Keywords
Zinnia elegans, tool-like receptor, extraction, gastric mucosal damage
This article is included in the Cell & Molecular Biology gateway.
Zinnia elegans, tool-like receptor, extraction, gastric mucosal damage
One of the chronic illnesses of the digestive system with a greater rate of clinical incidence and recurrence is a gastric ulcer (GU). Upper abdominal pain, stomach distension, belching, acid reflux, hematemesis, and melena may also be present in more severe cases. Additionally, it may result in consequences such as gastric perforation and bleeding, harming the patient's health and quality of life.1,2
The etiologies of the lesion are related to a disruption in the balance of factors that protect or damage the mucosal epithelium.3 Nonsteroidal anti-inflammatory drug (NSAID) misuse, smoking, Helicobacter pylori (H. pylori) infection, alcohol use, and psychological and physical stress contribute to the damage. In addition, excessive alcohol consumption is thought to be the main factor contributing to injury to the mucosal lining.4,5
The activation of neutrophil infiltration at the site of injury brought on by ethanol induction damages the gastric mucosa by increasing the production of proinflammatory, pro-oxidative factors, and enzymes, as well as free radicals.6 Interleukin-1 (IL-1), tumor necrosis factor (TNF), and toll-like receptor 4 (TL4), among other proinflammatory cytokines, were shown to have significant roles in the treatment of acute GUs brought on by ethanol in previous investigations. TNFα and IL1β are inflammatory cytokines that play a significant role as mediators of the acute inflammatory response to infectious agents.7–10
Zinnia elegans is one of the most widespread garden plants globally, with flowers of an extensive range of colors. Additionally, when compared to other garden plants; Zinnia has small flowers and a narrow foliage.11 The aerial parts of Z. elegans contain flavonoids as a significant compound as well as glycosides, saponins, anthocyanins, tannins, and phenols.12 Furthermore, studies have shown evidence of antifungal activity by Zinnia genus.13 Other studies have shown hepatoprotective activity14 as well as antibacterial and antiviral activity,15,16 and activity against cancer cells (demonstrated on cancer cell lines),17 besides the antioxidant properties.18 Moreover, antioxidants play a positive role in healing gastric ulcers and gastric infections with Helicobacter pylori.19 Therefore, due to the antioxidant and antibacterial properties of Zinnia elegans, it can probably be preventive against GU.
Therefore, we believe it is essential to investigate Zinnia elegans' ability to act as a protective barrier against GU. The present investigation evaluated the protection from Zinnia elegans extract on gastric mucosal injury in mice induced with 70% ethanol. In addition, to assess the gastroprotective properties of Zinnia elegans extract, the GU index, histology, and two inflammatory cytokines, namely TNFα and IL1β levels, were also analyzed.
The investigations were carried out following the guidelines established by the Ethics Committee at Al-Esraa University's College with approval number 437 on 7 January 2021. The animal study was carried out in accordance with the ARRIVE principles 2.0 and the ARRIVE Essential 10 checklist for pre-clinical animal research. Furthermore, all attempts were made to keep rats as comfortable as possible during experiments and sampling.
Zinnia elegans were collected in October 2021 from the local Iraqi market. The College of Science/University of Baghdad acknowledged the plant's authenticity. First, the plant material was dried at room temperature in the shade; then, the aerial parts were ground into a powder and weighed. Next, they were carefully cleaned using tap water, left to air-dry at room temperature, and ground into a powder material for further investigation.
The dried aerial parts of Zinnia elegans were mixed with ethanol (70%) using a Soxhlet apparatus for 8-10 h at 55-85 °C to extract the polar and non-polar compounds. The resulting ethanolic extract was concentrated under low temperature (40°C) and reduced pressure by a rotary evaporator. The obtained extracts were collected in stopper glass bottles and stored at 4°C.
The Iraqi National center for drug control and research provided male mice (28 ± 3 g) housed at 25±3°C, 30–70% humidity, and normal light/dark (12 h/12 h) cycle conditions; animals received essential lab nourishment. The entire study was conducted at the pharmacology laboratory of Al-Esraa University College at Specific Pathogen Free Animal Lab. Before experiments, all mice were permitted to acclimatize for one week. In this study, 32 animals were randomly allocated into four groups:
Group 1: Negative controls were given normal saline at a dose of 2.5 mL/kg for three days.
Group 2: Positive controls were given normal saline for three days, and on the third day, we gave them omeprazole (20 mg/kg).
Group 3: The experiment model was given normal saline for three days, and on the third day, were given ethanol (0.01 mL/g).20
Group 4: The treatment group was given Zinnia elegans extract (100/100 g) for three days, and on the third day, were given ethanol (0.01 mL/g).14
The induction of lesions in the animals’ stomach was done an hour before being euthanized under anesthesia by cervical dislocation; the stomachs were removed, and longitudinal gastric cutting was performed. After the stomach was slightly cleansed with ice-cold saline to remove the gastric contents, the more significant curvature was opened, and the degree of the gastric mucosal injury was assessed using the GU index. Then, the stomachs of each animal were divided into two moieties, one being preserved at -80°C for biochemical analysis and the other being submerged in 4% paraformaldehyde solution for histological studies.
Sigma-Aldrich (USA) provided the absolute ethanol. Omeprazole was purchased from Cipla Limited (India) B.No: DJ05551.
The Bioassay Technology Laboratory provided TNF-α, IL-1, and TL4 enzyme-linked immunosorbent assay (ELISA) kits (China); details are presented in Table 1.
| Providers | Kit | Catalog number | LOT | Manufacturing date | Expiration date |
|---|---|---|---|---|---|
| The Bioassay Technology Laboratory | TNF-α | E0117Mo | 202101001 | 2021/01/04 | 2022/01/03 |
| The Bioassay Technology Laboratory | IL-1b | E0192Mo | 202101001 | 2021/01/04 | 2022/01/03 |
| The Bioassay Technology Laboratory | TL4 | E1663Mo | 202101001 | 2021/01/04 | 2022/01/03 |
70% ethanol was used to cause gastric mucosal damage in mice.21
The phytochemical analysis of the alcoholic extract of the aerial parts of Zinnia elegans was assessed: saponins, alkaloids, tannins using 5% ferric chloride, terpenoids using 2, 4-dintrophenyl hydrazine, and steroids using Liebermann-Burchard test.22
The GU index was used to assess the degree of stomach mucosal damage: (0) represents no damage, (1) represents blood in the lumen, (2) represents pin-point erosions (erosion of 1 mm), (3) represents small erosions (1 mm ≤ erosion < 2 mm), (4) large erosions (2 mm ≤ erosion < 3 mm), (5) large area erosions (3 mm ≤ erosion).23
The injured stomach tissue was fixed in 4% paraformaldehyde solution for 24 hours, along with normal gastric tissues from the standard control group. After washing with tap water, the dehydration process used ethanol at increasing concentrations. The specimens were deparaffinized, rehydrated, cleaned in xylene, embedded in paraffin, sliced in 5-μm sections, mounted on clean glass slides, and then colored with hematoxylin and eosin.23 All gastric specimens were inspected blindly under a LIRI 2006 microscope and analyzed using a GMS image analysis system (Shanghai Optical Instrument Factory, China).
First, the stomach tissue samples were homogenized in phosphate buffer saline (pH 7.4). After that, the tissue homogenate was centrifuged at 2,000-3,000 RPM for approximately 10 min to obtain a supernatant for determining the levels of IL-1β, tumor necrosis factor-α TNF-α, and a TLR4 for later analysis using ELISA.
Phytochemical analysis of the ethanolic extract of Zinnia elegans' aerial parts revealed the presence of tannins, steroids, flavonoids, and terpenoids. Nevertheless, the extract was damaging to anthraquinones, alkaloids, saponins, and cardiac glycosides, as illustrated in Table 2.
The standard control group had no macroscopic defects, as seen in Figure 1a. The ethanol control group, on the other hand, showed significant morphological changes, such as redness and bleeding (Figure 1b). Zinnia elegans extract administered groups and the omeprazole control group revealed noticeable reduction in these modifications compared to animals in the ethanol control group (Figure 1c and d). In addition, Figure 2 shows that pretreatment of mice with Zinnia elegans extract (100/100 g) substantially lowered the GU index. The omeprazole control group's GU index was significantly much lower than the ethanol control group.
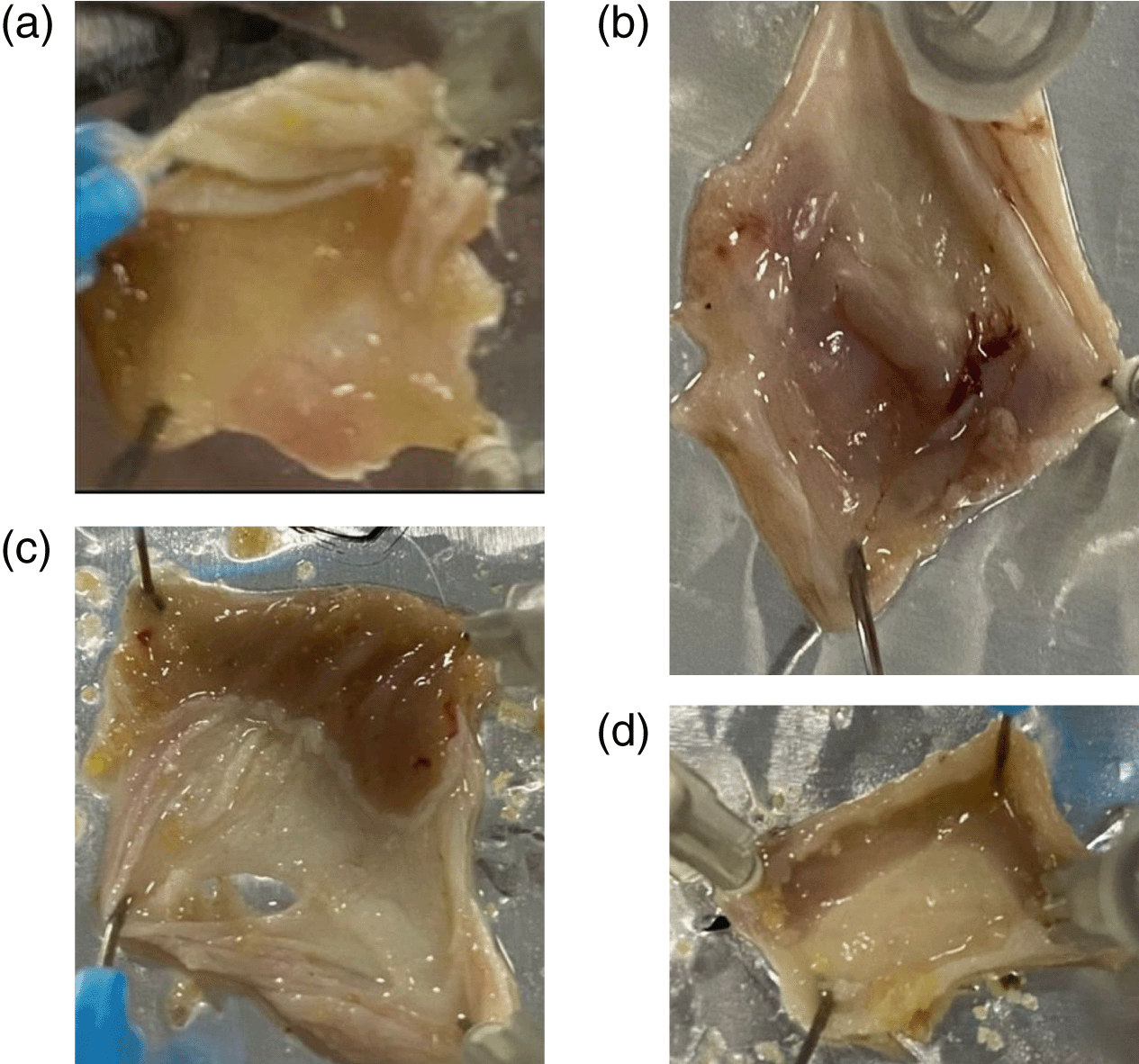
a: Received normal saline. b: Received normal saline and ethanol. c: Received normal saline and Omeprazole. d: Received Zinnia elegans extract.
Histopathological investigations of gastric mucosa from various groups are shown in Figure 3a-d. Mice who received normal saline are presented in Figure 3a. Histopathological analyses revealed that ethanol caused severe hyperemia and gastric mucosal epithelium shedding in the ethanol group (Figure 3b). Omeprazole (Figure 3c) also diminished these alterations. Zinnia elegans pretreatment (Figure 3d) showed the best prevention for the gastric mucosa cells by a significant reduction in these alterations.
IL1β (Figure 4a) and TNFα (Figure 4b) levels in the ethanol group were considerably more remarkable than in the control group due to gastric mucosal damage induced by ethanol. Omeprazole and Zinnia elegans pretreatment significantly reduced TNF and IL1 levels compared to the ethanol control group.
The results of this study showed that mice with the ethanol-induced group revealed a significant elevation in TLR4 level in stomach tissue compared to the standard control group. In contrast, mice pretreated with Zinnia elegans exhibited significant (p<0.05) down-regulation in TLR4 level compared to the ethanol group, which implies the potential effect of Zinnia elegans against damage associated with cytokines that lead to gastric injury, as shown in Figure 5.
The current work used ethanol-induced gastric mucosal injury in mice to assess the preventive efficacy of Zinnia elegans extract against gastric mucosal injury. The anti-ulcerative impact assessment was carried out using ethanol gastric mucosal damage in mice In addition, ethanol can affect the gastrointestinal mucosa, resulting in significant macroscopic damage such as redness, bleeding, and ulcers in a brief period.24 These modifications are similar to those observed in the present study. However, pretreatment with Zinnia elegans extracts significantly reduced these alterations.
Furthermore, the histopathological investigation revealed that Zinnia elegans extract pretreatment diminished hyperemia and mucous cell damage. These outcomes indicated that Zinnia elegans extract possesses a reasonable defensive role against ethanol-induced gastric abrasions on the stomach mucosa. Notably, the amount of proinflammatory cytokines in gastric tissues is a good indicator of the degree of gastric mucosal damage. TNFα and IL1β are two central proinflammatory cytokines that play a part in GU inflammatory response. TNF and IL-1 levels appear to rise due to ethanol-induced stomach mucosal injury.25,26 Nevertheless, pretreatments with Zinnia elegans significantly decreased IL1β and TNFα amounts, which showed the anti-inflammatory impact against gastric mucosa damage. Furthermore, earlier information has revealed that IL1β and TNFα can stimulate NF-κB. Stimulated NF-κB can result in numerous aspects, elevated COX-2, iNOS, and ICAM-1, increasing the severity of stomach mucosal damage.27 Furthermore, Zinnia elegans has been shown to inhibit the NF-κB pathway.28 EIGMD could be distinguished by an apparent oxidative stress marker that reflects the degree of the gastric mucosal damage.29 TNFα and IL1β are significant indicators for assessing the degree of gastric mucosal damage. Zinnia elegans therapy considerably lowered the TNFα and IL1β levels substantially higher than in the control group due to ethanol-induced gastric mucosal damage.30 Other factors may increase TNFα and IL1β levels and cell injury.31 TNFα and IL1β levels are connected with the severity of epithelial cell necrosis, injury, and death.32 Nonetheless, mice pretreated with Zinnia elegans presented lowered TNFα and IL1β levels.31,33 A small amount of TNFα and IL1β action can exacerbate gastric mucosal damage and influence the healing of gastric mucosal damage. Oral inoculation of Zinnia elegans remarkably reduced TNFα and IL1β levels. These facts suggest that Zinnia elegans' antioxidant activity could enhance the potential gastroprotection.34
In addition, more investigators discovered that Zinnia elegans offers an acceptable antioxidative impact.15,35 This outcome confirmed that Zinnia elegans might reactivate the antioxidant protection method or scavenge free radicals by itself, thereby enhancing the gastric mucosal defense system. In the present research, Zinnia elegans reduced TNFα and IL1β levels. Furthermore, Zinnia elegans, as a significant gastric protective aspect, provides excellent ethanol resistance and contribute to the acceleration of stomach ulcer repair.36 Again, oral administration of a Zinnia elegans pretreatment showed superior prevention for the gastric mucosa cells, enhancing gastric mucosa defense.
Additionally, Zinnia elegans also enhances angiogenesis in gastric mucosa and the healing of EIGMD.37–39 In conclusion, the outcomes of the current study indicated that Zinnia elegans had a significant defensive impact against ethanol-induced gastric mucosal damage in mice. Antioxidative and anti-inflammatory effects may be the means of this defense. Moreover, the alleviation of gastric mucosal damage by Zinnia elegans may be connected with the up-regulation of TNFα and IL1β levels. The GU index of mice pretreated with Zinnia elegans extract was significantly reduced. Compared to the ethanol control group, the GU index of the omeprazole control group was much lower. Further research is needed before the findings from this work can be used to develop a novel anti-GU substance that can be used in clinical medicine.
Based on the data, it can be inferred that Zinnia elegans ethanolic extract has an anti-inflammatory action that protects mice from ethanol-induced gastric mucosal damage. The present research suggests that a new anti-GU product could be developed as a different approach to treating gastric injury created by alcohol consumption.
Zenodo: Measurement of TNF-α, IL-1β and Tl4 levels in gastric tissue, https://doi.org/10.5281/zenodo.7156119. 40
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0)
Zenodo: The ARRIVE Essential 10: author checklist gor “Gastroprotective effect of Zinnia elegans extracts against ethanol-induced induced gastric mucosal damage through downregulation of TLR4 and inflammatory cytokines”, https://doi.org/10.5281/zenodo.7244086. 41
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)