Keywords
A. oryzae, QS, Virulence factors, Biofilm, KP-ESBL.
This article is included in the Cell & Molecular Biology gateway.
This article is included in the Pathogens gateway.
A. oryzae, QS, Virulence factors, Biofilm, KP-ESBL.
The severity of infectious diseases caused by bacterial strains that are resistant to treatment has made their advent a global problem today, including Klebsiella pneumoniae extended-spectrum beta-lactamases (KP-ESBL).1 KP-ESBL can hydrolyze beta-lactam antibiotics in addition to producing biofilms that hinder medications from penetrating cells.2 Biofilms comprise an extracellular matrix (polysaccharides, proteins, and extracellular doxyribonucleic acid [DNA]) that acts as a strong barrier for bacteria and makes them more resilient to environmental stress than planktonic cells.3 Additionally, biofilms aid KP-ESBL in spreading the infection and provide an environment that fosters the development of antibiotic resistance.4–6 Finding novel compounds that can suppress KP-ESBL virulence factors and biofilms is therefore urged in order to assist in fighting these bacteria.
Quorum sensing (QS) molecules, adhesion molecules, iron, and exopolysaccharides (EPS) can affect the formation of biofilms.7–9 Because QS is a regulator for the expression of capsule polysaccharides, the development of this biofilm is inversely related to the polysaccharide capsule's virulence factor.10 It is now crucial to find antibiofilm and antivirulence chemicals, especially those derived from natural sources. Biofilms have been shown to be inhibited by bioactive substances obtained from nature.11–14
Aspergillus sp., a filamentous fungus, is well known for its potent antibacterial properties.15–17 Inhibition of virulence factors and direct harm to the K. pneumoniae are known antibacterial mechanisms of Aspergillus sp. However, little is known about Aspergillus sp ability's to combat KP-ESBL biofilms. Our preliminary research proves Aspergillus crude protein which has the greatest biofilm inhibition is Aspergillus oryzae extracellular crude protein (AOEP). The aim of this study was to evaluate any potential antibiofilm properties of Aspergillus oryzae extracellular protein against KP-ESBL.
KP-ESBL (ID.100029) were obtained from the Laboratory of Microbiology, Faculty of Medicine, Brawijaya University, Malang, Indonesia. Luria Broth (LB) medium was used to cultivate the KP-ESBL strain, which was then incubated at 37°C for 16–18 hours. Sterile saline was diluted 100 times after being equalized with the Mc. Farland standard to produce a concentration of 106 CFU/mL. This bacterial suspension was then ready for testing. The fungal strain Aspergillus oryzae was provided by the Indonesian Culture Collection (Ina-CC). Preparation of Aspergillus oryzae begins with sub-culture and preservation of previously isolated Aspergillus oryzae. Sub-cultures were carried out from cryo to Luria Bertani solid medium. The culture was then treated in the form of H2O2 and without glucose, then stored at 37°C in an incubator. The nematode Caenorhabditis elegans was maintained on agar medium for nematode growth media (NGM) fed with Eschericia coli OP50. Gravid C. elegans were treated with hypochlorite to synchronize C. elegans culture at the first larval stage. Before being employed for infection, the C. elegans were then reared at 25°C until they reached the young adult stage.
100 mL of potato dextrose broth (PDB) medium were inoculated with 8-mm (diameter) Aspergillus oryzae mycelium and placed in a 250 mL Erlenmeyer flask containing 2% glucose. The flask was incubated for 72 hours at 27°C in a shaker incubator under static conditions (OD600 = 1.2). The culture was filtered using 0.22-micron filter paper after incubation (Whatman, Sigma Aldrich). As a source of extracellular protein, the supernatant was centrifuged at 12.000 rpm for 15 minutes at 4°C. Ammonium sulfate was used to precipitate extracellular proteins at saturation values of 80%. After one hour of stirring in the ice bath, ammonium sulfate was added to the supernatant. The crude protein extract was centrifuged at 4°C for 15 minutes at 12,000 rpm the next day after being maintained at 4°C overnight. After that, the complete protein precipitate underwent a twenty four hour-dialysis in a 0.01 M phosphate buffer at pH 7 using a 10×-sample volume. After that, the Aspergillus oryzae extracellular protein (AOEP) was prepared for the assay.
A growth inhibition test was conducted using microdilution broth. Briefly, fresh cultures were inoculated on LB medium at turbidity equivalent to 0.5 McFarland standard, 500 μL of each bacterial culture were added to a 96-well polystyrene flat-bed microtiter plate. The samples were added to the bacterial suspension in each well at final concentrations ranging from 0 to 150 g/mL. The growth control wells only contained bacteria on LB media and kanamycin as positive control. Double serial dilution of the Aspergillus oryzae extracellular protein (AOEP) tested sample was made starting from the first well by adding 50 μL of the tested sample, dissolved at 150 μg/mL. After incubation at 37°C for 24 hours, the absorbance was measured at 600 nm. The lowest absorbance value of the sample that could reduce more than 90% of the absorbance of the negative control was recorded as the MIC value. All experiments were performed in triplicate. Minimum bacterial concentration (MBC) for each sample was calculated by coating the contents of the first three wells, which showed no visible bacterial growth on the LB plate, and incubated for 24 hours.
The test well on a 96-well microplate received a total of 100 μL of Aspergillus oryzae extracellular protein (AOEP) at various concentrations (18.75, 37.5, 75, and 150 μg/mL). The negative control wells received 200 μL of mixed LB media and 1% glucose, while the positive control wells received 64 μg/ml of kanamycin. Each well was then filled with 100 μL of the KP-ESBL suspension. For 24 hours, the microplate was wrapped and kept at 37°C in an incubator. The microplate's contents were taken out the following day, thoroughly cleaned with sterile distilled water three times, and then dried. 200 μL of 0.1 % crystal violet dye was added to each well once the microplate had dried, and it was air dried at room temperature for 15–20 minutes. The microplate's contents were then cleaned with sterile distilled water and dried. After 15 minutes of incubation at room temperature, 200 μL of a 96% ethanol solution were added to each well, and the results were measured at 570 nm with a microplate reader.
Biofilm growth inhibition was calculated using the following formula:
(OD Control = Optical density control negative. OD Test = Optical density test)
Visual evaluation of AOEP's impact on KP-ESBL morphology was conducted using a scanning electron microscope (SEM, model Zeiss 224 EVO 50 VP, Germany). KP-ESBL bacteria were cultivated in LB broth and incubated for 24 hours in an aerobic environment at 37°C. A 1-mL volume of the bacterial suspension was obtained and treated with AOEP for two hours once it reached around 1×108 CFU/ml. Another 1 mL sample was taken from the culture and left untreated. These two bacterial samples were centrifuged after two hours for three minutes at 1400 rpm, and the pellets were then cleaned twice with 0.1 M phosphate buffer saline (PBS). KP-ESBL cells were exposed to 2.5% glutaraldehyde for two hours at 4°C for the SEM analysis. Samples were exposed to each concentration after fixation for one to two minutes in order to dehydrate them. The samples were then centrifuged at 1400 rpm for 10 min, after which the pellets were re-dispersed in 100% ethanol and air dried. The samples were coated with gold and palladium in an 80:20 ratio prior to examination under SEM at 20 kV. The working magnification was kept at less than 10 mm for better focusing.
Cultures of bacterial isolates left overnight were inoculated to 9.5 mL of LB broth along with 0.5 mL of cell lysate and incubated at 30°C for 24 hours. The late-log phase cells attached to the test tube walls were harvested by centrifugation at 8500 rpm for 30 min at 2°C. The filtered supernatant was added with three volumes of cold ethanol and incubated overnight at 2°C to precipitate the released EPS. The precipitated EPS were then collected by centrifugation at 5000×g for 30 min and dissolved in 1 mL of deionized water. Enzyme-free media culture added with PBS served as a control. The bacterial cells were removed, resuspended in sterile PBS, and read at 600 nm. The collected EPS was quantified using the phenol-sulfuric acid method.
Similar to the anti-infection screen, the liquid-based survival test was carried out with a few minor adjustments. A total of 30 young adult C. elegans were used in place of the N2 young adults that received treatment. As a result, the C. elegans were kept at 16°C to create gravid C. elegans, and they were given a hypochlorite treatment to develop eggs. In order to conduct an infection assay, eggs were sown on NGM agar and developed into sterile young adults of C. elegans at 25 °C. Every four hours following infection, both alive and dead C. elegans were counted. Each extract was examined in three wells, each representing about 100 C. elegans. In control wells, dimethyl sulfoxide (DMSO) was used in place of the extract, and Escherichia coli OP50 were fed. To examine the impact of AOEP on KP-ESBL pathogenicity to C. elegans, we performed a slow-killing survival experiment. On a 48-well microplate, KP-ESBL were first cultured for an overnight period at 37°C in the presence of AOEP (18.75, 37.5, 75, and 150 μg/mL). When 100 sterile young adult C. elegans were put into the well, the infection began. KP-ESBL was given DMSO treatment as a negative control in place of AOEP. After 48 hours, the C. elegans that were still alive were counted under microscope with a magnification of 100×.
The hot phenol method was used to extract total ribonucleic acid (RNA), where the DNA was removed using TURBO DNA-free (Ambion, Inc.), and the RNA quality was determined using a NanoDrop (ND-1000; Thermo Scientific) and an Agilent 2100 bioanalyzer with a Picochip (Agilent Technologies). After 35 qPCR cycles, the absence of contaminating DNA was determined by the absence of amplification products. A 1 μg of RNA, random hexamer primers (0.2 μg/L), and M-MulV-RT (20 U/L, Moloney murine leukemia virus reverse transcriptase; Thermo Fisher Scientific) were used to synthesize cDNA. Specific primers for mrkA 5′-CGGTAAAGTTACCGACGTATCTTGTACTG-3′, and wzI 5′-GCTTAYGCRGCYGGGTTAGTRGT-3′ designed with the Primer3Plus software (Primer3Plus is an open alternative). A master mix of the following components was prepared for light cycler reactions: 3.0 mL PCR-quality water, 1.0 μL (10 M), 10 μL 2× SYBR Green I Master Mix, 10 μL reverse primer, and 5.0 μL cDNA (50–100 ng). A multi-well plate was sealed with sealing foil, centrifuged for two minutes at 1500 g, and loaded into the LightCycler 480 instrument (Roche). For each sample examined, amplification was carried out in triplicate wells. All reactions had control reactions with no template (water) and minus-reverse transcriptase (RNA). Cycling conditions were as follows: denaturation (95°C for 10 minutes); amplification and quantification repeated for 45 cycles (95°C for 10 seconds, 57°C for 20 seconds, 72°C for 30 seconds with a single fluorescence measurement); melting curve (95°C for 10 seconds, 65°C for one minute with continuous fluorescence measurement at 97°C); and finally, a cooling step at 40°C for 10 seconds. After each run, a melting curve analysis was performed to confirm the specificity of the primers. For normalization, 16S rRNA was used as a reference gene, and relative gene expression was calculated using the 2Ct method.
The antimicrobial activity of AOEP was quantitatively assessed by measuring the turbidity at a wavelength of 600 nm. The results in Figure 1 represent crude proteins’ MIC and MBC values in various concentrations with kanamycin as positive control. The concentration of AOEP, which could inhibit > 90% of the bacterial population, represented MIC and was 300 μg/mL. The concentration used in the growth inhibition test of antibiofilm activity was sub-MIC, namely at 1/8 and 1/16 × MIC. This study used a 64-μg/mL dose of kanamycin as a positive control.
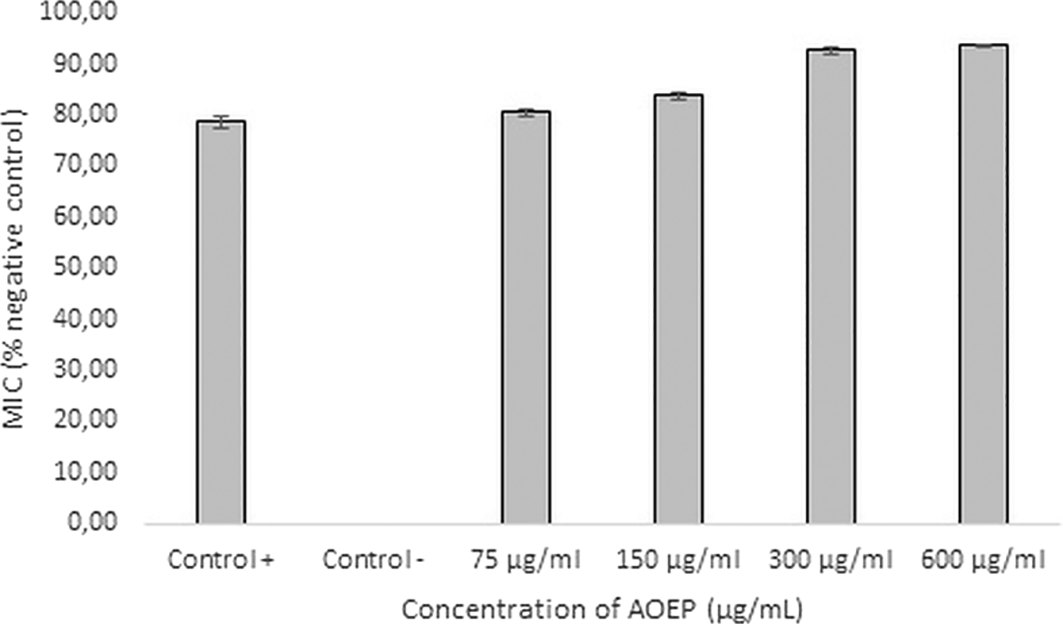
MIC at a concentration of 300 μg/mL was 92.74% (bold). Kanamycin as a positive control, was only able to inhibit KP-ESBL 78.51%. The negative control of KP-ESBL without AOEP exposure was 0%. Bars indicate the standard error, and the sign (*) above the bars indicates a significant difference (p < 0.05).
The highest concentration used for the AOEP inhibition test against KP-ESBL biofilms was 150 μg/mL, which was the MIC (p-value < 0.05). The Tukey Post Hoc test showed that there were significant differences between the overall treatment group and the negative control. The linear regression test results showed a R-value of 0.797, reflecting that AOEP could inhibit the growth of KP-ESBL in a dose-dependent manner. Furthermore, measurement of the inhibitory ability of biofilms and virulence factors used sub-MIC concentrations of 1/8 and 1/16 MIC, there were 18.75, 37.5, 75, and 150 μg/mL.
The microdilution method was used to test the inhibitory activity of the AOEP biofilm against KP-ESBL. Figure 2 displays the AOEP biofilm's inhibitory efficacy against KP-ESBL.

Inhibitory effect of AOEP on biofilms after co-incubation for 24 h with different concentrations of AOEP. The concentration of AOEP is given relative to MIC KP-ESBL. The AOEP biofilm inhibition concentrations of 18.75, 37.5, 75, and 150 μg/mL were 32.94%, 39.17%, 68.14%, and 72.18%, respectively. Bars indicate the standard error, and the sign (*) above the bars indicates a significant difference (p < 0.05).
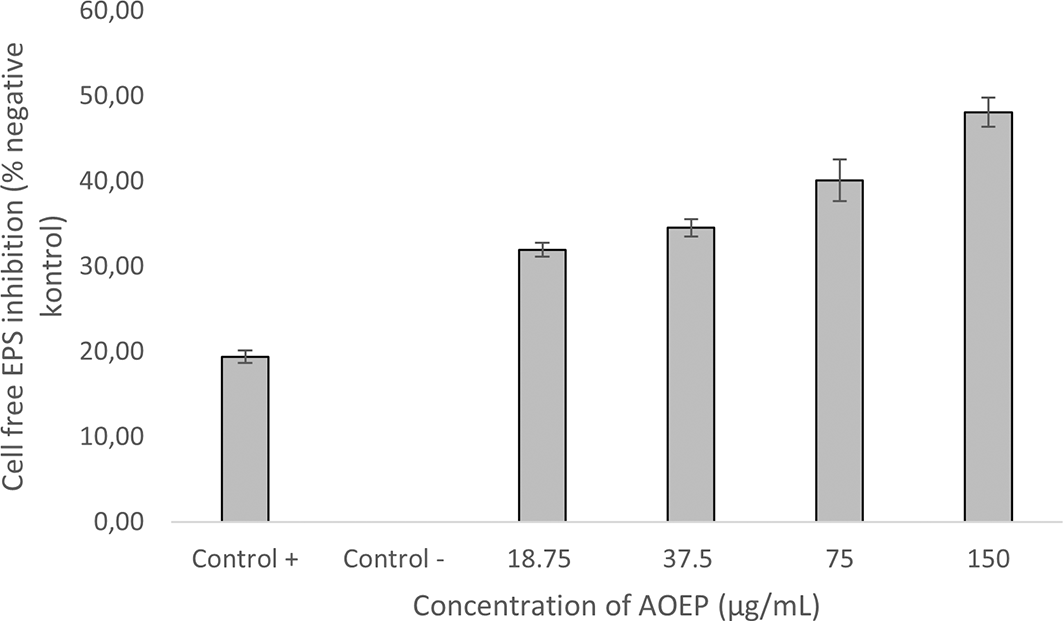
Inhibitory effect of AOEP on cell-free exopolysaccharide KP-ESBL after being incubated together for 24 hours with different concentrations: 18.75, 37.5, 75, and 150 g/mL. Bars indicate the standard error and the sign (*) above the bars indicates a significant difference (p < 0.05). KP-ESBL
AOEP was administered relative to the MIC. The MIC value of the K. pneumoniae strain was 300 μg/mL, so the highest concentrations for the anti-biofilm test were 1/8, 1/16 MIC. The biofilm inhibition value [100-(sample ABS/control ABS × 100)] for each AOEP concentration can be seen in Figure 2. In the crystal violet staining assay, KP-ESBL biofilms were significantly inhibited at concentrations of 1/4 × MIC (75 μg/mL) and 1/2 × MIC (150 μg/mL) (p < 0.05). Interestingly, AOEP inhibited biofilm formation at concentrations below the MIC. The ability of AOEP to inhibit biofilm formation in KP-ESBL exceeded the ability of the kanamycin (69.46 μg/mL and 37.9 μg/mL). The positive control had a biofilm inhibitory value of 37%, which was lower than the AOEP biofilm inhibitory level of 150 and 75 μg/mL. The negative control (KP-ESBL bacteria without AOEP exposure) showed the lowest biofilm inhibition value (0), which indicated that biofilm production was not inhibited at all. When administering the four concentrations of AOEP, the resulting OD value decreased significantly as the dose increased when compared with the OD of the negative control. This indicates AOEP inhibition of the KP-ESBL biofilm. Tukey test results indicated that there were significant differences between the overall treatment group against the negative control. The linear regression test results show that the R-value (0.957) that represented AOEP could inhibit the growth of KP-ESBL biofilm in a dose-dependent manner.
The assay was performed to test the ability of AOEP to reduce cell-free exopolysaccharide KP-ESBL.
KP-ESBL treated with AOEP 150 μg/mL could reduce the bond matrix with Congo Red dye by as much as 49% after staining and assessment with a spectrophotometer. As the dose of AOEP was reduced (75 μg/mL, 37.5 μg/mL, 18.75 μg/mL), its inhibition ability decreased (42%, 38%, 37%). Cell-free EPS might be reduced by 19% using AOEP 150 μg/mL and it surpassed the kanamycin (30 %). There was a significant difference, according to the one way ANOVA test (p-value < 0.05). The results of the Tukey Post Hoc test revealed that the overall treatment group and the unfavorable control group differed significantly. The findings of the linear regression test indicated that AOEP might inhibit the cell-free EPS KP-ESBL in a dose-dependent manner, and the R-value for this test was 0.896.
The delta-delta Ct method (2 DDCt) was used to quantify RT-qPCR results. Results are represented as “Target/adh3 fold change.” The results of gene expression analysis via RT-qPCR (Figure 4) showthat AOEP downregulated the gene expression for fimbriae mrkA, which acts as an adhesion molecule. Meanwhile, capsular EPS as measured by the wzI gene expression was increased.
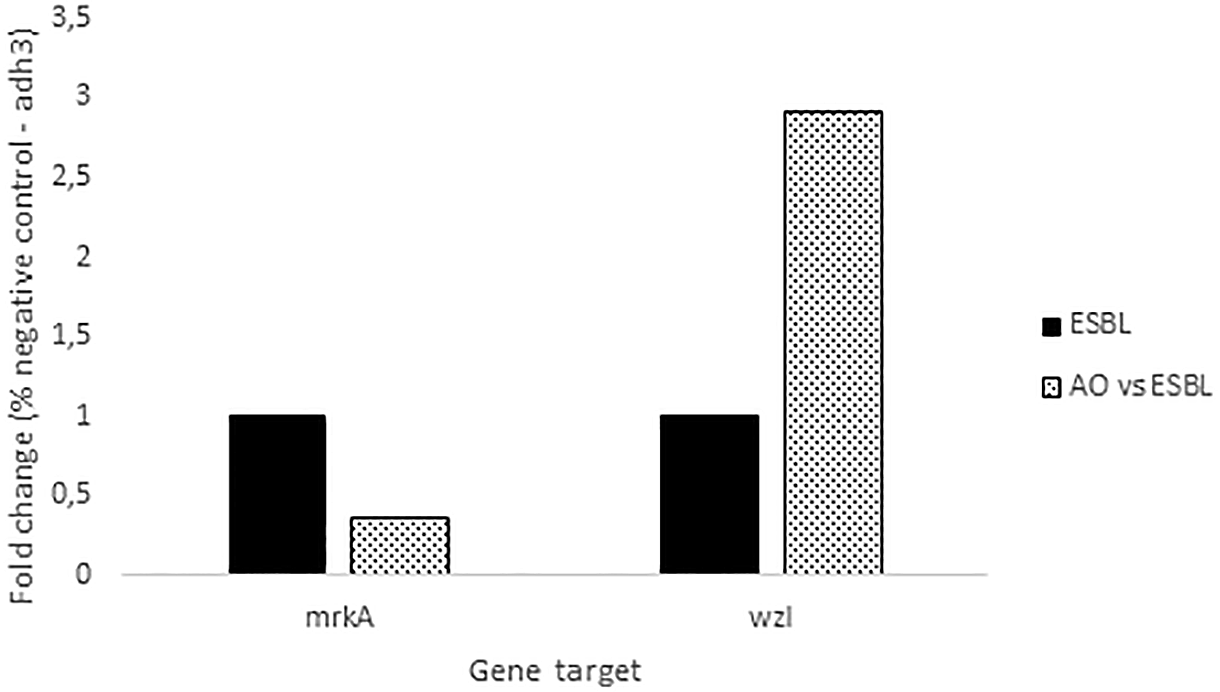
The expression of these two genes was measured in response to AOEP: mrkA (type 3 fimbrial shaft) and wzI (surface assembly of capsule). The expression of the adh3 house-keeping gene was used as an internal control for each sample. The concentration of AOEP treatment was 150 μg/mL, while the control group was KP-ESBL without AOEP exposure.
The impact of AOEP on cellular alterations was examined using SEM analysis. To supplement the information of the quantity of the biofilm, observation of the architecture of the biofilm mass using SEM was conducted. The impact of AOEP on the KP-ESBL biofilm structure was demonstrated by SEM data (Figure 5).
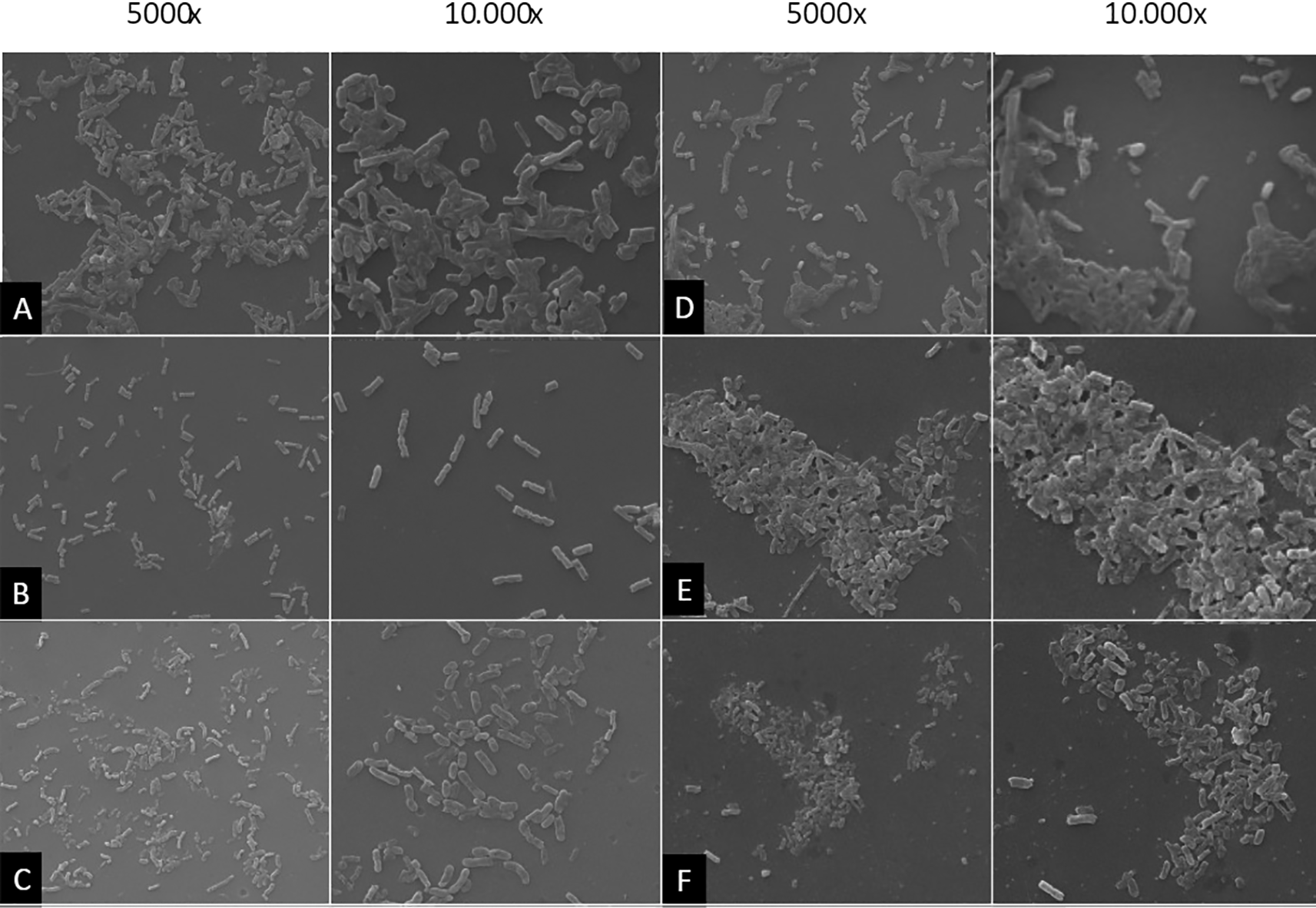
K. pneumoniae biofilms grew after incubation for 24 hours. AOEP was added with different concentrations: 150 μg/mL (B), 75 μg/mL (C), 37.5 μg/mL (D), 18, 75 μg/Ml (E) Positive control with the addition of the Kanamycin (F).
The negative control group showed bacterial colonies along with thick biofilms evenly distributed on the adhesion surface (Figure 5A). This was different from when the bacteria were treated with the 150 μg/mL AOEP (Figure 5B), the cells failed to aggregate, and there was a highly significant decrease in biofilm mass. In this group, the bacterial colonies were separated and became planktonic bacteria, and the adhesion surface was free of bacterial biofilms. Biofilm mass was also decreased in the AOEP 75 μg/mL (C) group, while the positive control group (Kanamycin, F) showed partial inhibition of the KP-ESBL biofilm. It can be seen that the biofilm structure of KP-ESBL was impaired due to the addition of AOEP when compared to the control. The control group was KP-ESBL which was not exposed to AOEP, as shown in Figure 5A. When bacteria stick together, the attachment of bacteria is more clearly facilitated by a thick mass of biofilm surrounding the bacterial colony. In this group, the biofilm appeared to cover all bacterial colonies on the surface of the adhesion medium. This appearance differed significantly from the group treated with 150 μg/mL of AOEP (Figure 5B) provides a clearer picture of the dispersal in the bacterial colonies treated with AOEP reflecting the impair the biofilm. The inhibition of biofilm mass formation in the group exposed to AOEP at a dose of 75 μg/mL (Figure 5C) also shows that the bacterial colony dispersed. However, the number of bacteria was higher than for a concentration of 150 μg/mL. At an AOEP dose of 37.5 μg/mL (Figure 5D), it was seen that some bacteria were separated, and some bacteria were attached (left). At 10000× magnification, a biofilm mass began to surround the bacteria and facilitated adhesion between bacteria and the adhesion medium.
Meanwhile, at the lowest concentration of AOEP, a dose of 18.75 μg/mL (Figure 5E), the presence of a thick biofilm was seen that matched the negative control. Interestingly, the sub-MIC ability of AOEP to reduce biofilm mass formation produced stronger effect than the kanamycin (Figure 5F). Overall, SEM results showed the highest reduction in biofilm mass formation occurred with a treatment of AOEP 150 μg/mL, which had a stronger effect than the kanamycin. These results indicate that AOEP can be used as a candidate antibiofilm agent at concentrations lower than MIC, especially against biofilm formation by KP-ESBL.
To observe the effect of AOEP on the infection caused by KP-ESBL, an in vivo study was conducted on C. elegans.
Figure 6 shows the percentage of C. elegans survival after 48 hours of exposure to KP-ESBL. Only about 4% of C. elegans infected with KP-ESBL survived up to 48 hours, while C. elegans exposed to E. coli OP50 88% survived until the end of the test. Surprisingly, the C. elegans that were exposed to AOEP and KP-ESBL (18.75, 37.5, 75, and 150 μg/mL) had significantly increased survival rates (17 – 68%) compared to the group of C. elegans that were only infected with KP-ESBL. The highest survival was in the 150 μg/mL group (68.25 ± SD 4.6). the one-way ANOVA test showed that there was a significant difference between negative control and treated groups (p-value < 0.05). The linear regression test results showed the R-value was 0.958. The analysis showed that AOEP could reduce the ability of KP-ESBL to infect C. elegans in a dose-dependent manner. Figure 7A shows the propidium iodide fluorescence micrograph of C. elegans (10× magnification) and infected with KP-ESBL. The C. elegans showed negative PI fluorescence when cultured under standard conditions with OP50 as a food source. There was an increase in the fluorescence intensity of propidium iodide when the C. elegans were infected with KP-ESBL and treated with AOEP (Figure 7B). The results of the in vivo survival assay showed that AOEP was able to reduce the virulence of KP-, which could be observed from the increased survival of C. elegans that were infected with KP-ESBL.
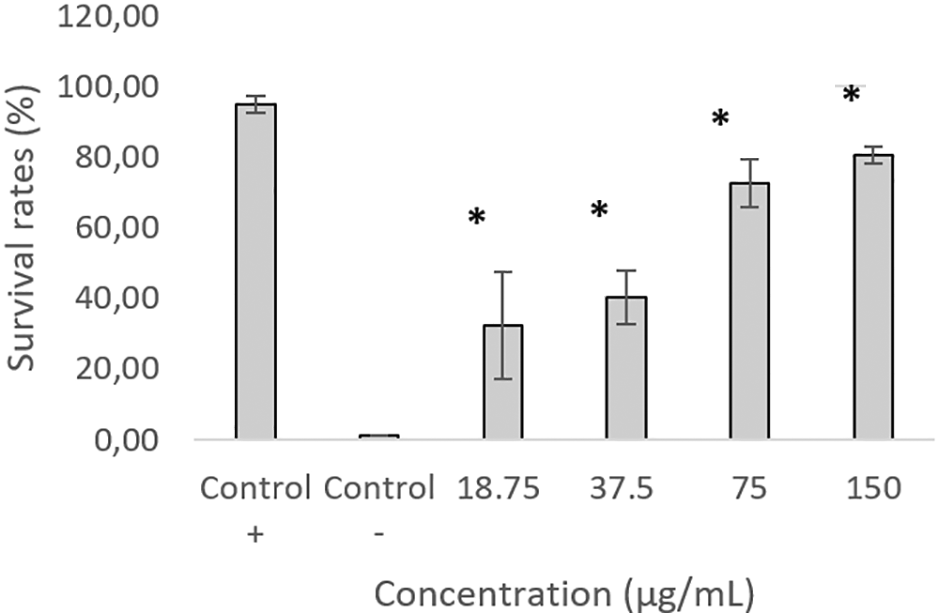
The graph shows the percentage survival rates when the test was carried out without exposure to AOEP with four different concentrations. KP-ESBL without AOEP was a negative control, while KP-ESBL with Escherichia coli OP50 (non-pathogenic) was a positive control. Results are expressed as mean ± SD.
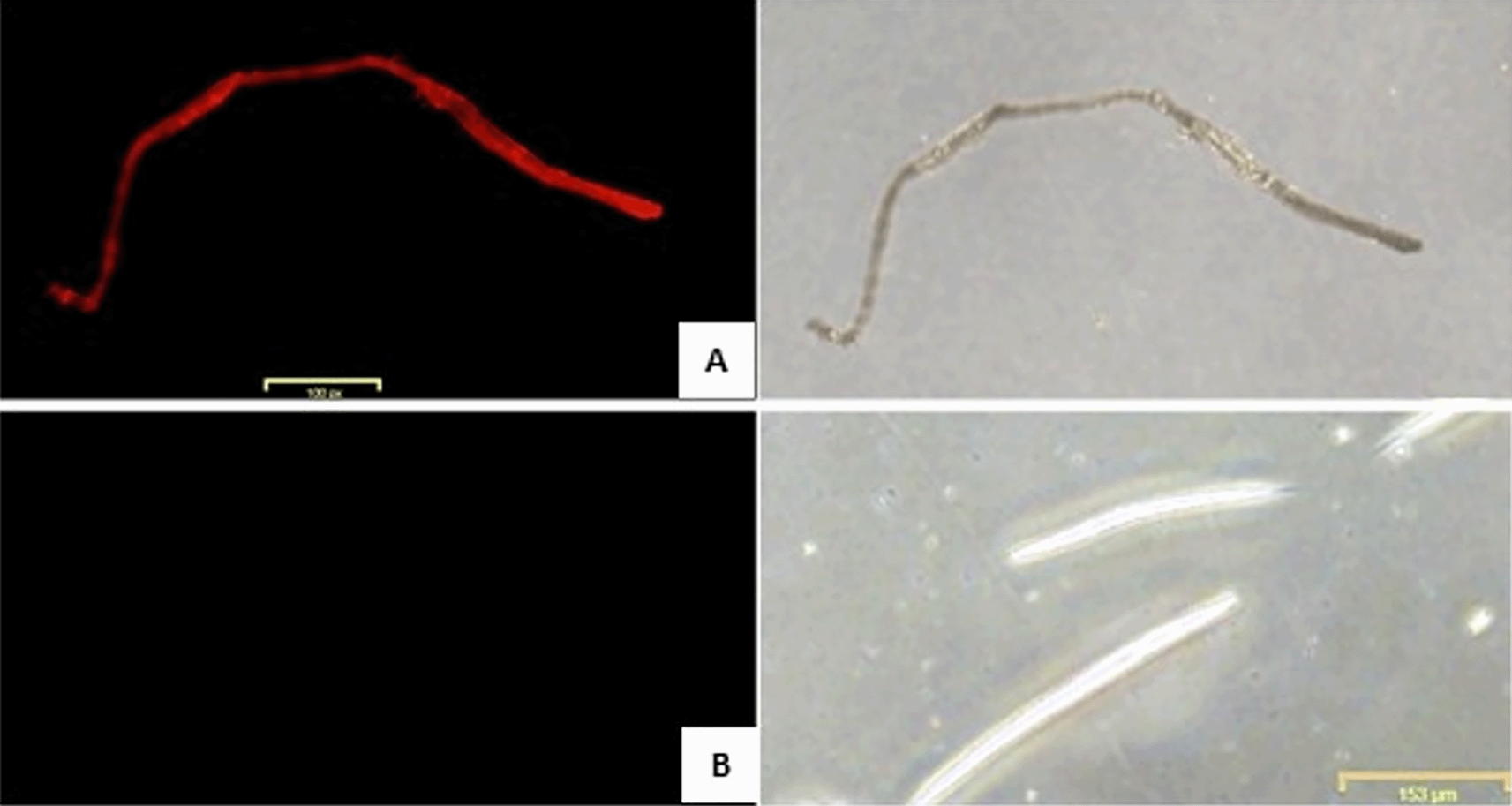
(A) C. elegans infected with KP-ESBL without AOEP administration. (B) C. elegans infected with KP-ESBL with AOEP administration. Propidium iodide fluorescence micrograph of C. elegans (100× magnification). C. elegans infected with KP-ESBL without AOEP died and showed positive red fluorescence when cultured (increased fluorescence intensity of propidium iodide indicated the death of the nematode parasiteof C. elegans).
A liquid chromatography-mass spectrometry LC-MS/MS study against AOEP was carried out in order to identify the A. oryzae molecule that contributes to KP-ESBL virulence and biofilm suppression. The results were displayed as a chromatogram, which showed the peak height and molecular weight of the sample substance. Figure 8 and Table 3 show the outcomes of the LC-MS/MS study.
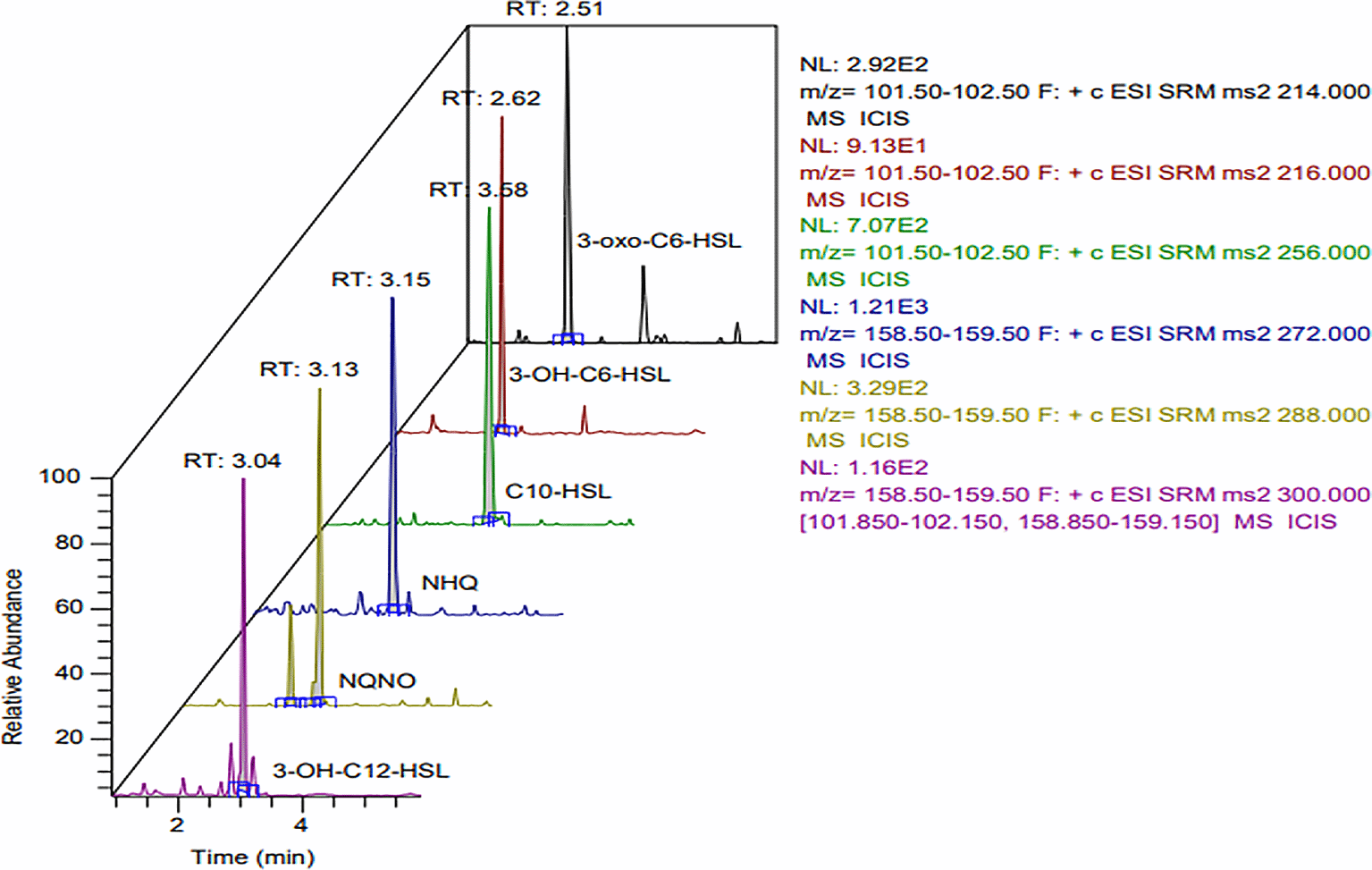
In order (from top to bottom), 3-oxo-C6-HSL, 3-OH-C6-HSL, C10-HSL, NHQ, NQNO, 3-OH-C12-HSL.
The chromatograms showed a number of substances with various peaks and molecular weights. Six compounds had prominent and high peaks (Figure 8). Based on the precursor ion (m/z), ion product (m/z), cone voltage, and impact energy, the six peaks were identified. The six peaks contained substances with properties resembling those of the QS substance K. pneumoniae. The six substances were NHQ, NQNO, 3-OH-C12-HSL, 3-oxo-C6-HSL, and C10-HSL. The six compounds were found to match the typical precursor parameters 3-oxo-C6-HSL, 3-OH-C6-HSL, C10-HSL, NHQ, NQNO, and 3-OH-C12-HSL (see Table 1).
| Control (+) | Control (-) | 18.75 | 37.5 | 75 | 150 | |
|---|---|---|---|---|---|---|
| %Inhibition* | 18.68 | 0.00 | 31.00 | 34.68 | 37.90 | 48.67 |
| 20.14 | 0.00 | 32.50 | 35.45 | 39.66 | 49.42 | |
| 19.32 | 0.00 | 32.29 | 33.45 | 42.75 | 46.15 | |
| Average | 19.38 | 0.00 | 31.93 | 34.53 | 40.11 | 48.08 |
| Standard error | 0.74 | 0.00 | 0.81 | 1.01 | 2.46 | 1.71 |
* The inhibitory effect of AOEP on cell-free EPS of KP-ESBL after incubation for 24 hours with different concentrations of AOEP exhibited inhibitory effect on cell-free EPS of KP-ESBL after incubation for 24 hours. The reduction of cell-free EPS by AOEP at concentrations of 18.75, 37.5, 75, and 150 μg/mL was 31.93%, 34.53%, 40.11%, and 48.08%, respectively. The kanamycin was only able to reduce 19.38% of cell-free EPS, while the negative control of KP-ESBL without AOEP exposure was 0%. The inhibition value of cell-free EPS by AOEP was better than that by kanamycin (48.08% versus 19.38%).
| Control (+) | Control (-) | 18.75 | 37.5 | 75 | 150 | |
|---|---|---|---|---|---|---|
| Survival rate | 95 | 2 | 31 | 47 | 66 | 83 |
| 97 | 1 | 28 | 34 | 77 | 79 | |
| 93 | 1 | 38 | 40 | 75 | 80 | |
| Average | 95.00* | 1.33 | 32.33* | 40.33* | 72.67* | 80.67* |
| Standard error | 2.00 | 0.58 | 5.13 | 6.51 | 5.86 | 2.08 |
QSSM: quorum sensing-like molecule.
The antibacterial ability of antibiofilm derived from natural sources can come from the production of enzymes, the formation of secondary metabolites or compounds that inhibit QS signals.18 QS inhibition can be mediated by receptor antagonists or quorum quenching enzymes.19 In this study, we searched for QS inhibitor compounds derived from the fungus A. oryzae. A. oryzae was harvested at a stationary phase in order to obtain the dominant secondary metabolite.20 A. oryzae produces secondary metabolites, such as asperfuranon, aspyridone, penicillin, isocoumarin, aspercryptin, and indole diterpene.21 However, from the LC-MS/MS analysis, we did not find any secondary metabolites or quorum quenching enzyme compounds from A. oryzae. This result is different from the LC-MS analysis from extract of A. meleus that produce AHL acylase, which can inhibit P. aeruginosa biofilms.22 In another study, A. niger produced cellobiose dehydrogenase which reduced the biofilm of Gram-negative bacteria.23
We discovered three new substances that are similar to the QS molecules of Gram-negative bacteria, which is interesting because we did not uncover secondary metabolites or quorum quenching enzymes. We propose those molecules, i.e., C10-HSL, 3-oxo-C6-HSL, 3-OH-C6-HSL and suggest them as QS molecules because, despite the fact that the three chemicals resemble the QS molecules found in Gram-negative bacteria, their activity is inversely related. We believe that A. oryzae’s QS molecules function as a competitive adversary. When it comes to attaching to AHL binding sites in LuxR, QS molecules compete with native AHL. One of the genes regulated by QS, the biofilm-encoding gene, is downregulated as a result of QS molecules binding to LuxR. According to this investigation, AOEP significantly reduced the KP-ESBL bacterial biofilm (74.24%). Aside from preventing the growth of biofilms, AOEP has also been demonstrated to lower EPS levels. EPS make up a robust biofilm matrix.3,22,24 The decrease in EPS synthesis was consistent with the structure of the KP-ESBL biofilm as determined by SEM. In the presence of AOEP, the bacterium cells were unable to aggregate. The matrix that holds bacteria together was also obviously thinner. Therefore, the absence of QS barriers may be the cause of the decline in biofilms. QS inhibitors (QSI) function by obstructing the binding sites for autoinducers. It also interferes with the formation of pili types 1 and 3 and cyclic diguanylate mono phosphate (c-di-GMP).25 As a result, QSI's inhibition will cause the expression of pili types 1 and 3 to be suppressed. Our findings are consistent with this notion. After exposure to AOEP, the expression of the pili type 3 gene (mrkA) was significantly reduced. This suggests that AOEP include QSI, which lowers mrkA expression. Sadly, there is no research to support our findings.
The expression of the wzI gene was assessed in order to support the mechanism through which AOEP inhibits KP-ESBL QS. Because QS controls the formation of capsular polysaccharides (CPS)10,26,27, inhibiting QS will impair its regulator role and cause the CPS to continue to be produced.26 The administration of AOEP in this investigation had no effect on the excretion of CPS by KP-ESBL. Through QS inhibition, it was discovered in this work that AOEP has antibacterial and antibiofilm activities against KP-ESBL. Because it synergistically reduces the expression of numerous virulence factors controlled by QS, QS inhibition in KP-ESBL is particularly helpful in the management of pneumonia.19
We chose C. elegans as a model because it is ideal for assessing QS inhibitors in order to further examine the therapeutic potency of AOEP on the infection caused by KP-ESBL. AOEP showed antibacterial and anti-QS action against KP-ESBL in in vitro experiments. The protective effect of AOEP against K. pneumoniae infection on C. elegans lends weight to these findings. The survival of infected C. elegans was generally increased by AOEP. We verified the anti-QS activity of AOEP in our investigation. Results from the C. elegans pneumoniae infection model demonstrate how AOEP can successfully reduce virulence by obstructing KP-QS ESBL's activity in in vitro investigations. It is possible to create new medicines for infectious diseases using K. pneumoniae QS inhibitors.
This study showed that the extracellular protein of A. oryzae posseses antimicrobial and antibiofilm activity against KP-ESBL. QSI is an AOEP compound that inhibits QS and degrade biofilms, EPS, and mrkA (type 3 pili). AOEP could protect C. elegans from KP-ESBL infection. AOEP is a potential source of natural antibiofilm agents against KP-ESBL.
Figshare: Aspergillus oryzae attenuates quorum sensing -associated virulence factors and biofilm formation in Klebsiella pneumoniae extended-spectrum beta-lactamases raw data, https://doi.org/10.6084/m9.figshare.20290929.28
This project contains the following underlying data:
- Biofilm inhibition.xlsx
- c. elegans survival rates.xlsx
- Exopolysaccharide.xlsx
- Minimum Inhibitory concentration.xlsx
- qRT-PCR.xlsx
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
We thank the Central Laboratory of Biomedik, Brawijaya University particularly to Suci Megasari for technical assistance.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Quorum sensing, Biofilm, Pseudomonas aeruginosa, Antibiotic Resistance, Bacteriophages, Microbiology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 07 Oct 22 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)