Keywords
desmoplastic small round blue cell tumours, s: malignant pericardial effusion, primary cardiac tumor, mediastinal metastasis, cardiac sarcoma, cardiac malignancy
This article is included in the Oncology gateway.
desmoplastic small round blue cell tumours, s: malignant pericardial effusion, primary cardiac tumor, mediastinal metastasis, cardiac sarcoma, cardiac malignancy
Desmoplastic small round cell tumor (DSRCT) is an extremely rare and aggressive malignancy. It was first described in 1987 in young males involving the scrotum and abdomen.1 The origin of this tumor is still unknown, and it tends to present as an intra-abdominal or retroperitoneal mass.2 It is thought that DSRCT has a mesothelial origin, but an epithelial origin has also been hypothesized.2
According to the National Cancer Institute database, not more than 200-450 cases have been published since this entity was first described. In addition, few cases of DSRCT have been reported as chest masses, among which three were described to have a primary cardiac origin,3,4 with one presenting in a pediatric age group.5
Here, we describe a case of a primary DSRCT affecting the pericardium and myocardium of a 71-year-old male patient and the treatment he received based on the most updated evidence. To the best of our knowledge, only three cases of cardiac DSRCT have been reported in the English literature. Nevertheless, clinicians should be aware of such tumors to prompt early diagnosis and avoid treatment delays.
A 71-year-old male, a retired mechanic, with a history of hypertension, dyslipidemia, diabetes mellitus, chronic hepatitis C infection related to remote intravenous drug use, splenectomy for traumatic splenic rupture, former smoker, and chronic T cell large granular lymphocyte (T-LGL) leukemia diagnosed two years prior, presented with complaints of shortness of breath and chest pain. The electrocardiogram showed low voltage tracing suggestive of pericardial effusion. He was found to have a large anterior and posterior pericardial effusion with diastolic collapse on echocardiography suggesting cardiac tamponade. Computed tomography (CT) chest demonstrated moderate pericardial and pleural effusions with enlarged mediastinal lymph nodes (LN), the largest measuring 2.3 × 2.2 cm, and a right infra-hilar node 2.1 × 1.5 cm, without axillary or another lymphadenopathy. The patient underwent pericardiocentesis followed by anterior thoracotomy to create a pericardial window with a biopsy of pericardial and myocardial mass.
Pathologic evaluation of pericardial fluid and surgical biopsy, including histological, immunohistochemical (IHC), and cytogenetic analyses, were performed. Cytology of the pericardial fluid showed sheets of atypical lymphocytes, suspicious for the lymphoproliferative disorder. Flow cytometry was negative for B or T cell lymphoma, T- LGL only comprised 2.7% of the cells, and CD56 (+) cells comprised 8%. The surgical biopsy consisted of multiple pieces of grey tan myocardial tissue, measuring 0.4 cm in greatest dimension, and two pieces of pick tan pericardial tissue, measuring up to 1 cm. Histologically, sheets of atypical, small, round to oval cells embedded in a dense stroma were noted. Immunohistochemical stains showed diffuse positivity for epithelial membrane antigen EMA, cytokeratin AE1/AE3, and Wt-1c, with focal positivity for desmin (peri-nuclear), CD99, and Fli-1 (shown in Figure 1). MOC31, TTF-1, and synaptophysin stains were negative. Cytogenetic analysis demonstrated EWSR1:WT1 fusion. The fluorescence in situ hybridization (FISH) study was positive for a rearrangement involving the EWSR1 gene at chromosome 22q12 (39% of cells). The morphological features, as well as the IHC profile, supported the diagnosis of DSRCT.

Original magnification (A/D) × 40; (B/C) × 4.
Pretreatment staging positron emission tomography (PET)/CT (shown in Figure 2) revealed moderate to large nonmetabolic pericardial effusion with abnormal enhancement of the myocardium and multiple metastatic hypermetabolic mediastinal and left supraclavicular lymphadenopathy. The patient completed a modified P6 outpatient regimen with cycles 1,2,3, and 6 of cyclophosphamide, doxorubicin, and vincristine and cycles 4, 5, and 7 with ifosfamide and etoposide. He tolerated chemotherapy well over four months without noted toxicities or dose delays. Follow-up CT chest/abdomen/pelvis without contrast showed significant improvement in the generalized mediastinal lymphadenopathy and pericardial effusion, reflecting a good response to treatment.
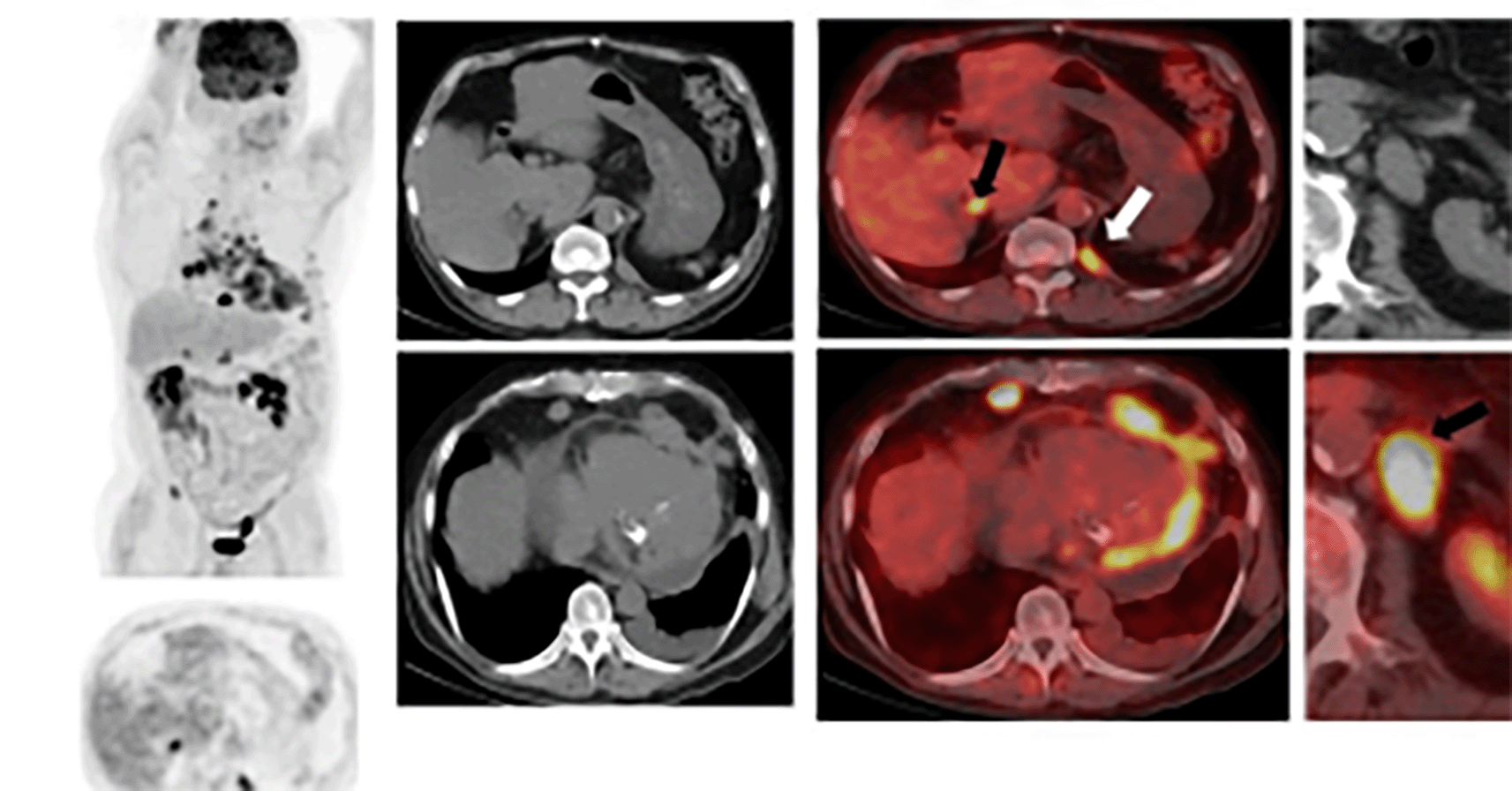
The relative paucity of tumor-free myocardium uptake compared to presumed regions of disease. Avid tracer uptake to numerous metastatic lymph nodes, including retrocrural lymph node (white arrow) and to both adrenal glands (black arrows).
The patient overall maintained good functional capacity and reported biking daily; however, he continued to present to the ED intermittently with complaints of pleuritic chest pain and dyspnea. Repeat CTA two months later showed disease progression with enlarged lymph nodes in the right mediastinum and a 2 cm pathologic lymph node in the lateral left epicardial fat pad that had previously measured 1 cm. The tumor was best characterized on magnetic resonance imaging (MRI) chest/thorax as an enhancing 3.7 cm infiltrative mass lesion about the anterior pericardium with likely infiltration of the adjacent right ventricular wall apex and numerous (more than 10) adjacent enhancing nodules seen involving the cardio-phrenic fat anteriorly, the largest on the right measured 2.1 × 1.9 × 2.1 cm, and the largest on the left 2.2 × 1.4 × 2.2 cm, which appeared contiguous with right periapical infiltrative pericardial mass (shown in Figure 3). The large pericardial effusion persisted, measuring up to 2.8 mm in maximum width without tamponade physiology. The patient received colchicine with an improvement in symptoms.

The patient presented again with complaints of abdominal pain and diarrhea two weeks after completing one cycle of second-line treatment with Irinotecan (20 mg/m2/day) and Temozolomide (100 mg/m2/day). As a result, he underwent urgent pericardiocentesis for early tamponade found on follow-up echo, followed by a repeat pericardial window.
Interval PET/CT revealed worsening disease with disseminated supraclavicular, mediastinal, and hilar lymphadenopathy with an invasion of the chest wall and possible invasion of the right ventricle (shown in Figure 4). The patient understood his poor prognosis and opted for completing two cycles of second-line chemotherapy. To date, it has been a total of 19 months since diagnosis.
DSRCT is a rare and highly malignant sarcoma commonly found in the abdomen, retroperitoneum, and pelvis, predominantly in adolescent males.6–8 Patients often present with symptoms related to abdominal distention, as the disease is often multifocal, with extensive lesions found in the peritoneum and mesentery.6,7,9 Concurrent sites of distant metastases at presentation commonly involve lymph nodes, the liver, and the lungs.6–10 Few cases of primary DSRCT have been reported in the lungs and pleura,11–13 with fewer reports of mediastinal cases.6,14,15 However, primary cardiac DSRCT is highly uncommon, with only three cases published in the literature.5–7
Histologically, DSRCT is characterized by nests of small, round, ovoid, or spindled cells embedded in an abundant desmoplastic fibrous stroma.16 Epithelial (keratin and epithelial membrane antigen EMA), mesenchymal (vimentin and desmin), and neural (CD56 and neuron-specific enolase NSE) markers are expressed on immunohistochemical staining of neoplastic cells, suggesting evidence of divergent differentiation.17 Immunohistochemical positivity for desmin, Wilms tumor 1 (WT1), keratin, and NSE is seen in the review of 32 tumors.1 In an analysis of 39 tumors with the histologic pattern of the desmoplastic small round cell, cytokeratin (PCK) is expressed in 95% of tumors, EMA in 96%, desmin in 100%, NSE in 72%, and WT1 in 89%.18 In our case, tumor cells show positive markers for desmin, WT1, PCK AE1/AE3, EMA, CD99, and CD56 consistent with immunohistochemical features of DSRCT. Also, the perinuclear dot-like pattern of desmin immunoreactivity is distinctive for DSRCT.19 The EWSR1:WT1 fusion protein, resulting from a reciprocal translocation between Ewing sarcoma RNA binding protein 1 (EWSR1) and Wilms tumor one gene (WT1) t(11;22) (p13;q12),20 constitutes the molecular hallmark of DSRCT.21 EWSR1:WT1 protein is thought to drive cell proliferation by acting through WT1 protein, a transcription factor involved in mesenchymal to epithelial differentiation during development.22 The observed immunoreactivity to the C-terminal region of WT1, present in our case, is diagnostic of DSRCT over Ewing sarcoma/primitive neuroectodermal tumor (EWS/PNET), which carries a similar EWSR gene rearrangement.23
Imaging studies regarding DSRCT can overlap with other aggressive peritoneal malignancies, such as neuroblastoma, malignant lymphoma, rhabdomyosarcoma, Ewing sarcoma, Wilms tumor, primitive neuroectodermal tumor, anaplastic synovial sarcoma.
Of the 400 DSRCT cases reported in the literature, roughly 200 are published in the radiology literature.24
CT and MRI are complementary studies in the setting of DSRCT; a retrospective study showed the peritoneal/omental masses with no identifiable origin of the tumor itself in 94% of the cases; furthermore, the majority (80%) demonstrating large (>5 cm) dominant soft-tissue deposit and multiple smaller foci25
CT and MRI typically demonstrated a heterogeneous soft-tissue enhancement with cystic degeneration; calcification is not a standard characteristic and is present only in 20% of the cases. Distant metastasis was seen in 25% of the cases; locally advanced disease was most familiar with further complications. These same characteristics have been seen in similar studies.15,26,27 Although CT scans in cardiac cases have yet to be completely documented, two of the three published cases had CTs prior to any intervention. The common findings were large pericardial effusion, mediastinal lymphadenopathy, and a heart mass. In all the cases, DSRCT was never the first diagnosis to be considered.5–7,28,29
Cases involving the mediastinum, and pleura, such as mesothelioma and localized fibrous tumors, make it hard to consider DSRCT as a possible diagnosis.
Mesothelioma usually presents as a unilateral pleural effusion, with thickening of the mediastinal pleura and circumferential/nodular pleural thickening with interlobar fissure as well; this can be seen in the image studies as an enhancement. On the other hand, fibrous tumors present as a homogeneous mass with intermediate to high attenuation on unenhanced CT scans.12–29
MRI has been useful in delineating the extension of the disease, including local invasion of osseous anatomical structures if surgical resection is being considered. MRI of DSRCTs often demonstrates high signal intensity on T2 weighted sequences vs. hypo- or iso-intensity relative to skeletal muscle on T1 weighted images. Heterogeneous T1 and T2 signal patterns and heterogeneous enhancement following gadolinium administration are attributed to fibrous stroma and internal degeneration, including necrosis, hemorrhage, and calcification.12,15,26,28,30
Regarding cardiac tumor involvement, the data for the utility of PET/CT as a diagnostic tool is limited, even unavailable. PET/CT shows the intensity of fluorodeoxyglucose (FDG) uptake, and the maximum standardized uptake value of the masses remains variable and is frequently as high as 12, indicating intensely avid hypermetabolism. PET/CT has no value in diagnosing DSRCT.30 However, PET/CT has been used to follow DSRCT lesions after the surgical or chemotherapeutic intervention, to verify tumor response to therapy, and to assess patient outcomes based on the changes in metabolic activity of lesions during therapy.26–30
There is no standard therapeutic regimen for DSRCT. The literature recommends various treatments, including multiple-agent chemotherapy, adjuvant radiotherapy, surgical procedures, and targeted drug therapy. In our index patient, the treatment was with chemotherapy utilizing the P6 regimen (consisting of seven courses of chemotherapy) with cycles 1,2,3 and 6 of cyclophosphamide (4200 mg/m2), doxorubicin (75 mg/m2), vincristine, and cycles 4, 5 and 7 with Ifosfamide (9 mg/m2) and Etoposide (500 mg/m2).
Due to the tumor's location, it becomes challenging to propose surgical management. However, the P6 regimen combined with debulking surgical and radiation therapy approaches is the best treatment strategy,31 which is well described in a review of 12 patients by Hassan et al. treated at the Mayo Clinic. Hassan et al. reported that the medical survival of patients managed with surgical resection was 34 months, compared with the ones that underwent biopsy alone were 14 months.32 Furthermore, T. Lal et al. compared the 3-year survival of the surgery group and the non-surgery group; 58% survived in the surgery group, while there was no survivor in the group that did not have surgery.15 Hence, it was proposed that effective cytoreduction and tumor resection would benefit patients without metastasis.
Jin et al. described the medical management for mediastinal DSRCT in a 51-year-old male patient who was treated with cyclophosphamide combined with doxorubicin and vincristine chemotherapy for four cycles. Repeat imaging showed distant metastasis, for which the patient was started on ifosfamide (9 g/m2) + etoposide (500 g/m2) every two weeks for three cycles. Subsequently, cyclophosphamide (200 g/m2) + adriamycin (75 g/m2) + vincristine (1.4 g/m2) were used for maintenance treatment for 4 cycles. Radiotherapy (a total of 70Gy of mediastinal Y field radiation) was administered. Nonetheless, there was no improvement; the patient had a total survival period of 17 months.15
Therapies for DSRCT are more limited due to surgical resection and radiotherapy difficulties. In addition, patients have progress of disease despite maximal chemotherapy. This should prompt further research into more effective chemotherapeutic options.
DSRCT is a rare and highly malignant sarcoma commonly found in the abdomen, retroperitoneum, and pelvis, predominantly in adolescent males. Patients often present with symptoms related to abdominal distention, as the disease is often multifocal, with extensive lesions found in the peritoneum and mesentery. Concurrent sites of distant metastases at presentation commonly involve lymph nodes, the liver, and the lungs. Few cases of primary DSRCT have been reported in the lungs and pleura, with fewer reports of mediastinal cases. However, primary cardiac DSRCT is exceptionally uncommon, with only three cases published in the literature.
Informed written consent was obtained from the patient to publish this case report and the accompanying images.
All data underlying the results are available as part of the article and no additional source data are required.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the background of the case’s history and progression described in sufficient detail?
Partly
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Yes
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Partly
Is the case presented with sufficient detail to be useful for other practitioners?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: DSRCT in general, T cell bispecific antibodies, radioimmunotherapy
Is the background of the case’s history and progression described in sufficient detail?
Yes
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Yes
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Yes
Is the case presented with sufficient detail to be useful for other practitioners?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Broad exposure to all sorts of tumors in an academic setting, including sarcomas and mediastinal tumors.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 28 Oct 22 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)