Keywords
Propolis extract, Candida Albicans, Antifungal activity, Vulvovaginal candidiasis
Propolis extract, Candida Albicans, Antifungal activity, Vulvovaginal candidiasis
Candidiasis affects approximately 138 million women worldwide each year, and is the second most common type of vaginal infection.1,2 Studies show that approximately 6000 women from different countries have a 9% prevalence of recurrent vulvovaginal candidiasis (VVC) and the likelihood of progression is high3; about 75% of women experience an episode of VVC during their childbearing years; about 40-50% experience a second VVC infection, and 5-8% of adult women have four or more episodes per year.4,5
The World Health Organization (WHO) considers C. albicans as one of the most recurrent causes of vaginal infections, because it is responsible for 80 to 92% of the cases of VVC.6 It is known that in the regions of Europe and Latin America, infection rates are 20 to 25%, of which 75% of adult women have contracted C. albicans at least once7; a study carried out in a Tertiary Care Hospital in India showed that out of 180 patients who underwent microbiological tests, 76-89% of the population studied had C. albicans as the most frequent pathology-causing species.8 In regions of the Americas, such as Mexico, a study of CVV was conducted on 412 people and found that 25% of them were positive. In the United States, 65% of hospitalised patients had fungal infections, and 78% of them are known to reach mortality depending on the risk factors presented by the patient.9 It is worth noting that C. albicans is considered to be the second most common cause of vaginal infections.10
In Peru, the most frequent species causing VVC is C. albicans, with a prevalence of 40-60% in adult patients.11 An obstetrician-gynecologist from the Instituto Especializado Materno Perinatal in Peru, after conducting a study, mentioned that, of all the cases found, 50% had vaginal discharge due to VVC and the vast majority were due to self-medication and overdose.12 In addition, the prevalence of VVC was found in 24% of pregnant women in the areas of Monsefu (Chiclayo) and 29.8% in Inkawasi (Ferreñafe), C. albicans always stood out as the aetiological agent for all these cases.11 In the Arzobispo Loayza National Hospital in Lima, of the total population investigated, 42.2% had a prevalence of vaginal infections.13
Current VVC treatment regimens have lost relevance in the face of antifungal resistance challenges,14 because the treatment of fungal infections such as VVC lacks specific drugs, unlike bacterial infections. These shortcomings require the production of a vaginal drug delivery system (VDDS) capable of attenuating, treating, and eliminating CVV clinically and effectively, thus avoiding the risk of disease recurrence and prolongation.15
Currently, amphotericin B and fluconazole are readily available azoles frequently used to treat candidiasis; amphotericin B is considered to be an excellent antifungal, but its level of toxicity is very high. However, their efficacy is poor due to the development of natural resistance by strains of C. albicans and non-albicans.16
Antifungal resistance is considered a consequence of inappropriate drug use, inadequate dosage, and incomplete treatment, as resistance to antifungal drugs has been increasing by up to 40% in recent years.16,17 These data are alarming in addition to the symptoms, recurrent misuse of medications, self-medication,18 short medication cycles or incomplete treatment of antifungal drugs19; these affect women who suffer from VVC as they have a poor quality of life that is unfavorable to their health.18 Therefore, the aim is to provide a solution to this common problem in women by considering the use of alternatives from natural products.
The aim of this study was to evaluate the antifungal activity of propolis extract against strains of Candida albicans isolated from patients diagnosed with acute and recurrent VVC, and to compare the antifungal activity of propolis extract and fluconazole.
The research was hypothetico-deductive, with a quantitative approach. The type of research was basic, and the design was experimental.20–22
The population consisted of Candida albicans strains isolated from patients diagnosed with VVC who were admitted to the gynaecology department of the María del Socorro Clinic in the district of Ate in Lima.
The sample size was 34 strains, a figure derived from the interaction between 17 strains of C. albicans (nine from acute VVC and eight from recurrent VVC) and two treatments (propolis extract and fluconazole), considering three replicates per strain, a total of 102 experimental units were obtained.23
It was suggested that there may be a significant difference between the antifungal activity of propolis extract and fluconazole against C. albicans strain types, this being the study hypothesis which will be tested by analysis of variance (ANOVA).
In the present investigation, to measure antifungal activity, the manual for susceptibility testing indicated by the Clinical and Laboratory Standards Institute (CLSI) in document M44 - A was used. Inocula of clinical strains of C. albicans were prepared and adjusted to the turbidity of the scale at 0.5 McFarland. Following the standardised susceptibility protocol established by Bauer et al.,24 the technique used was direct observation of the susceptibility of C. albicans strains.
The technique used was direct observation of inhibition halos and the instrument used was a data collection sheet to record the information obtained.
Prior to data processing and analysis, the following was applied:
Propolis extract
The propolis extract was collected with the support of beekeepers in the Oxapampa district, Cerro de Pasco region, Peru. Once the sample was obtained, we began with the separation of some components that were not part of the analysis, such as dust, wood chips, wood remains, among others that may alter the composition of the extract.25
Preparation of the extract
The characterization and preparation of the propolis at 50% concentration were done under aseptic conditions in a laminar flow cabinet at room temperature where impurities were discarded; then the sample was chopped and pulverized with the aid of a pestle and mortar.
The extract was prepared using 50 g of propolis in 100 mL of 70% ethanol, then the mixture was subjected to maceration for eight days at 37°C without contact with light. Subsequently, the sample was filtered with the help of Whatman No. 60 filter paper and then taken to a rotary evaporator at 60°C for the ethanol to be evaporated. The total extracts were transferred to the rotary evaporator until the solvent was eliminated and the solid obtained was placed in an oven for two hours at 70°C.
Finally, the ethanolic dry extract of propolis was obtained using the dilution and filtration technique to obtain propolis at a concentration of 50%. The selected propolis was visually identified according to its organoleptic characteristics, being predominantly brown in colour and lacking a defined aroma, to ensure its quality and purity.18
Method for obtaining clinical strains of Candida albicans
Permission was requested from the gynaecology service of the Clínica María del Socorro in the district of Ate in Lima to work with volunteer patients, who were previously informed of the study and agreed to sign the informed consent form.
Once authorization was obtained, samples of vaginal exudate were collected from the patients and taken by the doctor in charge of the area, who used a sterile dry vaginal swab to obtain the sample from the bottom of the vaginal sac, identifying the patients who presented acute and recurrent VVC; then, the samples were labelled with the corresponding type of infection and transported in sterile containers in a vertical position, in a cool environment and protected from light, to the laboratory of the aforementioned clinic. For isolation of the strains, the technique of depletion-streaking was used in Petri dishes with Sabouraud Dextrose Agar (SDA), and then incubated in an oven at 37°C.11
Candida albicans identification tests
After incubation, growth was observed on the surface of SDA, then a colony was taken for seeding on Chromogenic Candida agar to differentiate C. albicans from other Candida species, and microscopic observation was performed (see Figure 1 and Figure 2).
Note. a and b) Isolation on chromogenic agar.
In addition, for confirmation purposes, the growth of Candida sp. was validated with the germ tube test and then tested with the API 20 C AUX Candida test.10
a) Germ tube testing:
The germ tube test is highly useful in differentiating C. albicans and non-albicans Candida species. The procedure was performed with 0.5 mL of fresh human serum using the culture loop, inoculated with the strain under study and incubated at 37 °C. After three hours, a wet mount was prepared to observe under the light microscope, with 40× objective; the presence of the germ tube consisted of the filamentous extension of the yeast, where both C. albicans and C. dubliniensis formed a small extension similar to a “hand mirror” (Reynolds - Braude phenomenon). For this reason, the API 20 C AUX Candida test was used for confirmation.15
b) API 20 C AUX Candida test
The API 20 C AUX Candida test is an accurate yeast identification system consisting of 20 microtubes containing dehydrated substrates. These microtubes are inoculated into a minimal medium of API C medium and grow only yeasts capable of utilizing these substrates.
With 2 mL of saline and a young yeast culture, a suspension was made to a turbidity of McFarland scale 2.0. Then 100 uL of this suspension was added to the ampoule with API C Medium to proceed to fill the domes, always avoiding the formation of bubbles. The suspension was then kept in a humid chamber and incubated for 48-72 hours at 37°C.14
Finally, once the incubation time was over, the results were read and interpreted, observing growth by turbidity and with the help of the manufacturer's own numerical profile and the Apiweb identification program, the exact identification of the yeasts was carried out.
Preparation of culture environment
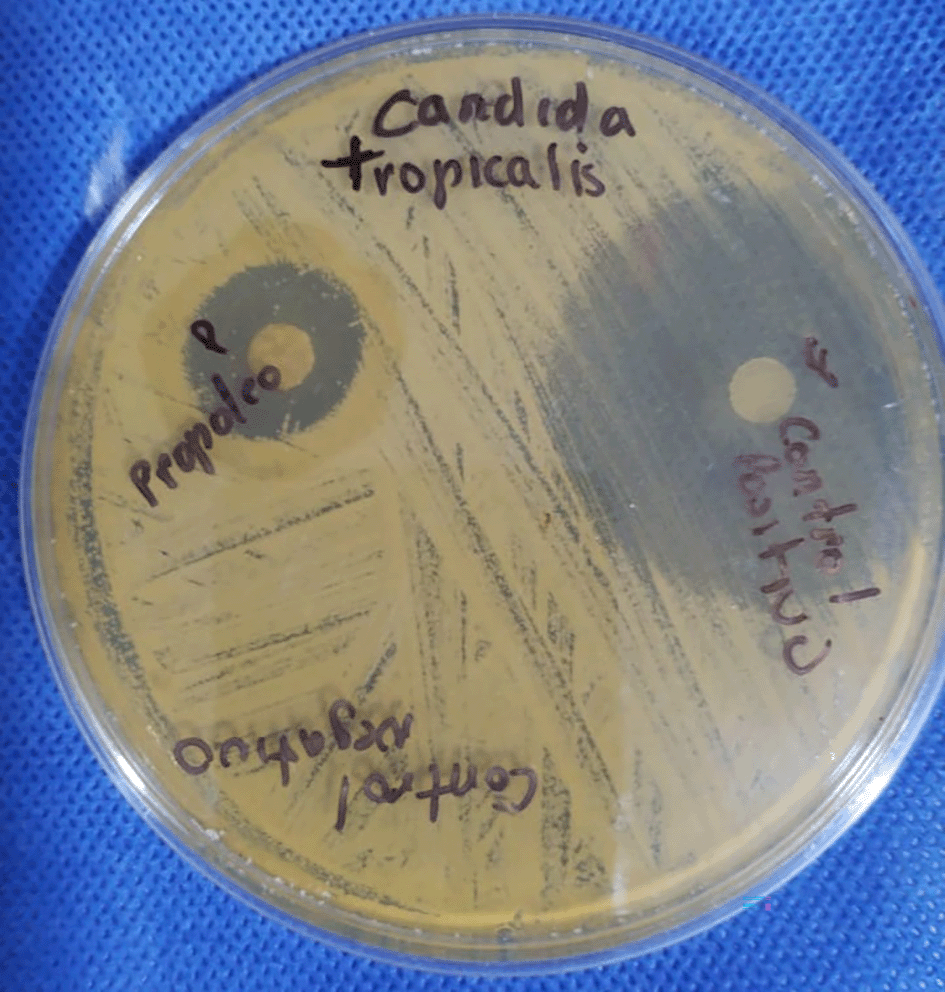
To measure antifungal activity, Müller Hinton Agar medium was used, which is validated for susceptibility testing as indicated by the Clinical and Laboratory Standards Institute (CLSI) M44 - A. Inocula of C. albicans clinical strains were prepared and adjusted to scale turbidity at 0.5 McFarland. The wavelength was 610 mm in the spectrophotometer for measuring the turbidity of the inoculum of the strains; it was then inoculated onto the agar surface with the help of a swab by streaking in three directions, allowed to dry for about 10 minutes and placed on fluconazole discs (positive control) and distilled water (negative control).9
Preparation of control groups
• Positive control: Fluconazole (25 mg/100 mL distilled water).
• Negative control: Distilled water (100 mL).
Quality control of the growth of C. albicans strains
One litre of Müller Hinton Agar was prepared according to the recommendation of the DIFCO manual26 by adding 20 g of glucose and 100 μL of stock solution of methylene blue. The latter solution is prepared by adding 0.1 g of methylene blue to 20 mL of distilled water (obtaining 5 μ/mL concentration). It was then autoclaved for 15 minutes at a temperature of 121°C and 15 lbs of pressure; following this, we let the temperature drop to about 50°C and poured 27 mL per Petri dish to reach 4 mm thickness, in 90 mm diameter plates. Two plates were considered for sterility control at 35°C for 24 hours.
Next, the previously grown C. albicans ATCC 90028 strain was taken to be seeded on agar. As described above, susceptibility testing was performed by adjusting the strain inoculum to 0.5 turbidity on the McFarland scale, seeding in three directions and placing a 25 μg disc of fluconazole for reading after 24 hours. To ensure quality control of strain growth, inhibition halos were observed.27
Once the C. albicans strains were obtained, the disc diffusion plate method was used, for which filter paper discs containing 200 μL of propolis extract and the respective controls were used, placing each embedded disc on a Petri dish with Müller Hinton agar and incubated for 24 hours at 37°C.10
After the incubation period, the antifungal growth inhibition halos were analyzed; the results were expressed by measuring the halos formed in the Petri dish, where the diameter of each zone was measured in millimeters, following the standardized susceptibility protocol established by Bauer, et al.24
Once the experimental procedure was completed, the data obtained were processed and a flow chart was elaborated for general data processing and analysis of the C. albicans strains.
The data collection process was carried out during September, October and November 2021, using a data collection sheet, and then tabulating the information in Microsoft Office Excel v. 2019 software, in which the information was recorded in a dataset.
The project was approved by Resolution N°1561-2022 by the president of the Institutional Research Ethics Committee of the Norbert Wiener Private University.
At the same time, written authorization was obtained from the Clinic María del Socorro to obtain samples and information through official letter N°017-2021-GMO-CMS.
The data collection instrument was applied at the time of measuring the growth inhibition halos of C. albicans strains by the effect of the antifungal activity of propolis extract and fluconazole, as well as recording the type of vvc and whether the causative agent was C. Albicans.
For the statistical analysis of the data, SPSS version 23.0 statistical software was used, in which analysis of variance (ANOVA) was used in order to establish whether the antifungal activity of propolis extract and fluconazole was equal in all studied strains; this was complemented with the Tukey significance test (α = 0.05) to define the differences in antifungal activity according to the types of C. albicans strains and the treatments used.
Participants were informed about the aim of the study, and those who agreed to participate signed an informed consent form, where they gave their approval for the use of the strains to be processed for the purpose of the study. All patients, carriers of the strain, were informed that their personal data would remain anonymous according to Law No. 29733 “Law on the protection of personal data”.28
Table 1 describes the antifungal activity of propolis extract and fluconazole against C. albicans strains isolated from patients with vulvovaginal candidiasis (VVC), showing mean inhibition halos of 15.2 mm for propolis extract and 18.0 mm for fluconazole.
Table 2 shows the existence of a statistically significant difference between the types of C. albicans strains that were inhibited by propolis extract and fluconazole. Thus, C. albicans strains isolated from acute VVC showed inhibition halos of a larger size on average than those from recurrent infection.
| Type of C. albicans strain | Mean | n | E.E. | ||
|---|---|---|---|---|---|
| Acute VVC | 18.87 | 54 | 0.20 | A | |
| Recurrent VVC | 14.08 | 48 | 0.22 | B |
Table 3 shows the existence of a statistically significant difference between the antifungal activity of propolis and fluconazole, the latter showing the largest average size of inhibition halos on C. albicans strains compared to propolis extract.
| Product | Mean | n | E.E. | ||
|---|---|---|---|---|---|
| Fluconazole | 17.86 | 51 | 0.21 | A | |
| Propolis extract | 15.10 | 51 | 0.21 | B |
H0-1: There is a significant difference between the antifungal activity of propolis extract and fluconazole against C. albicans strain types.
H0-2: There is no significant difference between the antifungal activity of propolis extract and fluconazole against C. albicans strain types.
In Table 4, the observed p-value was <0.0001, which is less than 0.05; therefore, we rejected the null hypothesis and affirm that there is a significant difference between the antifungal activity of propolis extract and fluconazole against the types of Candida albicans strains.
| FV | SC | GL | CM | F | p-value | Decision |
|---|---|---|---|---|---|---|
| Model | 777.42 | 2 | 388.71 | 174.73 | <0.0001 | |
| Strain type | 582.78 | 1 | 582.78 | 261.97 | <0.0001 | Reject H0-1 |
| Treatment | 194.64 | 1 | 194.64 | 87.49 | <0.0001 | Reject H0-2 |
| Error | 220.24 | 99 | 2.22 | |||
| Total | 997.65 | 101 |
The antifungal activity of propolis extract was evaluated against Candida albicans strains isolated from patients diagnosed with VVC; in the present investigation it was determined that propolis extract at a concentration of 50% showed antifungal activity on C. albicans strains. Studies by De La Cruz,13 and Joya et al.,3 who used ethanolic extracts of propolis against strains of the fungal species in question, differed from the present study in that they did consider using higher and lower concentrations of the product. The above suggests that a key factor in the sensitivity of C. albicans is its chemical composition, which is represented by proteins and polysaccharides. In addition, these microorganisms are known to have a cell wall, which is mainly composed of polysaccharides such as glucan and chitin. In addition, these species have a cytoplasmic membrane, where the antifungal agents carry out their function. In addition, the cytoplasmic membrane is composed of large amounts of carbohydrates and proteins in smaller proportions, which allow the entry and exit of substances such as secondary metabolites e.g., flavonoids, tannins, terpenes, and alkaloids.11,29
Regarding the specific objective of evaluating the antifungal activity of propolis extract against C. albicans strains isolated from patients diagnosed with acute and recurrent VVC, it was found that propolis extract showed inhibition of C. albicans strains isolated from acute and recurrent VVC. This finding is related to that reported by Adjapong et al.30 who compared the susceptibility of C. albicans strains isolated from acute and recurrent VVC, where the former showed greater sensitivity to fluconazole compared to those with recurrent infection, applying three doses of the antifungal agent.30 In the case of propolis, precise studies comparing its effect on both types of strains could not be found; however, it can be suggested that, in recurrent candidiasis, C. albicans expresses resistance to antifungal agents such as azoles, and therefore also to metabolites that induce their inhibition.31
Additionally, to compare the antifungal activity of C. albicans strains isolated from patients diagnosed with acute and recurrent VVC, it was found that C. albicans strains isolated from acute VVC showed a higher average inhibition halo than those from recurrent infection when exposed to propolis extract and fluconazole, and this difference was statistically significant. This finding is very similar to that reported by Adjapong et al.30 who compared the susceptibility of C. albicans strains isolated from acute VVC and recurrent VVC, where the former showed greater sensitivity to fluconazole compared to those with recurrent infection, applying three doses of the antifungal (2 μg/mL, 4 μg/mL and 8 μg/mL).30 In the case of propolis, precise studies comparing its effect on both types of strains could not be found; however, it can be suggested that, in recurrent candidiasis, C. albicans expresses resistance to antifungal agents such as azoles and, therefore, to the metabolites present in propolis extract that induce their inhibition as well.32
Finally, taking into account the specific objective of comparing the antifungal activity of propolis extract and fluconazole against strains of C. albicans isolated from patients diagnosed with VVC, it was possible to establish significant differences between the antifungal activity of propolis extract and fluconazole, the latter showing the highest average inhibition halo; this finding was very similar to that found by Capoci et al.7 who used propolis and fluconazole as a positive control for the inhibition of biofilms caused by C. albicans, the latter showing the greatest inhibitory effect against the strains of the microorganism in question.7 This suggests that, although the metabolites of the product studied may be involved in the growth and development of the infectious agent, antifungal agents have been shown to be more effective, as they not only affect the external component of the fungi, but also the cellular interior, causing a greater and faster inhibition. It should be noted that the metabolites described above, in most cases, stimulate the inhibition of biofilm formation, however, the antifungal agents par excellence, such as fluconazole, can pass through the cytoplasmic membrane of the fungus and affect cellular respiration and therefore cause its death more rapidly.32,33
It was concluded that:
• Propolis extract showed antifungal activity against strains of C. albicans isolated from patients diagnosed with both water-borne and recurrent vulvovaginal candidiasis.
• There was a significant difference between the antifungal activity of propolis extract and fluconazole against C. albicans strains isolated from patients diagnosed with vulvovaginal candidiasis.
• There was a significant difference between the inhibition halos of C. albicans strains isolated from patients diagnosed with acute and recurrent vulvovaginal candidiasis.
Zenodo: Antifungal activity of propolis extract against Candida albicans in patients with vulvovaginal candidiasis, https://doi.org/10.5281/zenodo.6981744.34
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
No
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Taking into account the area of study in which I am currently working, which is microbiology, immunology and molecular biology, I consider that this article does not meet the microbiological aspects necessary to be published. First, the method they use to determine susceptibility and resistance is obsolete. Currently there are new tools such as microplates to do this type of experiments. Second, the figures are not of good quality, nor are they appropriate for an original article. And finally, the methodology is confusing and not written properly.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 17 Oct 22 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)