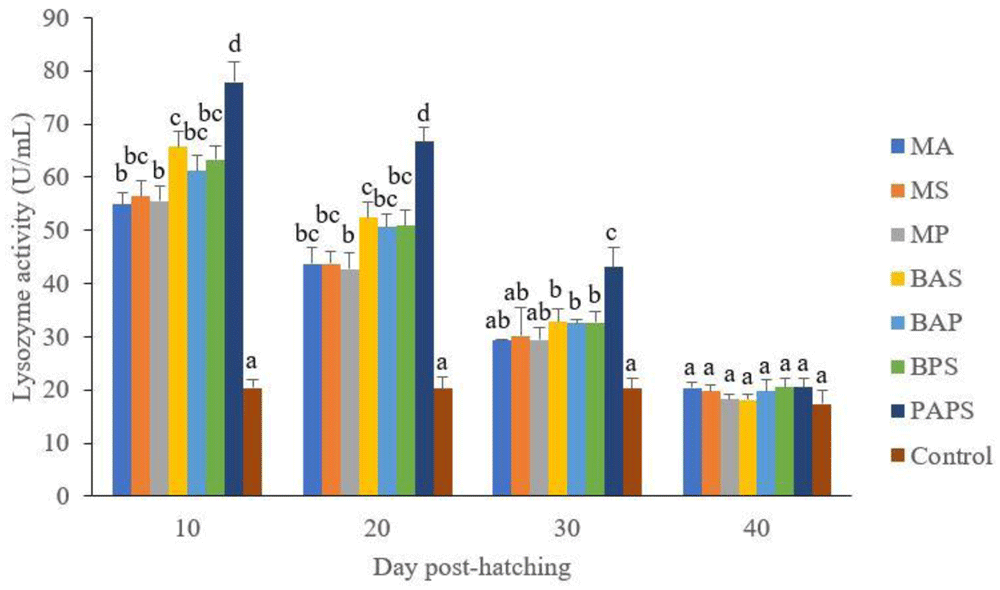
M: monovalent, B: Bivalent, P: Polyvalent vaccine, A: A. hydrophila, S: S. agalactiae, P: P. fluorescens. Values with different superscripts a,b indicate that their corresponding means are significantly different (P<0.05) according to one-way ANOVA followed by Duncan’s test.
Offsprings that were 10, 20, 30, and 40 days old from the vaccinated broodstock had higher RPS than those from the unvaccinated broodstock after being challenged with bacteria. The offsprings from the broodstock that were vaccinated with PAPS had the highest RPS when challenged with 3 bacteria simultaneously (a combination between A. hydrophila, S. agalactiae, and P. fluorescens) (Table 3) up to day 30. The RPS of the offspring vaccinated with PAPS were 86,11% (10 days old), 78,95% (20 days old) dan 56,41% (30 days old).
The offspring were produced by broodstock vaccinated with various types of vaccines through intramuscular (i.m.) injection (mean±SE).
Efforts to produce seeds that are immune to several diseases were the best alternative to increasing Nile tilapia production. Furthermore, PAPSs for A. hydrophila, S. agalactiae, and P. fluorescens were able to improve the broodstock’s immune response which was then transferred to the offspring. This process was carried out in other to produce offspring that possess both lysozyme and antibodies and a high survival rate post-challenge test using pathogenic bacteria. This was better than the other treatments that made use of the bivalent and monovalent vaccines.
The results from the observation of the broodstock for 28 days showed that the total leukocyte (Figure 1), phagocytic (Figure 2), antibody titer (Table 1), and lysozyme activity (Figure 3), started to increase in week two post-vaccination. The broodstock vaccinated with PAPS showed a higher increase in the immune response compared to the others that were vaccinated with the bivalent, monovalent vaccines, and was the lowest in the unvaccinated broodstock28,30,33,34,51. This showed that PAPS could increase the Nile tilapia broodstock’s immune response better than the other treatments.
The offspring produced from the broodstock that were vaccinated with PAPS had the highest antibodies (Table 2) and lysozyme activity (Figure 4) up to the 30th day post-hatching period and was the lowest in the offsprings from the unvaccinated broodstock (P<0.05). This demonstrated that their strong immune response was transferred to their offsprings27–29,33,34,52 through the egg yolk53.
The results from the challenge test using pathogenic bacteria (Table 3) showed that the offsprings that were produced using PAPS had a higher RPS compared to those from the offsprings produced from broodstocks that were treated using the monovalent and bivalent vaccines (P<0.05). This further showed that the vaccine treatment had adequately protected the fishes from bacterial diseases with an RPS that was greater than 60% up to the 30th day post-hatching period49. RPS of the offspring vaccinated with formalin-inactivated vaccine in this study was higher at same time and lasted longer than the findings of Nurani et al.34 on days 10 and 20, closely similar to the Sukenda et al.18 and Pasaribu et al.54, but higher on day 20. The high RPS in the offspring during the challenge test using pathogenic bacteria in PAPS treatment was due to the broodstock’s high number of leukocytes, phagocytic activity, the amount of antibody, and lysozyme activity transferred to the offsprings for protection against diseases. Meanwhile, in the control (unvaccinated), there was no transfer of immunity from the mother. In addition, the offspring hasn't been able to produce their own immune response, so the total leukocyte, phagocytic activity, antibody, and low lysozyme activity caused low offspring SR during the challenge test. Compared to the Abu-elala et al.33 study, the offspring RPS was higher and could last up to 3 months, whereas in this study, the PAPS RPS vaccine was lower and only lasted up to days 30. The low RPS of the PAPS vaccine can be improved by the use of adjuvants, the use of quality tilapia broodstock, proper nutrition in terms of quality and quantity, and the application of biosecurity in the hatchery33.
The role of leukocytes which consist of neutrophils, lymphocytes, and monocytes, is to infiltrate the infected area for rapid protection55, stimulating the production of antibodies through the recognition of foreign bodies, including vaccines and pathogens during the challenge test in this study. The phagocytic activity occurs during phagocytosis, which involves antibodies and complements during opsonization. Furthermore, the total leukocyte parameter increases in line with other immune responses, such as the antibacterial lysozyme, which triggers the complement system and phagocytic cells56–58. It encourages phagocytosis by activating leukocytes and polymorphonuclear macrophages or through opsonization59. The high number of leukocytes and a large amount of lysozyme in the treatment using PAPS which is similar to an infection by a pathogen indicated the success of PAPS in triggering the fish’s immune system when developing an immune response.
The offsprings produced by the broodstock that were vaccinated with PAPS were protected from infections by A. hydrophila, S. agalactiae, and P. fluorescens. However, the monovalent vaccines only protected the offsprings from one type of bacteria. This is one of the advantages of applying PAPS. The results of this study revealed that the application of PAPS produced broodstock and offspring with better immune responses than the bivalent and monovalent vaccines. Therefore, the development of a polyvalent vaccine is more prudent than that of bivalent or monovalent because of its ability to target more than one species of bacteria31,51,52,60–63. The use of this type of vaccine caused the fish to respond to multiple antigens and form an immune response, thereby making it a strategic method in controlling bacterial diseases commonly found in culture and breeding environments33,34,52,64. Additionally, the application of polyvalent vaccines is more practical than the monovalent containing only one type of antigen. This showed that PAPS provided the most effective protection against diseases caused by pathogenic bacteria that often affect fishes, and thus is an ideal candidate for developing a polyvalent vaccine against bacterial infection.
The results show that the application of the polyvalent vaccine against A. hydrophila, S. agalactiae, and P. fluorescens increased the antibody, lysozyme, total leukocytes, and phagocytic activity in Nile tilapa broodstock which was transferred to their offsprings, leading to a high RPS during the challenge test. Therefore, it is possible to produce seeds of Nile tilapia that are immune to diseases caused by A. hydrophila, S. agalactiae, and P. fluorescens. This process could be carried out through the vaccination of the broodstocks using a polyvalent vaccine against A. hydrophila, S. agalactiae, and P. fluorescens.
OSF: Underlying data for ‘ Transfer of maternal immunity using a polyvalent vaccine and offspring protection in Nile tilapia, Oreochromis niloticus ’. https://doi.org/10.31219/osf.io/cnqdg65
The project contains the following underlying data:
Data on broodstock immune response, offspring immune response, and offspring RPS in tilapia, O. niloticus can be accessed on OSF
Data are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
Research using fish in Indonesia has not been regulated and therefore it does not require animal ethics. However, this research has received approval from the Ministry of Education and Culture of the Republic of Indonesia (No.: 004/PL.22.7.1/SP-PG/2019). In addition, this study applies the principle of the International Animal Welfare standards including the assurance of fish welfare during maintenance and the use of drugs during sampling.
Special gratitude also goes to the Director of Pangkep State Polytechnique of Agriculture, South Sulawesi, Indonesia for allowing the sample analysed in the laboratory.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Aquatic animal health, microbiology
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: fish immunology, fish diseases, aquaculture, aquaculture extension
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
References
1. Shirajum Monir M, Yusoff SM, Mohamad A, Ina-Salwany MY: Vaccination of Tilapia against Motile Aeromonas Septicemia: A Review.J Aquat Anim Health. 32 (2): 65-76 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Aquatic animal health, microbiology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 4 (revision) 10 Nov 23 |
read | ||
|
Version 3 (revision) 09 Mar 23 |
read | ||
|
Version 2 (revision) 15 Jun 22 |
read | ||
|
Version 1 24 Sep 21 |
read | read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)