Keywords
Histopathology, fixation, honey, formalin, neutral buffered
Histopathology, fixation, honey, formalin, neutral buffered
Fixation is an initial and critical tissue processing step for microscopy in histopathology.1 Fixation preserves the tissues' life-like condition by preventing autolysis and bacterial putrefaction.2 10% neutral buffered formalin (NBF) is the preferred fixative of choice because it is readily available and internationally accepted. Its preparation is easy, fast, cheap, and has long-term storability.3,4 However, the International Agency for Research on Cancer (IARC) and US Environmental Protection Agency (EPA) classified formaldehyde, which is the primary component of formalin, as a human carcinogen.5
Therefore, safer alternative fixatives should replace formalin, such as natural fixatives that are eco-friendly, economical, and readily available substances. Examples of natural fixatives are honey, sugar, jaggery, molasses, saline, rose water, and coconut oil. Honey is a mixture of sugars, minerals, trace elements, vitamins such as vitamin C, and antioxidants such as pinobanksin, pinocembrin, hydrogen peroxide, chrysin, and catalase.6 Combined, these compounds give honey its anti-autolytic, antimicrobial, antiviral, antimutagenic, and antioxidant effects. These honey features are known for several centuries.7 Bee honey is acidic and also possesses preserving, dehydrating, and tissue hardening properties.3 In addition, it can penetrate the deepest tissue.3 These properties make the honey a potential tissue fixative. However, tissues fixed in honey at low pH are less rigid after fixation and have homogenized connective tissue, a breach in the continuity of sections, folding of the tissue sections, and growth of molds over some time.1,3,8,9
The present study was designed to find a safe substitute fixative for formalin without compromising the quality staining criteria of the tissue sections. The hypothesis was that neutral honey fixative or other experimented honey fixed rat tissues better or similar to that of 10% NBF. Neutral honey is honey where the pH is around seven whereas natural honey usually has a low pH. It is recommended that the pH fixatives be kept near seven in order to achieve optimal results.10 We thought that increasing the pH of the honey might overcome the disadvantages associated with low pH honey fixatives. To the best of our knowledge, neutral honey has not been experimented with to fix histological tissues. Thus, we aimed to examine the effectiveness of neutral buffered honey and other types of honey fixatives to fix histological tissues.
The study was ethically approved by the Ethics Committee for Animal Use in Research, College of Medicine and Health Sciences, Sultan Qaboos University, Oman (Ethical Clearance number SQU/EC-AUR/2021-2022-15). Three Wister rats (200 g) were retained for two days in a room measured setting (a temperature of 20 ± 2°C, relative humidity of about 60%, with a 12-hour light-dark cycle). They were provided ad libitum with an additive-free standard diet (Oman Flour Mills, Muscat, Oman) and tap water. The rats were euthanized with an overdose of ketamine (75 mg/kg) and xylazine (5 mg/kg) via intraperitoneal injection. All efforts were made to ameliorate any suffering of animals as all procedures were carried out per the national, and international laws and policies. In addition, this study is reported in line with The Animal Research: Reporting on in vivo Experiments (ARRIVE) guidelines.11,27 The cost of formalin, Sumer honey, and date honey were obtained from local suppliers.
Two common natural honeys were used as fixatives, namely Sumer and date. Sumar is the most well-known and one of the finest types of Omani natural honey. It is light black in color and produced from Omani Dwarf bees. It is very expensive honey as it is produced in small amounts and usually found in the caves of the mountains, therefore, it is difficult to acquire. Date honey is very common in Oman as it is extracted from dates. It is slightly brown in color and not expensive. In this study, we evaluated nine groups of fixatives, including neutral buffered Sumer honey, neutral buffered date honey, formalin, Sumer honey, date honey, alcoholic formalin, alcoholic Sumer honey, and alcoholic date honey. The pH for these fixatives were 7.20, 7.25, 7.27, 3.62, 3.56, 5.15, 3.95, 5.0, and 5.85, respectively. These groups were compared with the gold standard fixative, which is 10% NBF. The neutral buffer was made with sodium phosphate monobasic (0.4 g), sodium phosphate dibasic (0.65 g), formalin (10 ml), and distilled water (90 ml). 10% fixatives contain 10 ml (Sumer and date honeys and formalin) and 90 ml of distilled water. 10% alcoholic fixatives contain 10 ml (Sumer and date honeys and formalin) and 90 ml ethanol. In this study, we have chosen experimental conditions such as a temperature of 18–23°C, 24 hours fixation time, tissue thickness of 3 mm, and fixation volume of 1:10. In fact, 1:10 fixation volume has been reported by many researchers and found to be the most recommended ratio.12,13 In addition, during our pilot study we used 5, 10, and 15% honey fixative concentrations for 24 and 48 hours fixation time and found that 10% for 24 hours fixation preserved tissue morphology well.
Nine samples were fixed in each group: three uniform-thickness samples (3 mm) from each organ (liver, kidney, and stomach) were obtained from the rats. In order to have a uniform thickness for all groups, tissues were cut using the Cutmate forceps (Forceps for 2/3/4 mm tissue blocks, Code No 62359, Milestone s.r.l, Sorisole, Italy). The tissues were immediately placed in fixatives for 24 hours at room temperature (18–23°C). After fixation, all tissues were given the same treatment. As previously described, all tissues were processed using an automated histoprocessor (Spin Tissue Processor Microm STP 120; Thermo Scientific, Walldorf, Germany).14 Processing included dehydration in ethanol, clearing in xylene, and infiltration in paraffin wax. Afterward, 3 μm sections were cut using a rotatory microtome (Leica RM2135, Nussloch, Germany). One slide was obtained from each organ (i.e., three from the liver, three from the kidney, and three from the stomach). Thus, for all three organs, nine slides were obtained and for all nine groups, 81 slides were obtained. Differences in microtomy were observed.
Hematoxylin and eosin (H&E) and special stains
All 81 slides were stained with H&E.14 In addition, a number of special stains were used to stain slides in different groups. Jones' Methenamine silver stain (JMS) to stain glomerular basement membranes in the kidney slides. Gordon and Sweets method was used to stain reticular fibers in the liver slides. The periodic acid–Schiff (PAS) method was used to detect basement membranes of glomerular capillary loops and tubular epithelium.14 All special stains were performed using the Ventana BenchMark Special Stains system (Ventana Medical Systems, Inc., Code No: 06657389001, Tucson, AZ, USA). Positive controls (human kidney for JMS and PAS, and human liver for G&S; Pathology Department, Sultan Qaboos University Hospital) were run simultaneously.
Immunohistochemistry
Three additional kidney tissue samples cut at 4 μm using a rotatory microtome (Leica RM2135; Nussloch, Germany) were obtained for vimentin staining. Slides were deparaffinized, rehydrated and epitope retrieved by pre-treatment (PT) link (Code PT200, Agilent Dako, CA, USA). All slides were then washed three times in phosphate buffered saline (PBS) each for a min. Following which, slides were incubated with a polyclonal primary antibody against vimentin (host species: rabbit; Abcam Cat# ab137321, RRID:AB_2921312) at 1:1000 dilution for 30 minutes. Slides were then washed with PBS three times each for 5 min followed by incubation for 30 min with a secondary antibody (Envision Flex, High pH (Link), Hrp. Rabbit/Mouse; Agilent Cat# K8000, RRID:AB_2890017) and then washed with PBS three times each for 5 min. After that, the reaction was visualized using 3,3′-Diaminobenzidine (Code No K3468, Dako, CA, USA) for 2 min. Next, slides were counterstained with Mayer's hematoxylin for 2 min and then washed for 2 min in running tap water. Finally, slides were dehydrated, cleared, and mounted in dibutyl phthalate polystyrene xylene. Placental tissue previously known to be positive was used as a positive control for vimentin (Pathology Department, Sultan Qaboos University Hospital).
All slides were examined by light microscopy (BX40, Olympus Optical Co, Tokyo, Japan) attached to a camera (DP71 controller, Olympus Optical Co, Tokyo, Japan). For H&E evaluation (Table 1), if the score was ≤ 2, graded as inadequate, and if the score was 3-5, graded as adequate.15,16 For special stains, specificity, and intensity, were used and graded either negative (0), weak (1), moderate (2), or strong (3). For special stains, specificity, and intensity, were used and graded either negative (0), weak (1), moderate (2), or strong (3). For immunohistochemistry, the intensity was used and graded either negative (0), weak (1), moderate (2), or strong (3), and for the background is the inverse. Three senior biomedical scientists working in a histopathology laboratory blindly evaluated all the slides, such that all slides had three results for each parameter. Then the average score of each parameter was obtained. The data were analyzed using IBM SPSS Statistics (RRID:SCR_016479) software version 23 (IBM Corp., Armonk, New York, United States). For Fisher's exact test: we compared two staining criteria, for example, adequate and inadequate for nuclear staining. For the ANOVA test, we compared more than two criteria: the grade (negative, weak, moderate, and strong) of specificity for each fixative group. The comparison was with the NBF as the gold standard in all fixative groups. A P-value of less than 0.05 was considered statistically significant.
There were no noticeable differences in sectioning, formation of ribbons, and floating on the water bath in all groups. There was no significant difference in the nuclear staining and uniformity of staining among all the groups (Table 2).27 By contrast, 10% neutral buffered Sumer honey, 10% neutral buffered date honey, 10% Sumer honey, and 10% date honey fixed tissues had significantly inferior cytoplasmic staining compared to 10% NBF (Figure 1).
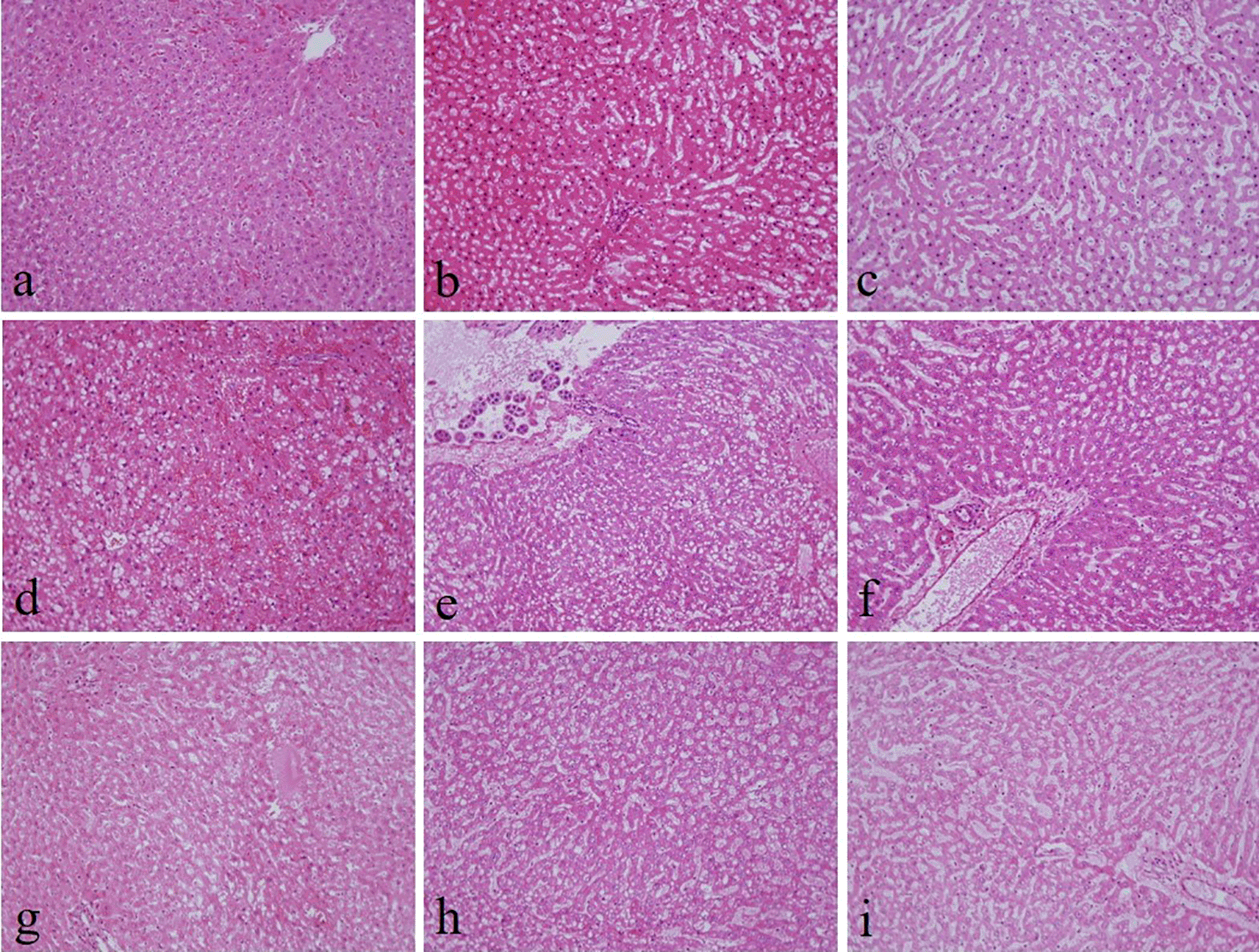
(a) 10% neutral buffered formalin, (b) 10% neutral buffered Sumer honey, (c) 10% neutral buffered date honey, (d) 10% formalin, (e) 10% Sumer honey, (f) 10% date honey, (g) 10% alcoholic formalin, (h) 10% alcoholic Sumer honey and (i) 10% alcoholic date honey (magnification, ×200).
The intensity and specificity of JMS in 10% Sumer and date honeys and 10% alcoholic Sumer honey were similar to that of 10% NBF (Figure 2). In addition, the specificity and intensity of all groups for PAS were comparable with 10% NBF. However, the intensity of PAS in 10% Sumer honey and 10% alcoholic date honey were inferior in comparison with 10% NBF (Figure 3). All honey groups showed weak staining of the reticulin fibers using the Gordon and Sweets method (Table 3). Immunohistochemical staining with vimentin showed comparable findings with 10% NBF as there were no significant differences noted in intensity and background for all groups (Figure 4). In addition, no background staining was observed in all groups.
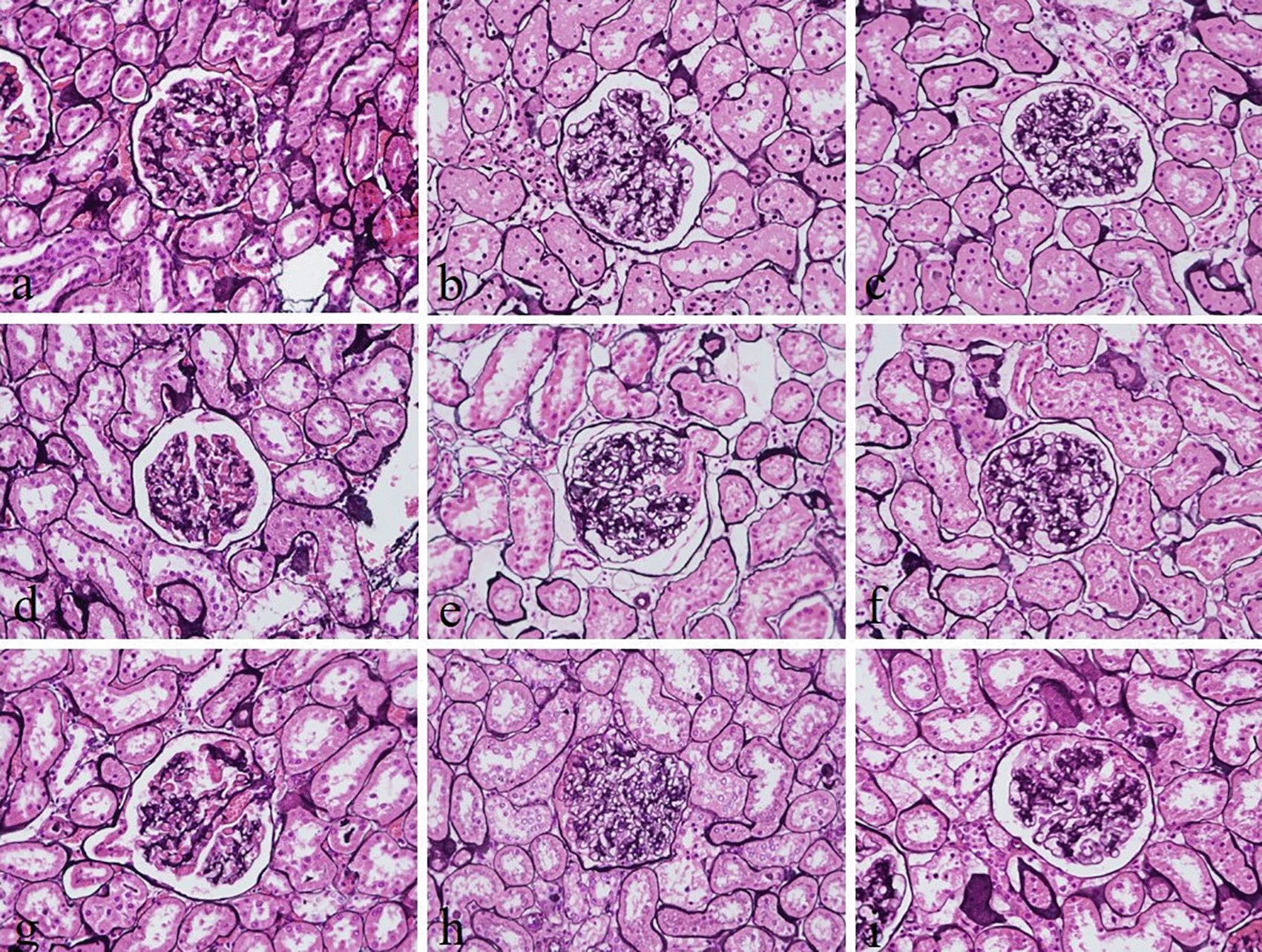
(a) 10% neutral buffered formalin, (b) 10% neutral buffered Sumer honey, (c) 10% neutral buffered date honey, (d) 10% formalin, (e) 10% Sumer honey, (f) 10% date honey, (g) 10% alcoholic formalin, (h) 10% alcoholic Sumer honey and (i) 10% alcoholic date honey (magnification, ×400).
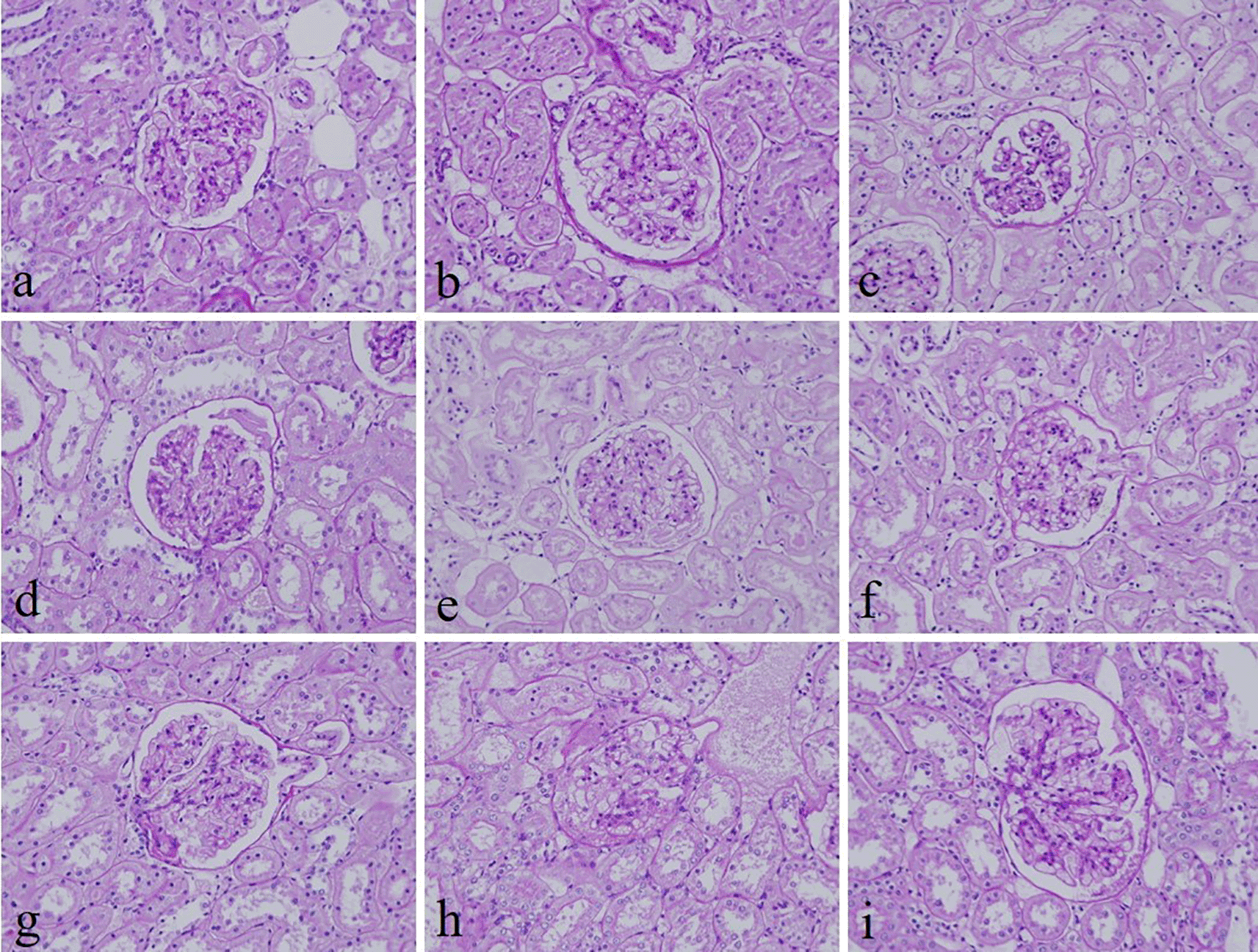
(a) 10% neutral buffered formalin, (b) 10% neutral buffered Sumer honey, (c) 10% neutral buffered date honey, (d) 10% formalin, (e) 10% Sumer honey, (f) 10% date honey, (g) 10% alcoholic formalin, (h) 10% alcoholic Sumer honey and (i) 10% alcoholic date honey (magnification, ×400).
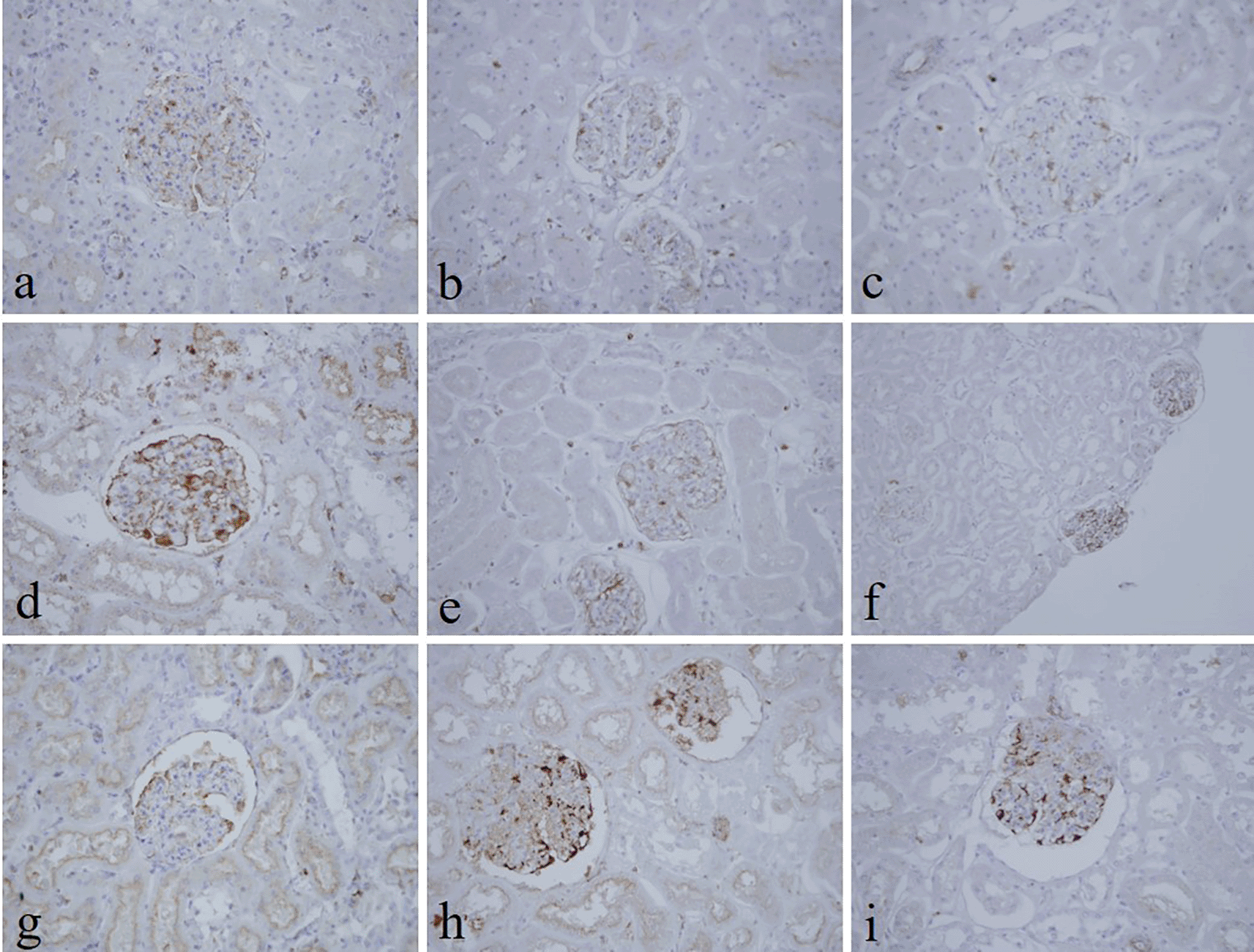
(a) 10% neutral buffered formalin, (b) 10% neutral buffered Sumer honey, (c) 10% neutral buffered date honey, (d) 10% formalin, (e) 10% Sumer honey, (f) 10% date honey, (g) 10% alcoholic formalin, (h) 10% alcoholic Sumer honey and (i) 10% alcoholic date honey (magnification, ×400).
Sumer honey is more expensive than formalin. One liter of Sumer costs 60 Omani Rial (OMR), equivalent to 155.79 USD, whereas one liter formalin costs OMR 1.6, equivalent to USD 4.15. One liter of date honey costs USD 5.19 (OMR 2.00).
The aim of the present study was to find a safe substitute fixative for formalin, which is a human carcinogen. To the best of our knowledge, this is the first study to evaluate honey as neutral buffered honey similar to that of the NBF. The honeys used in this study had overall similar findings to that of NBF.
The present study evaluates three important aspects of honey fixation: H&E, special stain, and immunohistochemistry. In both types of honeys and in all groups, H&E results showed that the overall quality of tissue staining was comparable with 10% NBF. The nucleus was well preserved with precise nuclear details. It is known that honey contains ascorbic acid and various vitamins, carbohydrates, minerals, and trace elements. Thus, it gives honey a low pH. Low pH fixative is suitable for nuclear staining.17
The findings of the present study are in line with other studies that have reported that 10% honey, using rat liver and kidney tissues at room temperature for 24-hour fixation, gave comparable results with those obtained by formalin-fixed control tissues.18 Another similar study that used the same staining criteria to evaluate honey as a substitute for formalin found that 10% honey is as good as 10% NBF and suggested that honey is a safe alternative for formalin.6 Recently, two studies in cytology showed that 20% honey fixed oral smears had acceptable nuclear and cytoplasmic staining, well-preserved cell morphology, clarity, and uniformity of staining comparable to ethanol, which is the gold standard fixative in cytology, with no statistical difference between both fixatives.19,20 However, in the current study, cytoplasmic staining was inadequate in neutral buffered Sumer honey, neutral buffered date honey, 10% Sumer honey, and 10% date honey. We thought by increasing the pH in neutral buffered Sumer honey and neutral buffered date honey fixatives would enhance cytoplasmic staining. The results of the present study are in concordance with another similar study where they compared formalin fixed tissues with honey fixed tissues and concluded that the nuclear details are well established compared to cytoplasmic details.9
Reticular fibers in all groups were not well demonstrated. The staining was weak. This finding disagrees with another study, which reported that reticular fibers using silver impregnation were well demonstrated using 10% honey as a fixative.20 However, the demonstration of glomerular basement membranes in the kidney by JMS in 10% Sumer and date honey and 10% alcoholic Sumer honey were similar to 10% NBF fixed sections. Srii et al., evaluated the efficacy of 10% honey as a fixative agent to preserve cellular and structural characteristics using different special stains. They found that Masson's trichrome and Van Gieson staining results are similar to those fixed by formalin.21 Similarly, the specificity and staining intensity of PAS on honey fixed tissues were comparable with 10% NBF.4 Our results align with this study where PAS stain in all groups revealed similar findings with 10% NBF. However, the intensity for only 10% Sumer honey and 10% alcoholic date honey was inferior compared to 10% NBF.
In this study, both types of honey in H&E and special stains showed the absence of red blood cells in the liver, kidney, and stomach tissues. When red blood cells are exposed to hydrogen peroxide, which is a component of honey, this would make cellular changes that lead to alterations in phospholipid organization and cell shape, and membrane deformability. Hydrogen peroxide has the ability to form a covalent complex with hemoglobin and spectrin, which are specific structures of red blood cells.22 This may explain why honey masks the staining of red blood cells.
In the current study, vimentin as an immunohistochemical marker was evaluated. In routine immunohistochemistry, this marker is used as an internal control for detecting cytoplasmic staining.23,24 Vimentin in all honey groups showed similar findings of 10% NBF as similarly reported by Özkan et al., in which honey fixed ki-67 and vimentin markers in different fresh tissues, including endometrium, breast, placenta, uterus, omentum, suprarenal, stomach, and lung similar to NBF.2 Gunter and Bryant reported good staining levels without antigen retrieval for common leukocyte antigen, cytokeratin AE1/AE3, and epithelial membrane antigen in breast tumor samples treated with honey.25 In addition, the demonstration of vimentin and pan-cytokeratin in gingiva tissues using 10% honey was similar to formalin-fixed tissues.25 Similarly, the immunohistochemical demonstration of pan-cytokeratin was comparable to using 20% honey fixative in fresh goat oral mucosa.26
In comparison with formalin, the cost of Sumer honey is very expensive, however, date honey costs are almost similar to formalin. Thus, the cost-benefit balance between the safety of laboratory workers in histopathology and good quality staining should be considered.
Several limitations of our study are worth noting. First, we should point out that large tissues or small biopsies were not evaluated; differently sized tissues would produce more meaningful results. Second, only one immunohistochemical tumor marker was assessed; a wide range of immunohistochemical markers would improve our knowledge on how useful honey is as a potential fixative. Third, a limited number of special stains was evaluated. Fourth, the sample number was small. Finally, DNA/RNA extraction from honey-fixed specimens was not assessed.
Neutral honey is potentially a safer substitute fixative for formalin, however, further experiments on larger and different specimens and additional special stains and immunohistochemical markers should be conducted.
Zenodo: Effectiveness of neutral honey as a tissue fixative in histopathology. https://doi.org/10.5281/zenodo.6591173.27
This project contains the following underlying data:
- Author_Checklist_-_Full.pdf (ARRIVE checklist)
- H&E slides evaluation.xlsx (81 H&E slides evaluation)
- Figure 5. jpg (Gordon and Sweets stained-liver sections of different fixative groups: (a) 10% neutral buffered formalin, (b) 10% neutral buffered Sumer honey, (c) 10% neutral buffered Date honey, (d) 10% formalin, (e) 10% Sumer honey, (f) 10% Date honey, (g) 10% alcoholic formalin, (h) 10% alcoholic Sumer honey and (i) 10% alcoholic Date honey (x400))
- Figure 6. jpg (Hematoxylin and eosin-stained stomach sections of 10% neutral buffered formalin (magnification, x200))
- Figure 7. jpg (Hematoxylin and eosin-stained stomach sections of 10% neutral buffered Sumer honey (magnification, x200))
- Figure 8. jpg (Hematoxylin and eosin-stained stomach sections of 10% neutral buffered date honey (magnification, x200))
- Figure 9. jpg (Hematoxylin and eosin-stained stomach sections of 10% formalin (magnification, x200)).
- Figure 10. jpg (Hematoxylin and eosin-stained stomach sections of 10% Sumer honey (magnification, x200))
- Figure 11. jpg (Hematoxylin and eosin-stained stomach sections of 10% date honey (magnification, x200))
- Figure 12. jpg (Hematoxylin and eosin-stained stomach sections of 10% alcoholic formalin (magnification, x200))
- Figure 13. jpg (Hematoxylin and eosin-stained stomach sections of 10% alcoholic Sumer honey (magnification, x200))
- Figure 14. jpg (Hematoxylin and eosin-stained stomach sections of 10% alcoholic date honey (magnification, x200))
Zenodo: ARRIVE checklist for ‘Effectiveness of neutral honey as a tissue fixative in histopathology’. https://doi.org/10.5281/zenodo.6591173.27
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
An earlier version of this article can be found on bioRxiv (https://doi.org/10.1101/2021.04.27.437988).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Veterinary pathology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 3 (revision) 09 Nov 23 |
read | ||
|
Version 2 (revision) 21 Jul 23 |
read | read | |
|
Version 1 07 Sep 22 |
read | ||
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)