Keywords
Moringa oleifera leaf extract, Sjögren's syndrome, Salivary gland, Apoptosis
This article is included in the Plant Science gateway.
Moringa oleifera leaf extract, Sjögren's syndrome, Salivary gland, Apoptosis
Sjögren’s syndrome (SS) is a systemic, complex, and multifactorial autoimmune disease. The triggering events of autoimmune disease and the pathophysiology of SS are unknown, but it is thought that both genetic and environmental factors play an important role (Igoe and Scofield 2013). The incidence of SS ranges between 0.01% and 0.72% globally (Brito-Zerón et al. 2016). Women are more likely to have SS compared with men, with a percentage value of 9:1 and a mean age of 56 years (Stefanski et al. 2017). The prevalence of SS is also found to be higher in the elderly population (Haugen et al. 2008). SS predominantly appears in women around or after menopause, although it can develop at any age (Brito-Zerón et al. 2009; Haugen et al. 2008). In Indonesia, there are no epidemiological data related to SS cases; only systemic lupus erythematosus and rheumatoid arthritis (RA), thus SS does not have adequate data.
Oxidative stress occurs in many autoimmune diseases, along with the excess production of reactive oxygen species (ROS) and reactive nitrogen species, which are related to the inflammatory process. The sources of such reactive species include nicotinamide adenine dinucleotide phosphate hydrogen (NADPH) oxidases (NOXs), the mitochondrial electron transport chain, nitric oxide (NO) synthases, and other enzymes (Smallwood et al. 2018). Various cluster of differentiation/CD4+ T cell subsets emerge to contribute to primary Sjögren’s syndrome (pSS) pathogenesis, including the T helper (Th) 1, follicular T helper, and T helper 17 (Th17) cells. T helper 17 cells play a role in mucosal barrier physiology and pathogen-associated inflammatory responses. T helper 17 cells producing interleukin 17 (IL-17) are also found in salivary gland lesions and are high in peripheral blood (Verstappen et al. 2018). Interleukin 17 promotes the production of metalloproteinase matrix 9 (MMP-9) and harms the mice's corneal barrier (Chauhan et al. 2009; de Paiva et al. 2009). In SS patients there is an increase in MMP-9 gene expression and plasma levels (Hulkkonen et al. 2004). MMP-9 is an important inflammatory mediator involved in SS immunopathogenesis (Pflugfelder SC 2014). B lymphocyte cells also play an important role in the pathogenesis of SS by several mechanisms, namely, as cytokine producers, antigen-presenting cells, and autoantibody secretors (Nocturne and Mariette 2018). Autoantibodies that are characteristic of pSS include anti-Ro/Sjögren's syndrome antigen type A (anti-Ro/SSA) antibodies, which can be detected in 70–100% of patients with SS, and anti-La Sjögren syndrome type B antigen (anti-La/SSB), which has detection rates ranging from 40 to 90% (Wenzel et al. 2001). Anti-La antibodies are not always positive, however, the combination of the two is more likely to lead to SS than anti-Ro antibodies alone (Scofield et al. 2018). Anti-Ro antibodies are a more specific diagnostic marker and are included on Sjogren's syndrome criteria when compared with anti-La (Shiboski et al. 2017).
Sjogren's syndrome primarily affects the salivary and tear glands. In the ACR/EULAR classification criteria for the diagnosis of primary Sjogren's syndrome, the presence of focal lymphocytic sialadenitis in the labial salivary glands and a focal score ≥ 1 foci/4 mm has the highest score, which is 3. The diagnosis of Sjogren's syndrome is established if the total score is ≥4.
Currently, there is no effective drug for the management of the etiology of SS (Shen et al. 2019; Vivino et al. 2019). Therapeutic approaches are limited to topical and systemic to treat sicca and systemic symptoms, with the aim of improving quality of life (Carsons et al. 2017; Shih et al. 2017). Although diagnostic criteria and guidelines for the management of SS have been developed, gaps remain with respect to effective specific therapies and their impact on patients (Romão et al. 2018). The imbalance of cytokines and their pathological effects is one aspect that can be a target for therapy (Sambataro et al. 2017).
Moringa oleifera (MO) is reported to have anti-inflammatory, antimicrobial, antioxidant, anticancer, cardiovascular, hepatoprotective, anti-ulcer, diuretic, antiurolithiasis, and anthelmintic functions (Farooq et al. 2012). M. oleifera leaf extract, ripe or still soft, exhibits strong antioxidant activity against free radicals, prevents oxidative damage to key biomolecules, and provides significant protection against oxidative damage. Furthermore, various animal safety studies involving M. oleifera leaf extracts have shown a high level of safety (Mahmood et al. 2010; Stohs and Hartman 2015). The ethyl acetate fraction of M. oleifera extract in in vitro studies contained high levels of phenols and antioxidant activity. This fraction can inhibit the production of cytokines by macrophages in vitro triggered by cigarette smoke extracts, such as tumor necrosis factor (TNF), interleukin-6 (IL-6), and interleukin-8 (IL-8). In addition, it inhibits the expression of RelA, which is a gene involved in the NF-κB (activated B-cell kappa-light-chain-enhancing nuclear factor) p65 inflammatory signaling pathway (Kooltheat et al. 2014).
As aforementioned, M. oleifera leaves have antioxidant activity, so there is a need for research to assess whether there is any effect of M. oleifera (Kelor) leaf extract on salivary gland damage and the levels of IL-17, MMP-9, malondialdehyde (MDA), epithelial necrosis, and caspase-3 levels in primary SS model rats.
An experimental laboratory technique with a post-test only control group design was performed. The sampling method used in this study was purposive sampling. Male mice, BALB/c strain, aged 8–10 weeks, body weight (BW) 26–27.5 g, no physical disability, and normal activity were the inclusion criteria. Mice that died during the treatment period was the exclusion criteria. Sample size was determined by Federer's formula [(n − 1) (t − 1) > 15], so the obtained minimum samples number was six mice for each group. Two mice were added to each group to anticipate mice death, so that the final sample size was 32.
Thirty-two samples were divided into four treatment groups: 200 mg/kg BW MO-ethanol leaf extracts with 1.23 mg/kg BW dexamethasone group (T2), 1.23 mg/kg BW dexamethasone alone group (T1), normal control group/C- (without induction of Ro antigen (SSA) and extract of MO-ethanol), and negative group/C+ (with induction of Ro antigen (SSA) on day 42). The treatment in the form of dexamethasone and MO-ethanol leaf extracts given for 14 days. MDA, IL-17, MMP-9, and caspase-3 levels and salivary gland epithelium damage (histopathological changes) were observed 14 days post-Ro antigen (SSA) induction. The method used to measure MDA level was Thiobarbituric Acid Reactive Substance (TBARS) while IL-7 and MMP-9 were ELISA. Some of the salivary gland was used for histological preparations using the paraffin method without Harris hematoxylin–eosin (HE) staining for the examination of caspase-3 with the standard procedure of immunohistochemical staining. The salivary gland epithelium damage examination used the HE staining of histological preparation.
Histopathological preparations were observed and scored according to the following categories:
Score 0: No necrosis
Score 1: Mild necrosis
Score 2: Moderate necrosis
Score 3: Severe necrosis.
Interventions of BALB/c mice, histopathological preparations for epithelial necrosis, and histopathological reading of epithelial necrosis were conducted at the Experimental Animal Care Center (PAU UGM, Yogyakarta), Histology and Cell Biology Laboratory (Faculty of Medicine, UGM, Yogyakarta), and Anatomical Pathology and Histology Laboratory (Faculty of Medicine, UNS Surakarta), respectively. The authors were unaware of the allocation group so that all the mice were handled, monitored and treated in the same way while conducting the experiment.
This mice model was conducted for 42 days. BALB/c mice were immunized with a short peptide of 60-kD Ro antigen (SSA) that triggered an immune response and formed anti-Ro antibodies. There was a decrease in mouse salivary flow and T lymphocyte infiltration (both CD4+ and CD8+ T cells), in immunized mice similar to SS in humans (Scofield et al. 2018). BALB/c mice have a similar response to the nucleotide-binding and oligomerization domain/NOD-like receptor gene, C3H/HeJ. The C3H/HeJ gene has implications for the pathogenesis of SS (Kim et al. 2017; Sellers 2017). The average BW of BALB/c mice at 8, 9, and 10 weeks old was 26.2±1.4, 27.0±1.4, and 27.4±1.4 g, respectively. Mice were reared on a diet containing 6% fat according to the LabDiet® 5K52 feed formulation.
Mice were kept in four cages made of plastic tubs covered with wire at the top. The conditions during acclimatization and treatment were controlled in a fixed environmental range, namely in a room that had 12 hours of light and 12 hours of darkness with a room temperature ranging from 23-26°C with the aim that the test animals could adapt according to the animal's biological time and the conditions to be occupied during the experiment. Temperature, water supply, the number of mice in the cage, and the change of husks were all done the same for all groups of mice. Adaptation to mice with care in cages with a size of 28 × 30 × 12 cm so that they can move freely and not be stressed.
At the end of the study, euthanasia and removal of salivary glands were performed. We made 3 mm horizontal incision on the skin placed 1 mm below the ear lobe to expose the glands underneath. After identifying the parotid gland, we used the curved forceps to pull the gland out, used a scalpel to separate the gland from the surrounding tissue. The tissue was then put into a container containing 10% neutral buffer formalin. The sample was then made preparations with Harris Hematoxylin Eosin staining.
The leaf extracts were washed with tap water and dried at 24°C for a day and subsequently in an oven for two consecutive days at 45°C. The extracts was then ground using a mechanical blender and stored in a vacuum container. M. oleifera leaves were completely crushed using 90% ethanol (ethanol: distilled water, 9:1) and then put into a shaking aspirator bottle for 3 days at 24°C. The residue was filtered using Whatman filter paper No. 1, and the filtrate was condensed using a rotary evaporator at 40°C. The condensed residue softened and became dark green in color, and was then freeze-dried. The freeze-dried extracts were weighed, and stored in closed containers, before being properly labeled and stored at −20°C. Administration of M. oleifera leaf extract in ethanol at 200 mg/kg BW for 14 days showed anti-inflammatory activity (Both et al. 2017; Karthivashan et al. 2016).
The normality test used was the Shapiro–Wilk test. Numerical data were subsequently analyzed using ANOVA, then processed using the Tukey HSD (Honest Significant Difference) post hoc test for normally distributed and homogeneous data and the Games–Howell post hoc test for non-homogeneous data. The Kruskal-Wallis test was conducted on an abnormal distribution and continued with Mann-Whitney post hoc test. Independent t-tests were performed to compare the treatment group with other groups. The statistical analysis used was SPSS 22 for windows and a p value<0.05 was considered statistically significant.
Differences in MDA levels based on groups can be seen in Table 1. The C(−) control group had the lowest MDA level (1.55±0.2 mg/ml), whereas the C(+) group had the highest MDA level (10±0.5 mg/ml). Based on a one-way ANOVA, there was a significant difference in MDA levels with C(−) as reference (p< 0.05). Levene’s test value was p=0.03, and the Games–Howell post hoc test was conducted (Table 2).
| Groups | MDA levels |
|---|---|
| C(−) | 1.55±0.2 |
| C(+) | 10±0.5 |
| T1 | 5.38±0.43 |
| T2 | 2.98±0.32 |
| p value | <0.001** |
| MDA | ||||
|---|---|---|---|---|
| C(−) | C(+) | T1 | T2 | |
| p value | Reference category | <0.001** | <0.001** | <0.001** |
| 95% CI | Reference category | −9.05 to −7.87 | −4.34 to −3.32 | −1.82 to −1.04 |
| Mean difference | Reference category | −8.46 | −3.83 | −1.43 |
The difference in MDA levels with the T2 group as a reference compared to other groups is shown in Table 3. It exhibits the significant difference in MDA levels of the T2 treatment group post-Ro (SSA) antigen induction when compared to the C(−), C(+), and T1 groups (p<0.001). This was the most striking difference between the T2 and the C(+) cluster with p<0.001 (95% CI 6.58–7.47). It is clear that the T2 group was more effective in reducing MDA levels post-Ro (SSA) antigen induction than the other groups.
Differences in IL-17 levels based on groups can be seen in Table 4. Based on a one-way ANOVA, there was a significant difference in IL-17 levels with C(−) as reference (p<0.05). Levene’s test value was p=0.92. The Tukey HSD post hoc test was subsequently conducted (Table 5).
| Groups | IL-17 levels |
|---|---|
| C(−) | 30.94±2.69 |
| C(+) | 82.04±3.52 |
| T1 | 65.93±3.15 |
| T2 | 40.95±3.28 |
| p value | <0.001** |
| IL-17 | ||||
|---|---|---|---|---|
| C(−) | C(+) | T1 | T2 | |
| p value | Reference category | <0.001** | <0.001** | <0.001** |
| 95% CI | Reference category | −5.75 to −5.49 | −2.32 to −1.85 | 1.23 to −0.90 |
| Mean difference | Reference category | −5.62 | −2.08 | −1.06 |
Table 5 shows that IL-17 levels post-Ro (SSA) antigen induction in the C(+), T1, and T2 groups were significantly different from the C(−) control group with p<0.001. This showed effective treatment in reducing IL-17 levels. Differences in IL-17 levels between the T2 group and the other groups is shown in Table 6. The table demonstrates that there is a significant difference in IL-17 levels of the T2 treatment group post-Ro (SSA) antigen induction when compared with the C(−), C(+), and T1 groups with p<0.001. The most significant difference was between the T2 and C(+) groups with p<0.001 (95% CI 37.44–44.74). It can be concluded that the T2 group was more effective in reducing IL-17 levels post-Ro (SSA) antigen induction than the other groups.
| T2 group | IL-17 level post-Ro (SSA) antigen induction | ||
|---|---|---|---|
| C(−) | C(+) | T1 | |
| F value | 0.52 | 0.013 | 0.02 |
| t value | −6.67 | 24.17 | 15.54 |
| p value | <0.001** | <0.001** | <0.001** |
| 95% CI | −13.24 to −6.78 | 37.44–44.74 | 21.53–28.42 |
Differences in MMP-9 levels based on groups can be seen in Table 7. According to a one-way ANOVA, there was a significant difference in MMP-9 levels with C(−) as reference (p<0.05) and Levene's test value p=0.28, and then the Tukey HSD post hoc test was conducted (Table 8). Table 8 shows that MMP-9 levels post-Ro (SSA) antigen induction in the C(+), T1, and T2 groups were significantly different from the C(−) control group with p<0.001. This showed effective treatment in reducing MMP-9 levels. Differences in MMP-9 levels between the T2 group and the other groups are revealed in Table 9.
| Groups | MMP-9 levels post-Ro (SSA) induction |
|---|---|
| C(−) | 7.68±0.67 |
| C(+) | 30.71±1.15 |
| T1 | 15.71±1.28 |
| T2 | 11.08±0.84 |
| p value | <0.001** |
| MMP-9 | ||||
|---|---|---|---|---|
| C(−) | C(+) | T1 | T2 | |
| p value | Reference category | <0.001** | <0.001** | <0.001** |
| 95% CI | Reference category | −24.40 to −21.64 | −9.41 to −6.64 | −4.78 to −2.02 |
| Mean difference | Reference category | −23.02 | −8.03 | −3.4 |
| T2 group | MMP-9 level post-Ro (SSA) antigen induction | ||
|---|---|---|---|
| C(+) | C(+) | T1 | |
| F value | 0.92 | 0.68 | 1.21 |
| t value | −8.96 | 39.04 | 8.58 |
| p value | <0.001** | <0.001** | <0.001** |
| 95% CI | −4.21–2.59 | 18.55–20.70 | 3.47–5.78 |
Table 9 displays that there are significant differences in MMP-9 levels of the T2 treatment group post-Ro (SSA) antigen induction when compared to the C(−), C(+), and T1 groups with p<0.001. The most significant difference was between the T2 and C(+) groups with p<0.001 (95% CI 18.55–20.70). It can be seen that the T2 group was more effective in reducing MMP-9 levels post-Ro (SSA) antigen induction than the C(−), C(+), and T1 groups.
Differences in caspase-3 levels based on groups can be seen in Table 10. Based on a one-way ANOVA, there was a significant difference in caspase-3 levels with C(−) as reference (p<0.05) and Levene's test value p=0.01. The Games–Howell post hoc test was subsequently conducted (Table 11).
| Groups | Caspase-3 levels |
|---|---|
| C(−) | 1.77±0.43 |
| C(+) | 7.39±0.11 |
| T1 | 3.85±0.2 |
| T2 | 2.83±0.14 |
| p value | <0.001** |
| Caspase-3 | ||||
|---|---|---|---|---|
| C(−) | C(+) | T1 | T2 | |
| p value | Reference category | <0.001** | <0.001** | <0.001** |
| 95% CI | Reference category | −5.75 to −5.49 | −2.32 to −1.85 | 1.23 to −0.90 |
| Mean difference | Reference category | −5.62 | −2.08 | −1.06 |
Table 11 shows that caspase-3 levels post-Ro (SSA) antigen induction in the C(+), T1, and T2 groups were significantly different from the C(−) control group with p<0.001. This showed effective treatment in reducing caspase-3 levels. Differences in caspase-3 levels between the T2 group and the other groups is shown in Table 12. Table 12 shows that there are significant differences in caspase-3 levels of the T2 treatment group post-Ro (SSA) antigen induction when compared to the C(−), C(+), and T1 groups with p<0.001. The most significant difference was between the T2 and C(+) groups with p<0.001 (95% CI 4.43–4.69). It can be concluded that the T2 group had more effective results in reducing caspase-3 levels post-Ro (SSA) antigen induction than the other groups.
| T2 group | Caspase-3 level post-Ro (SSA) antigen induction | ||
|---|---|---|---|
| C(−) | C(+) | T1 | |
| F value | 4.52 | 0.23 | 1.98 |
| t value | −20.67 | 72.93 | 11.74 |
| p value | <0.001** | <0.001** | <0.001** |
| 95% CI | −1.17−9.52 | 4.43–4.69 | 0.83–1.21 |
A total of 11 mice were induced by Ro (SSA) antigen, and their salivary glands were collected. These samples were prepared with Harris hematoxylin and eosin staining and evaluated using a scoring system, which can be seen in Figure 1. Differences in salivary gland epithelial necrosis based on groups can be seen in Table 13. Table 13 shows that there is a significant difference in salivary gland epithelial necrosis scores between various groups with p<0.001, and then a Mann–Whitney post hoc test was conducted (Table 14).
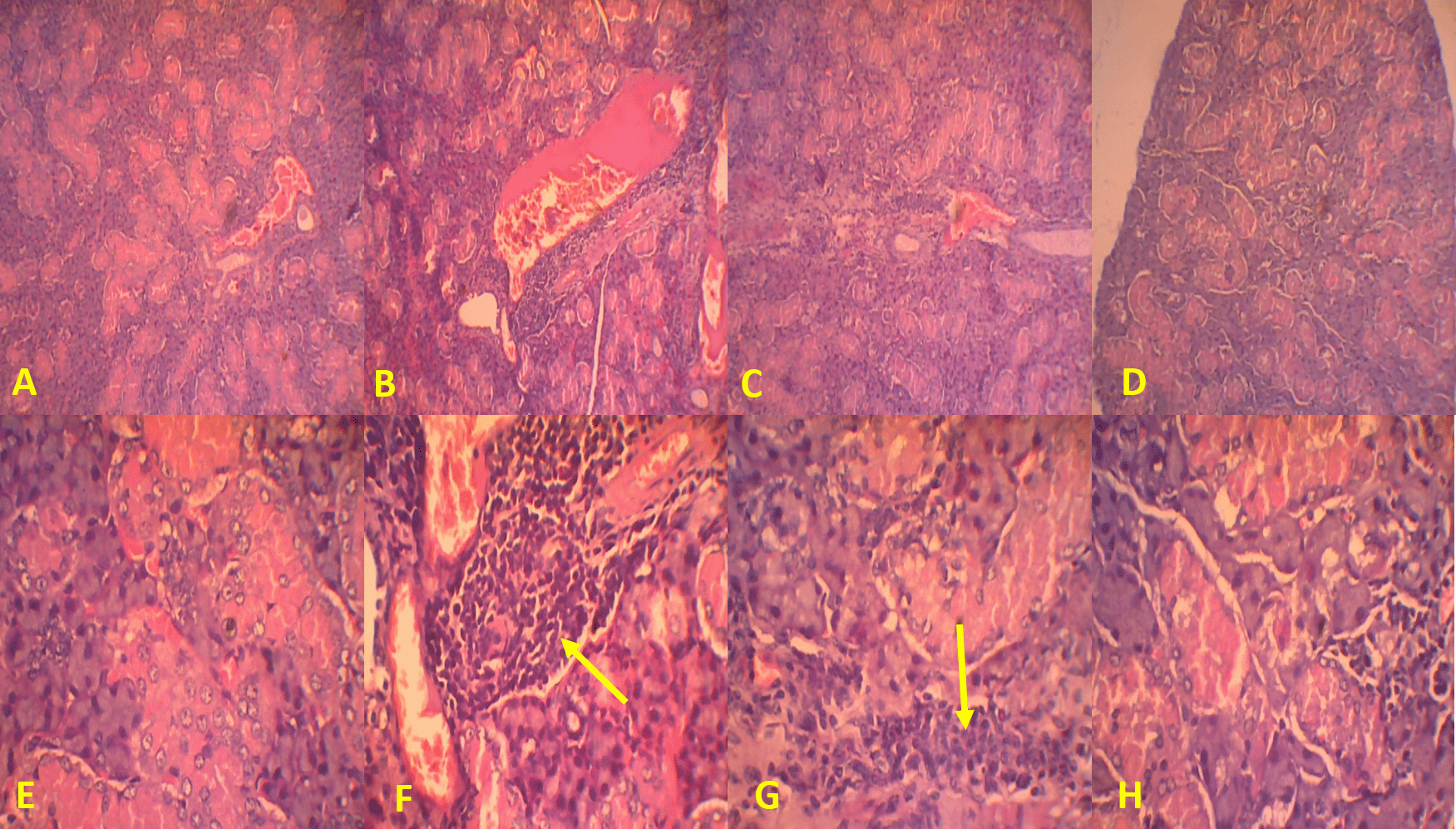
(A, B, C, and D) Magnification at 100× and (E, F, G, and H) 400×. Arrows indicate lymphocytic infiltration.
| Groups | Score of salivary gland epithelial necrosis, n (%) | |||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| C(−) | 8 (100) | 0 (0) | 0 (0) | 0 (0) |
| C(+) | 0 (0) | 0 (0) | 5 (62.5) | 3 (37.5) |
| T1 | 0 (0) | 0 (0) | 5 (62.5) | 3 (37.5) |
| T2 | 0 (0) | 4 (50) | 4 (50) | 0 (0) |
| p value | <0.001** | |||
| Groups | Score of salivary gland epithelial necrosis post-Ro (SSA) antigen induction p value | ||
|---|---|---|---|
| T1 | T2 | C(+) | |
| T1 | |||
| T2 | 0.01* | ||
| C(+) | 1.00 | 0.01* | |
| C(−) | <0.001** | <0.001** | <0.001** |
Table 14 shows that dexamethasone administration, with or without MO-ethanol extract, was more effective in preventing damage, upon histopathological examination, to salivary gland epithelial necrosis 14 days post-induction of Ro antigen (SSA). There was also a significant difference after being given MO-ethanol extract at a dose of 200 mg/kg BW in reducing the degree of salivary gland epithelial necrosis.
The pathogenesis of SS involves autoantigen presentation, B and T cells, and autoantibody-mediated mechanisms of tissue injury (anti-SSA/Ro and anti-SSA/La antibodies). This mechanism causes cell fragmentation. TNF-α triggers endothelial cell adhesion molecules such as E-selectin that in turn trigger polymorphonuclear (PMN) cell arrest, which also involves IL-17 (microcirculation) and initiation of inflammatory responses (MMP-9 secretion, lysozyme, and caspase-3 pathways). PMN cells, especially neutrophils, trigger the activation of MMP-9 that degrades collagen leading to basement membrane damage. Neutrophils also secrete lysozyme enzymes that can affect mitochondrial oxygenation and the GMP cycle, triggering epithelial necrosis. The inflammatory process makes cells swell, so that the cell membrane ruptures, which leads to epithelial necrosis. Caspase-3 activation, which acts as an executor, promotes DNA fragmentation and ends with cell death through apoptosis. Basement membrane damage, epithelial necrosis, and epithelial cell apoptosis result in destruction to the salivary and lacrimal glands. Macrophages and lymphocytes are the main source of IL-6, especially in the inflammatory process. Together with IL-1 and TNF-α, IL-6 is able to activate T cells, induce an acute inflammatory response, and increase C-reactive protein (CRP) synthesis by hepatocytes. CRP is one of the inflammatory mediators that lowers nitric oxide synthase levels in the endothelium, which encompass endothelial dysfunction. NFκB has activity in initiating the inflammatory process and increasing proinflammatory cytokines (TNF-α), adhesion molecules, and NADPH transcriptions. Angiotensin II increases ROS production through NOX stimulation via a type 1 receptor (AT1R/Angiotensin II type 1 receptor). Angiotensin II and TNF-α have activity to stimulate NFκB activation in ROS-dependent pathways, which can further increase the production of cytokines and other proinflammatory chemokines. The formation of more ROS than antioxidants causes oxidative stress. MDA is used as a reference for the emergence of indicators of oxidative stress (Both et al. 2017; Brito-Zerón et al. 2009; Nakamura et al. 2018; Pflugfelder SC 2014; Verstappen et al. 2018).
M. oleifera leaves exhibit antioxidant activity due to their high polyphenol content. They exhibit strong antioxidant activity against free radicals, thus prevent oxidative damage to major biomolecules and provide protection against oxidative damage. M. oleifera leaf extract is a prospective indicator of oxidative stress according to decreasing serum MDA levels. This would prevent the apoptotic process that is characterized by a decrease in caspase-3 levels in serum, and epithelial necrosis followed by a decrease in serum MMP-9 and IL-17 levels so that lacrimal and salivary gland damage can be minimized (Charoensin 2014; Verma et al. 2009).
This study showed that administration of 200 mg/kg BW of MO-ethanol leaf extract in SS model mice could significantly reduce MDA levels compared to the negative control group. This is in accordance with Nadimin’s study (2016), which aimed to determine the effect of M. oleifera extract on MDA levels during pregnancy in Makassar, Indonesia. This study was divided into two denominations: the intervention and the control. It was found that MDA levels in the control group were greater than those in the intervention cluster (p=0.033) (Nadimin 2016). The powder form of MO leaves could also reduce MDA levels in pregnant women, with p=0.028 (Misrawati and Marliah 2018). Several other studies have also shown that administration of MO-ethanol extract can reduce MDA levels in various diseases (Albrahim and Binobead 2018; Almufazar 2018; Wulandari et al. 2017).
This study showed that administration of MO-ethanol leaf extract significantly reduced IL-17 levels. The effect of MO-ethanol leaf extract, which can reduce IL-17 levels, was also useful in cases of inflammation due to ultraviolet-B/UV-B exposure (El Shanawany et al. 2019). The study by Ma et al. (2018) on psoriasis-induced mice showed a decrease in IL-17 levels (p<0.05) when given MO seed extract. A previous study showed that M. oleifera significantly reduced serum levels of IgG/Immunoglobulin G, IL-2, and IL-17 in sheep coinfected with Fasciola gigantica and Clostridium novyi (Engsuwan et al. 2021). Several studies addressing other cytokines including TNF-α, IL-6, IL-8, IL-1β, IL-10, NO, and PGE2 (prostaglandin E2) have been conducted. Kooltheat et al. (2014) found that MO can abolish the production of monocyte-derived macrophage factors, such as TNF-α, IL-6, and IL-8 (Xiao et al. 2020). Wardhani (2020) found that M. oleifera has a hepatoprotective effect by inhibiting TNF-α, IL-1β, IL-6, and IL-10. M. oleifera also inhibits fatty liver disease by inhibiting lipogenesis via the NF-κB pathway as characterized by decreased LDL-R/low-density lipoprotein receptor, SRB1c, DGAT2/diacylglycerol o-acyltransferase 2, and PPARγ/peroxisome proliferator activator γ and increased insulin sensitivity (Wardhani 2020).
Xie et al. (2021) evaluated the inhibitory effect induced by the alkaloids contained in M. oleifera on the proliferative and migratory phases in in vivo or in vitro methods on human prostate cell cancer (PC3). This study shows that M. oleifera will inhibit proliferation and induce cell apoptosis which causes cell cycle arrest. Furthermore, M. oleifera suppresses the migration of prostate cancer cells and inhibits the expression of MMP-9 (Xie et al. 2020). The study of Xie et al. (2020) also showed that western blotting results of Moringa oleifera alkaloids extract treatment at 200 g/ml inhibited the expression of MMP-2 (p<0.05) and MMP-9 cell migration-associated proteins compared to the control. Both studies were in accordance with the recent study, especially regarding the MO-ethanol leaf extract effect that can reduce MMP-9 levels compared to the control group.
Several studies, which analyzed the anti-inflammatory effect of MO leaf extract on caspase-3 levels, have been conducted. The study of Mousa et al. (2019), which aimed to determine the anti-inflammatory effect of MO leaves on thioacetamide intoxicated rats, showed that MO-ethanol leaf extract is able to downregulate caspase-3. The study by Bahr and Farouk (2016), which aimed to determine the hepatoprotective effect of MO leaf extract on experimental animals combined with lornoxicam, obtained significant results in reducing caspase-3 levels (p<0.05). Rijal et al. (2016) observed changes in caspase-3 expression (apoptosis) in PCG (primary congenital glaucoma) trabecular cell cultures treated with Moringa oleifera leaf extract. This study showed a significant change in caspase-3 expression (apoptosis) after administration of methanol extract of Moringa oleifera leaves at doses of 20, 30, and 40 g/ml in primary congenital glaucoma trabecular meshwork cell cultures (Wulandari et al. 2019). In accordance with these studies, this research showed that MO-ethanol leaf extract could significantly reduce caspase-3 levels.
In this study, it was shown that administration of MO-ethanol leaf extract significantly reduced salivary gland epithelial necrosis scores in the treatment group compared to the control group. It occurred due to the anti-inflammatory mechanism of MO-ethanol. The study conducted by Fatmawati et al. (2019), which observed histopathological features of the pancreas in diabetic rats induced by streptozotocin, showed changes in Langerhans insula repair compared to the hyperglycemic control group and also restored weight loss to normal. The study conducted by Kamaliani et al. (2018) found that the administration of M. oleifera ethanol extract (200 mg/kg BW) in diabetic Wistar rat kidneys causes fatty degeneration compared to the control group. They explained that a dose of 200 mg/kg BW was an optimal dose without causing necrosis. Ijioma et al. (2018) also investigated several doses of MO-ethanol leaf extract (200, 400, and 800 mg/kg BW) in the stomach of aspirin-induced rats, which showed epithelial surface protection, characterized by more mucus granules and better results than those of Cimetidine in which patches of intact superficial cells were observed.
This study had several strengths. Firstly, the study proved that MDA, IL-17, MMP-9, and caspase-3 levels and salivary gland epithelium decreased significantly when given MO-ethanol extract compared to those given only Ro (SSA) antigen and dexamethasone. MO-ethanol extract could be a complementary therapy. Secondly, the study was expected to form the basis for further research. Thirdly, the research will hopefully become a protocol in human clinical trials and could inspire future researchers to conduct research using human samples.
However, the study also had several limitations. Firstly, many other dependent variables such as markers of salivary gland damage (IFN-γ/interferon gamma, IL-6, BAFF/B-cell-activating factor, TGF-β/Transforming Growth Factor-β, LAMP3/Lysosome-associated membrane glycoprotein 3) were not observed. Secondly, the study did not observe any variation in dose of MO-ethanol leaf extract. Further research can be conducted with MO-ethanol leaf extract dose variations to determine minimum and maximum doses that can be given as supportive therapy in SS. Finally, the study method used IHC (immunohistochemistry) and ELISA (enzyme-linked immunosorbent assay) techniques; other techniques such as immunofluorescence could be performed in further research.
Administration of 200 mg/kg BW MO-ethanol extract 14 days post-induction by Ro (SSA) antigen significantly reduced MDA, IL-17, MMP-9, and caspase-3 levels and salivary epithelium damage (histopathological changes). MO-ethanol at a dose of 200 mg/kg BW can inhibit the apoptosis process of SS.
The experiments were approved by the ethical guidelines by Dr. Moewardi Hospital Ethical Committee (approved number: 178/II/HREC/2022).
Conceptualization, Agus Susanto; Data curation, Agus Susanto, Ambar Mudigdo and Brian Wasita; Formal analysis, Agus Susanto; Funding acquisition, Agus Susanto; Investigation, Agus Susanto and Brian Wasita; Methodology, Agus Susanto; Project administration, Agus Susanto and Brian Wasita; Resources, Agus Susanto and Brian Wasita; Software, Agus Susanto; Supervision, Bambang Purwanto and Ambar Mudigdo; Validation, Ambar Mudigdo and Brian Wasita; Visualization, Brian Wasita; Writing – original draft, Agus Susanto; Writing – review & editing, Agus Susanto and Bambang Purwanto.
Data repository name: [Raw Data Agus Joko Susanto.xlsx]. https://10.6084/m9.figshare.21388788.org/ (Nadimin 2016).
The project contains the following underlying data:
Data repository name: ARRIVE checklist for ‘[ARRIVE Guidelines-Author Checklist -.pdf]’. https://10.6084/m9.figshare.21388866.org/ (Albrahim and Binobead 2018).
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
The authors thank Fatna Andika Wati and Dyah Rohmania Agustiana for their indispensable assistance in manuscript preparation.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Pharmacology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 07 Nov 22 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)