Keywords
18F-FDG, PET/CT, Colorectal cancer, Predictive value
This article is included in the Oncology gateway.
18F-FDG, PET/CT, Colorectal cancer, Predictive value
Colorectal cancer (CRC) is one of the main causes of high worldwide oncological mortality, and its metastasis to the lymph nodes (LN) is an important prognostic factor.1 Globally, CRC is the third most commonly diagnosed cancer, with an estimated 1.9 million (10%) new cases and 935,173 (9.4%) deaths.2 Furthermore, CRC is the second leading cause of cancer mortalities in 2020, according to the WHO GLOBOCAN database.3 In South-Central Asia, 102,987 (63%) new cases of CRC and 59,206 (36%) mortality incidences were registered in 2020.2,4
Positron-emission tomography/computed tomography (PET/CT) is a hybrid diagnostic method that shows the value of metabolic processes of the tissue at the molecular level in the tomographic mode. The advantage of PET/CT is to visualize viable tumor tissue and assess its biological activity by the degree of radiopharmaceutical agent accumulation in tissues and can be used to measure the hypermetabolic focus of visceral adipose tissue (VAT) activity. 18F-Fluorodeoxyglucose (18F-FDG) is now extensively used to assess functional VAT activity during PET/CT; therefore, it can identify accumulation loci and further detect metastases.5
Although the predictive role of 18F-FDG PET/CT in detecting metastases has been poorly studied, the studies on the reported prognostic value for various cancer locations have yielded inconsistent findings.6–11 Thus, VAT has been shown to increase the CRC risk, but the relationship between VAT and the predictive outcome in CRC is ambivalent. VAT is closely associated with dysregulated visceral fat activity increasing adipokines related to systemic inflammation, and can play a role in oncogenesis and metastatic lesion.1,12 The increased inflammatory condition of VAT activity might influence the status of LN in CRC patients.13–17
Byung Wook Choi et al. were among the few to retrospectively show the predictive value of metabolic parameters on 18F-FDG PET/CT in classical rectal adenocarcinoma.12 Another study by Sung Hoon Kim et al. retrospectively showed the prognostic factor of 18F-FDG PET/CT for LN metastasis in rectal cancer,18 whereas Kisoo Pahk et al. retrospectively showed the predictive value of functional VAT activity measured by preoperative 18F-FDG PET/CT for regional LN or distant metastasis in CRC patients.1
Given that the findings of these studies have been inconsistent in showing the exact maximum standardized uptake value (SUVmax) readings indicative of a higher risk of metastases, more data is needed to verify whether PET/CT can assist in early metastases identification in CRC patients. Therefore, the objective of this study was to quantitatively define functional VAT activity via 18F-FDG PET/CT in patients with CRC and its predictive potential for early LN metastases detection.
Approval was obtained from Local Bioethics Commission of the Medical Centre Hospital of President’s Affairs Administration of the Republic of Kazakhstan (approval #17/2020 on 24 January 2020) and Local Ethical Commission of the Al-Farabi Kazakh National University (approval #102 IRB – A102 on 28 May 2020). All patients routinely provided written informed consent for all medical tests and examinations and written informed consent was obtained for participation in the current study. We minimized selection bias by enrolling all patients for whom data were available in the database and of sufficient quality. When calculating the sample size, we allowed the maximum standard deviation. The significance level (α) was 0.05, and the study power was 80%, with a confidence probability of 95% (t=1.96). This study follows the TREND guidelines.19
We enrolled 534 patients with CRC, among which 60 patients had no metastases, 175 patients had metastases, 98 patients had a postoperative relapse with high metabolic activity, and 201 patients had a primary cancer disease progression. Patients who had metastases, postoperative relapse with high metabolic activity and primary cancer disease progression were excluded from the study. In total, we have prospectively evaluated 60 patients with a histologically confirmed diagnosis of adenocarcinoma who underwent 18F-FDG PET/CT in the Nuclear Medicine Unit of the Diagnostic Center of the Medical Centre Hospital of President’s Affairs Administration of the Republic of Kazakhstan (Nur-Sultan) during the period time between November 2015 and June 2021.
The study included 60 patients (age 39–81; median 60 (interquartile range (IQR) 55–68) years; 46 women) after a surgical treatment and courses of Folfiri and Folfox chemoradiotherapy according to the regimen. During the initial screening for eligibility, patients with histologically unverified colon cancer or with metastases confirmed at the baseline examination were excluded from the study. We also excluded patients with concurrent cancers. Tumor, lymph nodes, and metastasis (TNM) staging system along with American Joint Committee on Cancer (AJCC) stages of recruited patients are shown in Table 1. As Table 1 presents, there were no patients with AJCC stage IV, whereas adenocarcinoma was identified in 100% of patients. Of note, patients were classified into AJCC stages at their baseline examination, after which they were subjected to treatment and then underwent baseline PET/CT. By the time enrolled patients underwent baseline PET/CT, they had completed their treatment, had no signs of cancer or metastases, and this baseline PET/CT was considered as day 0 of the research.
Patients underwent 18F-FDG PET/CT at the initial enrollment and then again at a follow-up medical examination scheduled six months or more (median 12, IQR 6–40) after the baseline examination. All images were reconstructed using dedicated workstations and software (image evaluation system Wizard). Patients’ data were anonymized and de-identified before studies.
18F-FDG was produced at the Republican Diagnostic Center (Nur-Sultan, Kazakhstan) and was used on the day of the study due to the ultra-short shelf life (109 minutes). The whole-body 18F-FDG PET/CT images were completed using PET/CT scanner (Biograph TruePoint PET·CT, Siemens Medical Solutions USA Inc., USA) and carried out in conformity with the accepted clinical protocol of 18F-FDG PET/CT examination.20 Prior to PET/CT procedure and the corresponding 18F-FDG injection, patients fasted for at least 6 hours, and the glucose serum level in all patients was <11 mmol/l. The average activity dose of the injected 18F-FDG was 252.55 MBk, ranging from 132.5 to 465.3 MBk. The average effective radiation dose was 8.75 mSv, with a range from 6.8 to 17.1 mSv. CT scans were obtained following PET emission scanning. PET/CT study protocol included a topogram, a low dose CT to eliminate signal attenuation and anatomical correlation, and the collection of PET data. Duration of PET data collection depended on the patient’s height and weight, but usually completed within 25–40 minutes. Once PET data were obtained, CT and PET images were reconstructed and stored in the transaxial, coronal, and sagittal slices.
Image analysis was performed using the extended Siemens workspace (Biograph TruePoint PET·CT operating manual) in a region of interest (ROI). We calculated the standardized uptake value accumulation (SUV) in VAT automatically with the software using the formula:
VAT areas were identified by using predefined Hounsfield units (HU), ranging from [-70] to [-110] from background CT images. To measure the VAT activity, ROI (1.00 mm for each measured point) was divided into regions according to the topographic structure, including eight subdomains of abdominal regions (RE – epigastric region, RLH – left hypochondriac region, RRL – right lumbar region, RU – umbilical region, RLL – left lumbar region, RRI – right inguinal region, RP – hypogastric (pubic) region, RLI – left inguinal region) and pelvic cavity (P). They were located on three consecutive sections of the abdominal cavity to exclude the kidneys’ extra physiological absorption of 18F-FDG. We measured SUVmax in the axial plane for each area, and the average SUVmax of each area was calculated separately. All images were reconstructed in transaxial, sagittal and coronal multiplanar planes and read visually. With these functional parameters, the analysis was carried out by the status of metastatic LN lesions.
The primary end-point of this analysis was SUVmax of selected nine locations at baseline and follow-up. Image analysis was performed by determining the maximum standardized uptake value (SUVmax) VAT accumulation in each abdominal and pelvic cavity point. Each measured point was 1.00 mm and varied depending on the volume of VAT of the measured area. VAT areas were identified from background CT images, and SUVmax was defined on PET images, including a hypermetabolic focus on 18F-FDG PET/CT. We report SUVmax values for nine locations of the VAT, whereas the SUVmax at baseline and follow-up was a mean of several loci for each area with a 1-mm shift.
We first tested all variables for normality using the Kolmogorov-Smirnov test. Quantitative variables following the normal distribution pattern are presented as means (M) with the corresponding standard deviation (SD); alternatively, we reported medians with the corresponding IQR. SUVmax values for different locations and at different time periods (baseline or follow-up) were then compared with nonparametric tests, such as the Mann-Whitney U-test or Wilcoxon test. Because, in total, we selected nine locations to report SUVmax values, we tested SUVmax values for each location in the univariate analyses with regard to sex, primary tumor location, and other variables, using either Mann-Whitney U-test (for two groups) or Kruskall-Wallis test (for three or more groups). We also used a similar approach to compare groups depending on metastases status, including positive (pLM) patients in whom metastases were detected at a follow-up visit and negative (nLM) who showed no metastases. In such an analysis, we compared baseline SUVmax as a predictor. In addition, we tested age and sex as predictors of showing pLM at follow-up. Locations with significant differences between groups with regard to SUVmax and other tested predictors (age, sex) showing significant associations with LM status, were then tested in a logistic regression analysis, first crude, and then adjusted for other significant predictors, where we report the odds ratios (OR) of developing metastases at follow-up with the corresponding 95% confidence intervals (CI).
Finally, we applied receiver operating characteristic (ROC) curve analysis to assess the diagnostic performance of quantitative variables when predicting a categorical outcome. The optimal cut-off value of the quantitative variable was estimated using the Youden’s J statistic. All statistical analyses were performed using StatTech v. 2.7.1 (StatTech LLC, Russia).
There were more women in the studied group (n=46). The most prevalent primary tumor location (PTL) was the rectum (n=21), n=20 patients had the PTL in the colon, including n=6 as ascending, n=6 as descending and n=8 as transverse, whereas n=19 patients had tumor in the sigmoid, as presented in Table 2. With regard to tumor AJCC classification, most patients were classified as stage II (n=31) and III (n=27), with no patients having stage IV. At the baseline examination, the overall mean SUVmax was 0.80, with a significant difference in a nine-group comparison (p=0.016), whereas the highest accumulation level was found in RP (0.89) and the lowest in RLI (0.68). Sex affected the SUVmax in RLH (p=0.043) and RLL (p=0.048) locations, yielding higher readings in women compared to men. We also found differences in baseline SUVmax for colon, sigmoid and rectum in RRL (p=0.006), RU (p=0.016) and RLL (p=0.004), but not for a histological grade, TNM or AJCC stage (Table 2).21
At the follow-up examination of the 60 patients recruited initially, metastases developed in 16 (27%) patients, and these were classified as positive lymphatic metastasis (pLM), whereas the remaining 44 (73%) patients were classified as negative lymphatic metastasis (nLM). Such metastases location included LN of the neck, mediastinum, chest, peritoneum, retroperitoneum, and pelvis. We tested whether baseline SUVmax was different in those who developed metastases compared to those who did not. We found that such differences were statistically significant but not for all locations, only for RRL (1.29 vs. 0.82, p=0.032) and RU (1.00 vs. 0.74, p=0.041) (Table 3), indicative of some predictive potential of SUVmax in these two locations for metastasis at follow-up.
The median SUVmax of all locations increased from 0.8 at baseline to 0.94 at follow-up (p<0.001). We did not find a statistically significant SUVmax increase when considered separately in out of nine locations (Table 3), mostly because the sample size of each location was only 1/9 of the overall sample. We found the trend of SUVmax increase overall when stratified nLM to pLM, but it was insignificant. In addition, follow-up SUVmax for colon nLM equaled 0.93, with no difference compared to pLM (1.12; p=0.72). Similarly, we failed to confirm statistically significant differences of SUVmax when comparing nLM (0.93) with pLM (0.98) for sigmoid (p=0.62) and rectum (0.92 for nLM and 1.05 for pLM) (p=0.68).
In the univariate analysis, age and sex were not associated with metastases at follow-up (median age in nLM 59 vs. 63 years in pLM, p=0.12). We then tested whether baseline SUVmax of the selected two locations found to be significantly associated with metastases at follow-up, including RRL and RU, could predict metastases in the unadjusted and adjusted for age regression models. In the model adjusted for age, the OR for positive metastases at follow-up for RRL was non-significant and equaled 2.88 (95% CI 0.79; 10.70), and this model accounted for only 8% variability, whereas the OR for RU in a similar model adjusted for age was significant and equaled 5.42 (95% CI 1.20; 24.50), with an even greater R2 (0.13).
Of the nine locations in which we tested SUVmax as a predictor of metastasis on the follow-up visit, the highest areas under the curve (AUC) were found for RLH, RRL, RU and RRI. For RLH, SUVmax of 0.74 yielded the greatest AUC (0.668; 95% CI 0.505 – 0.831) with quite high sensitivity (75%) and specificity (61%). Although this model was statistically significant (p=0.049) (Figure 1), we failed to identify SUVmax corresponding to high sensitivity (80% or above) with acceptable specificity. When a high sensitivity of 80% was reached, we observed a dramatic fall in specificity. The corresponding SUVmax value with the highest AUC (0.682; 95% CI 0.520 – 0.843) for RRL was 1.05, for which sensitivity reached 69% and specificity was as high as 77%. This model was also statistically significant (p=0.032) (Figure 2). SUVmax value with the highest AUC (0.672; 95% CI 0.509 – 0.835) for RU was 0.85, for which sensitivity equaled 63% with almost similar specificity (61%). This model was also statistically significant (p=0.043), and Figure 3 illustrates AUC for this location. Finally, SUVmax with the highest AUC (0.679; 95% CI 0.517 – 0.841) for RRI was 0.78, for which sensitivity reached 69%, but specificity was only 61%, but statistically significant (p=0.035). Figure 4 reflects AUC for this analysis. Finally, PTL, tumor stage system, tumor grade and staging on LM did not affect SUVmax.
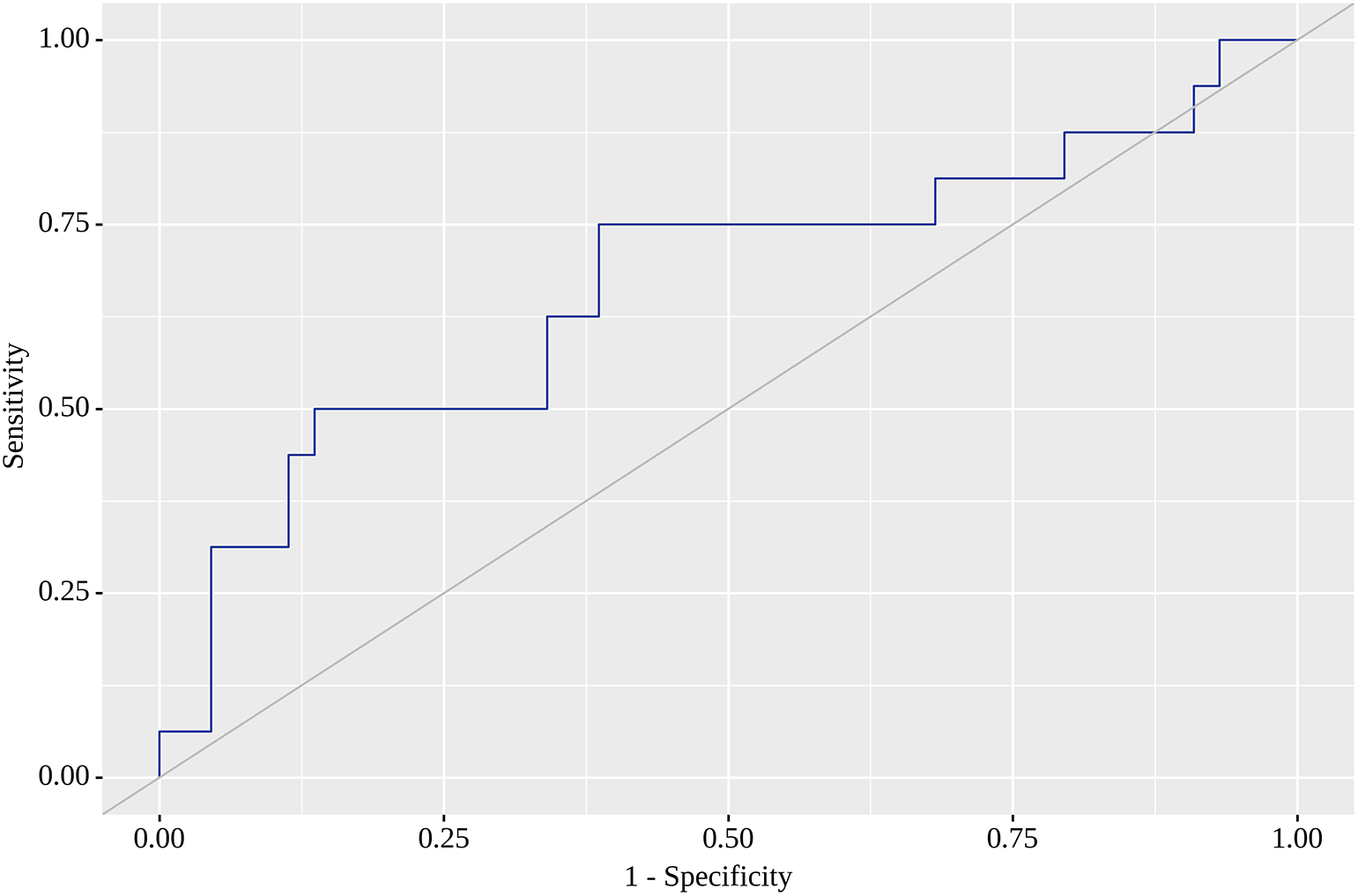
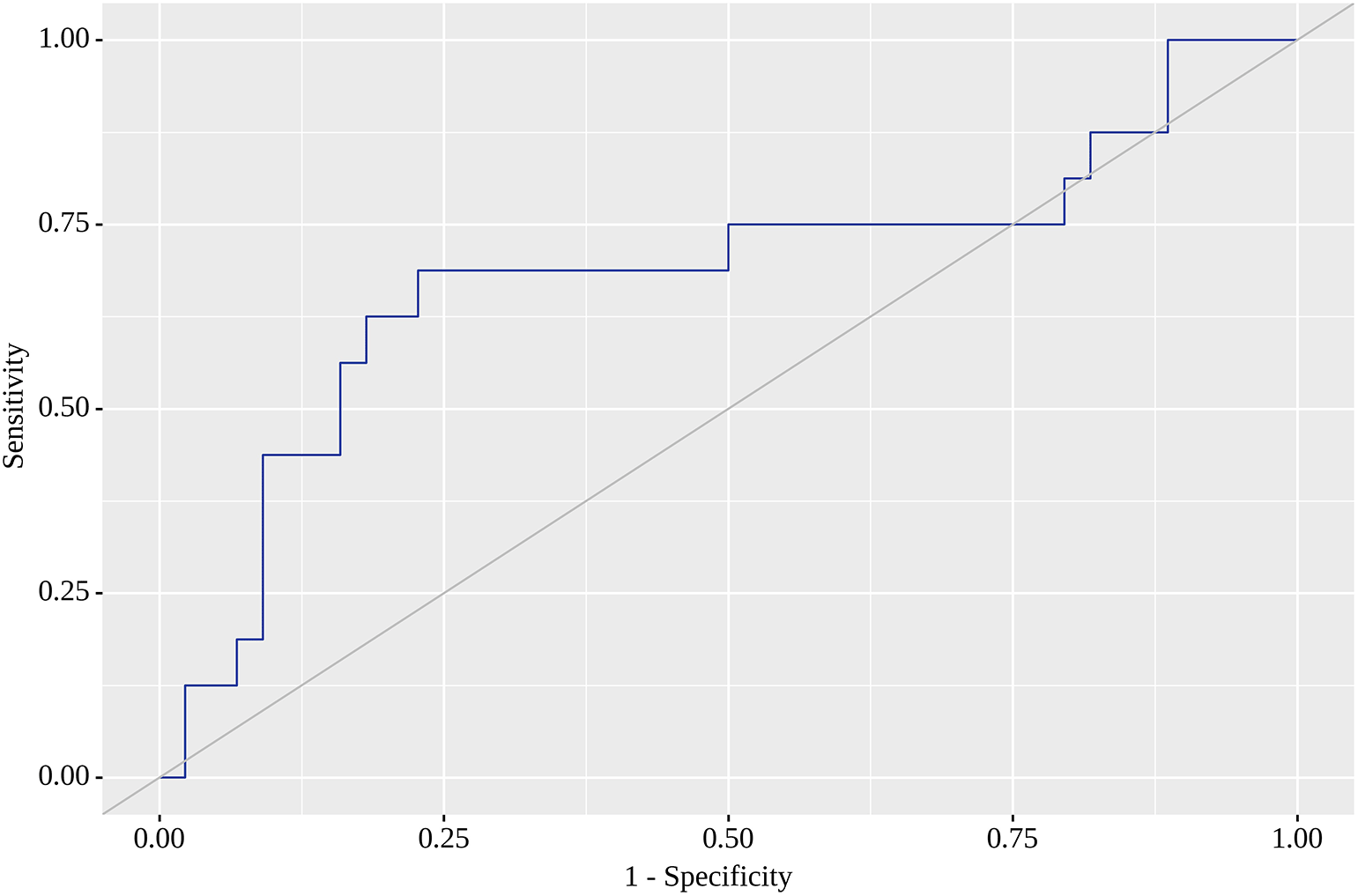
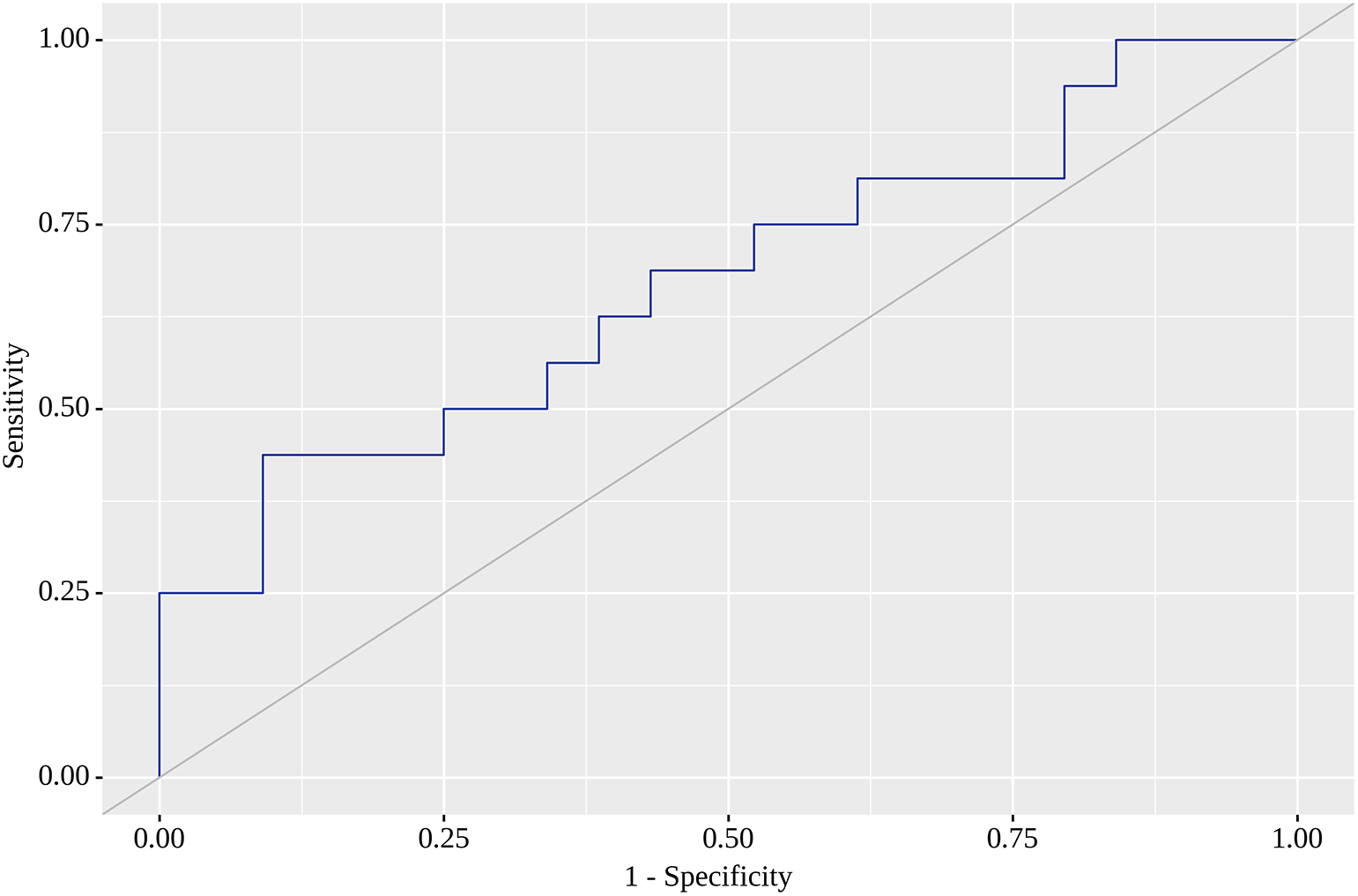
The current prospective observational cohort study is one of very few to identify the location of greater 18F-FDG accumulation by functional VAT activity as early markers of later metastases indicative of the metastatic status of CRC patients. In a cohort of 60 patients in adjusted regression models and ROC analysis we showed that 18F-FDG accumulation in RLH, RU and RRL and RRI were predictors of later metastases in CRC patients with moderate, but statistically significant sensitivity and specificity values. The threshold value of SUVmax 0.74 for RLH resulted in 75% sensitivity and 61% specificity, whereas the corresponding SUVmax for RRI was 0.78 with a sensitivity of 69% and a specificity of 61%. We also found that a threshold value of SUVmax 1.050 resulted in 69% sensitivity and 77% specificity for accumulation in RRL, whereas the SUVmax value of 0.85 warranted 63% sensitivity and 61% specificity for RU. In our analysis, 18F-FDG accumulation in the remaining tested five locations was not associated with later metastases risk.
The predictive value of 18F-FDG PET/CT for CRC has been reported in a number of preceding studies, reporting different SUVmax values. Byung Wook Choi et al. retrospectively emphasized the predictive value of metabolic parameters on 18F-FDG PET/CT in classical rectal adenocarcinoma in 149 patients on two models (AUC 0.778 and 0.762, p=0.04; 0.814 and 0.779, p=0.83).12 One more study by Sung Hoon Kim et al. retrospectively showed the prognostic value of 18F-FDG PET/CT for LN metastasis in rectal cancer in 166 patients, nodal SUVmax 2.356, AUC 0.698 (p=0.04), 0.720 (0.033), 0.806 (p=0.04).18 Finally, Kisoo Pahk et al. retrospectively showed the prognostic role of functional VAT activity evaluated by preoperative 18F-FDG PET/CT for regional or distant LN metastasis in 131 CRC patients; however, the ratio of visceral to subcutaneous fat activity (VAT/SAT) was evaluated, while the ratio of SUVmax 1.88, AUC 0.862, sensitivity 84.6%, specificity 78.8%, p<0.001.1 Emir Sokolović et al. showed the predictive metabolic value of SUVmax with metastatic CRC patients, and concluded that SUVmax could be used as a novel predictive factor of disease progression among metastatic CRC patients. Average ±SD progression-free survival with a SUVmax above 4.1 was 11.3±9.37 months, and a SUVmax below 4.1 was 19.6±12.05 months (p=0.001).22 Esra Arslan et al. showed the predictive potential of 18F-FDG PET/CT and KRAS mutation in CRC, where the mean SUVmax with primary tumor was estimated to be 21.1±9.1 (range= 6.0–47.5) and tumor mean SUVmax with a KRAS mutation (24.0±9.0) was found to be significantly higher than those without a KRAS mutation (17.7±8.2) (p=0.001).23
A number of prior reports ascertained the relationship between visceral adiposity and the prediction of CRC.24 Nevertheless, the outcomes were versatile and did not reach consent. These analyses used CT to measure VAT volume as a surrogate marker of VAT activity. But, VAT volume is reportedly unrelated to the visceral fat inflammatory process,25 whereas the identification of VAT volume by CT may not be satisfactory in affecting the current functional VAT activity.5 Therefore, a functional imaging modality like 18F-FDG PET/CT could be more suitable for evaluation of functional VAT activity than CT.
Previous research on functional VAT activity and 18F-FDG PET/CT concentrated on systemic inflammatory diseases, such as atherosclerosis or chronic obstructive pulmonary disease.5 Liang-qian Tong et al. illustrated the association between pulmonary 18F-FDG metabolism and smoking history in 347 healthy adults with chronic obstructive pulmonary disease where differences in the pulmonary SUVmax according to smoking status were analyzed. The mean SUVmax of current smokers was higher than that of ex-smokers with a medium (1.03±0.14 vs 0.88±0.16) or larger tobacco burden (1.08±0.15 vs 0.89±0.11) (p=0.012, p<0.001, respectively). However, there were no differences between the mean SUVmax of ex-smokers (0.91±0.13) and current smokers (0.91±0.16) with a smaller tobacco burden (p=0.888). The mean SUVmax of ex-smokers and current smokers with less tobacco burden were both significantly higher than that of non-smokers (0.78±0.13) (p<0.001, p<0.001, respectively).26
This research used 18F-FDG PET/CT to demonstrate the practical application of functional VAT activity for cancer disease, which can provide molecular data about inflammatory processes in CRC LM.
The current analysis has some limitations. Firstly, despite its prospective design, the study sample was limited, although patients were consecutively recruited for several years. Secondly, we could only enroll patients from a single nuclear medicine unit and only in the country’s capital. However, PET/CT is not yet widely available elsewhere in the country; therefore, the current sample is comprised of patients who were forced to travel to the capital for the examination, thus, representing a population from almost the entire country. Thirdly, predictive value was evaluated for SUVmax only, and other crucial factors such as grade, and location of the primary tumor could not be analyzed. Further prospective research data with larger populations will be necessary to verify our outcomes.
Finally, functional VAT activity evaluated by 18F-FDG PET/CT is substantially associated with LM. Furthermore, it is a useful factor for the prediction of LM. Implementing the results into practical medicine will help practitioners choose tactics and control CRC patients.
Open Science Framework: ‘Raw Underlying Data Colorectal Cancer for Predictive Value’. https://doi.org/10.17605/OSF.IO/NSZFK.21
This project contains the following underlying data:
Open Science Framework: TREND checklist for ‘Predictive value CRC’ https://doi.org/10.17605/OSF.IO/PRM95.19
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Nuclear Medicine
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Radiology and Nuclear Medicine in Cancer Diagnosis
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 10 Oct 22 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)