Keywords
D-dimer, Ferritin, Fibrinogen, IL-6, Prothrombin Time
This article is included in the Coronavirus (COVID-19) collection.
D-dimer, Ferritin, Fibrinogen, IL-6, Prothrombin Time
The SARS-CoV-2 virus is a β-coronavirus with an enveloped, positive-sense, and single-stranded RNA. The COVID-19 pandemic due to SARS-CoV-2 virus poses a serious threat due to systemic inflammation and coagulopathy. Interleukin-6 (IL-6) is a cytokine that has a positive pleiotropic effect on inflammation. This cytokine can amplify the coagulation system to activate the epithelial cells, monocytes, and neutrophils,1 that can inhibit anticoagulant protein S and antithrombin and enhance the activity of clotting factor VII, von Willebrand, and fibrinogen.
Previously considered, increased ferritin was associated with hemophagocytic lymphohistiocytosis/macrophage activation syndrome in COVID-19. However, the absence of five out of eight characteristics in the previous COVID-19 study explains the differences between the disease.2 Several retrospective studies have been conducted to observe the relationship between IL-6, PT, fibrinogen, and ferritin with COVID-19 progression.3–7 In this current prospective cohort study, we aimed to investigate the effectiveness of these parameters in COVID-19 progression.
In this prospective cohort study conducted from July 2020 to January 2021, COVID-19 patients aged ≥18 years at the Pertamina Central Hospital Modular Extension Simprug (Simprug Modular Extension Hospital, SMEH, Jakarta, Indonesia) were included. Patients with COVID-19 were detected based on nasopharyngeal and oropharyngeal swab examination by polymerase chain reaction (PCR). Inclusion criteria: aged 18 years or older; moderate and severe COVID-19 (according to COVID-19 WHO guideline confirmed by positive BALF PCR SARS-CoV-2); willingness to provide blood sample. Exclusion criteria: history of chronic bleeding; undergoing hemodialysis; undergoing plasma convalescent clinical trial, or taking immunomodulation therapy (particularly IL-6 and intravenous immunoglobulin therapy).
Patients who fulfilled the criteria were observed on the first day of hospital admission, and blood samples (i.e., first sample collection) were collected to analyze IL-6, D-dimer, ferritin, fibrinogen, and prothrombin time. The collected blood samples were stored in the fridge at -20° celcius at Clinical Pathology laboratory of Pertamina Central Hospital.
After collected, IL-6 was analyzed in the Integrated Laboratory of the Faculty of Medicine, Universitas Indonesia. During transferring IL-6 samples were maintained at constant temperature -20° celcius accoding to Guidance on regulations for the Transport of Infectious Substances Guidance on regulations for the Transport of Infectious Substances 2007. IL-6 was analyzed using an ELISA IL6 Vmax microplate reader. Complete blood count, D-dimer, fibrinogen, and prothrombin time (PT) were analyzed using Sysmex CS2100i. Ferritin was analyzed using Cobas 601 Roche in the Clinical Pathology laboratory of Pertamina Central Hospital.
The severity of the illness was classified according to the WHO interim guidance (version 18th May 2020)8 as moderate, severe, or critical illness. Patients were observed up to day 14th or considered completed when the patients improved, worsened, died, or were discharged earlier. Afterward, samples were collected at the end of the observation (i.e., second sample collection). Worsening was defined as COVID-19 disease severity progression from moderate to severe, critical illness or death. All patients received antibiotics, antivirals, corticosteroids, and anticoagulants according to the standard hospital therapy (Fondaparinux, LMNH, or UFH).
All collected data were analyzed using Anaconda package program9 and reported in the text, table, or figures. All p-values < 0.05 were considered statistically significant. Correlation test was used to analyze correlation between IL-6 with ferritin and coagulopathy. Method of cutoff points obtained using area under curve method. Cutoff points by receiver operator characteristic obtained by calculating the optimum sensitivity and specificity.
This study has been approved (approval date: June 29th 2020) by the ethical committee, Universitas Indonesia (approval number: KET-650/UN2.F1/ETIK/PPM.00.02/2020), and Pertamina Central Hospital (approval number: 3315/B00000/2020-S8).
During the study period from July 2020 to January 2021, 374 severe and moderate COVID-19 patients were treated at the SMEH. Of those, 117 patients were randomized, 42 were excluded due to incomplete data, one received convalescent plasma therapy, and 1 had lysis of blood samples. The remaining 73 patients were further analyzed.
The characteristics of the study subjects are summarized in Table 1. Of the 73 COVID-19 patients, 61 (84%) were classified as having severe COVID-19 and 12 (16%) were classified as having moderate COVID-19. The majority of the patients were male and the mean age of patients was 61 years (SD±12.74). Most of the patients were overweight, hospitalized at day 6th of the illness with duration of 7 days. As the final observation outcomes, most of the subjects with moderate disease experienced improvement, and subjects with severe disease experienced worsening on day 14 of the illness. The majority of the patients had at least one comorbidity, with hypertension was the most common. All subjects were given the combination of remdesivir, favipiravir, and oseltamivir. They also received corticosteroids (dexamethasone or methylprednisolone) and anticoagulants (unfractionated heparin, enoxaparin, or fondaparinux).
The variable distributions were analyzed according to disease severity and the worsening or improvement of patients' condition, as shown in Table 2.
Correlation coefficient analysis using the Spearman method showed p≥0.05 between IL-6 and ferritin, fibrinogen, D-dimer, and PT (Table 3).
| IL-6 and | Correlation coefficient | p |
|---|---|---|
| Ferritin | 0.08 | 0.50 |
| D-dimer | −0.13 | 0.27 |
| Fibrinogen | 0.01 | 0.91 |
| Prothrombin time | 0.03 | 0.77 |
Figure 1 and Table 4 displayed the analysis of the receiver operating characteristic (ROC) curve (AUC) for correlation between elevated IL-6, ferritin, fibrinogen, D-dimer, and PT levels with COVID-19 patients' deterioration.
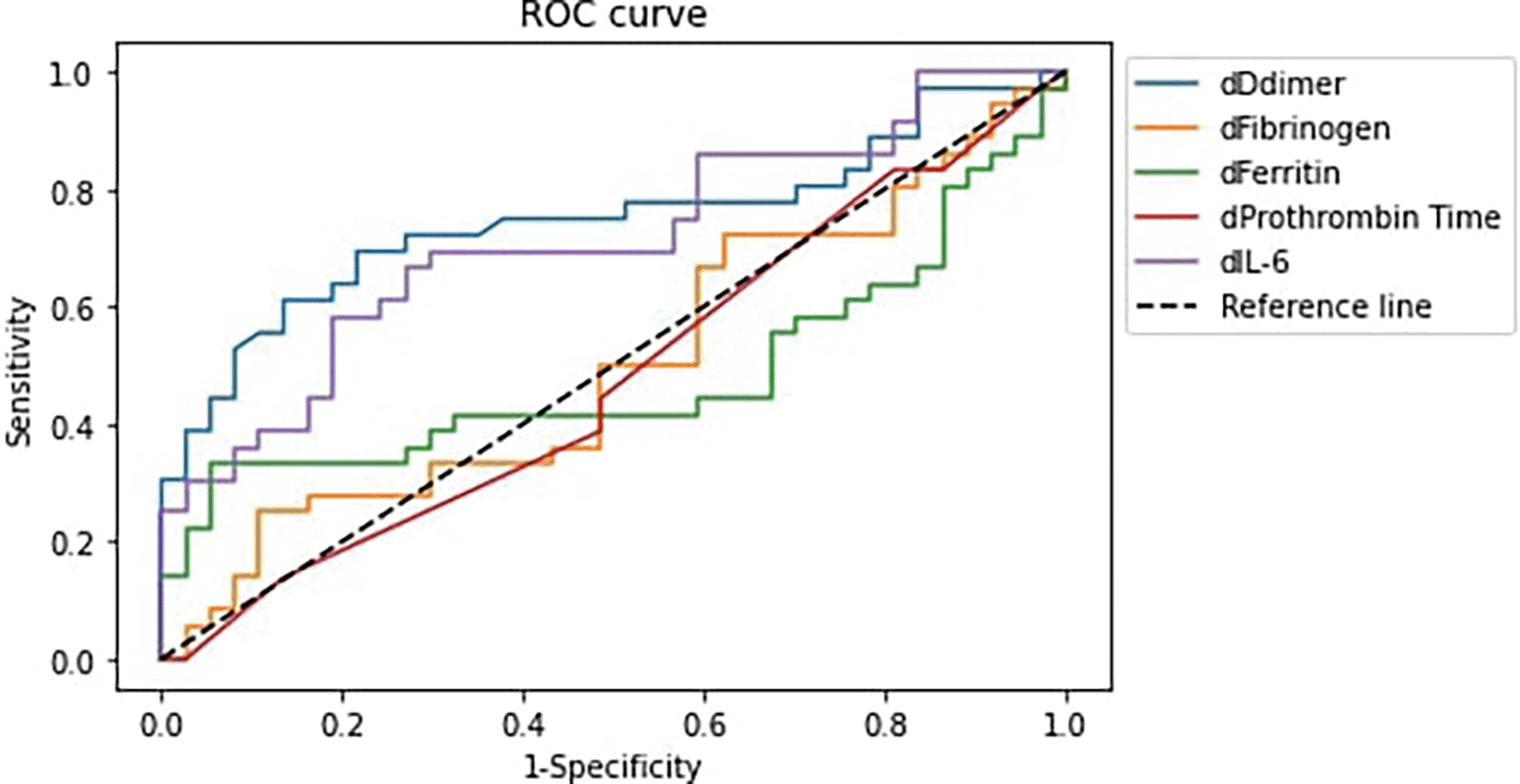
Notes: (1) dDdimer: D-dimer difference, dFibrinogen: Fibrinogen difference, dFerritin: Ferritin difference, dProthrombin Time: Prothrombin Time difference, dIL-6: IL-6 difference. (2) Difference is defined as the value at the end of observation – value at initial observation.
| Variables | AUC | 95% CI |
|---|---|---|
| D-dimer | 74.77% | 63.48–86.07% |
| Fibrinogen | 50.15% | 36.81–63.49% |
| Prothrombin Time | 47.67% | 34.36–60.99% |
| Ferritin | 48.42% | 35.10–61.75% |
| IL-6 | 71.32% | 59.48–83.17% |
We found no correlation between IL-6 and other variables. Thus, as one of the disease severity index components, we aimed to investigate the correlation between the oxygen saturation (SpO2)/fraction of inspired oxygen (FiO2) ratio and IL-6, ferritin, fibrinogen, D-dimer, and PT (Figures 2 and 3). As a result, our study demonstrated the correlation between IL-6, ferritin, fibrinogen, D-dimer, and PT and SpO2/FiO2 ratio as the severity determinants.
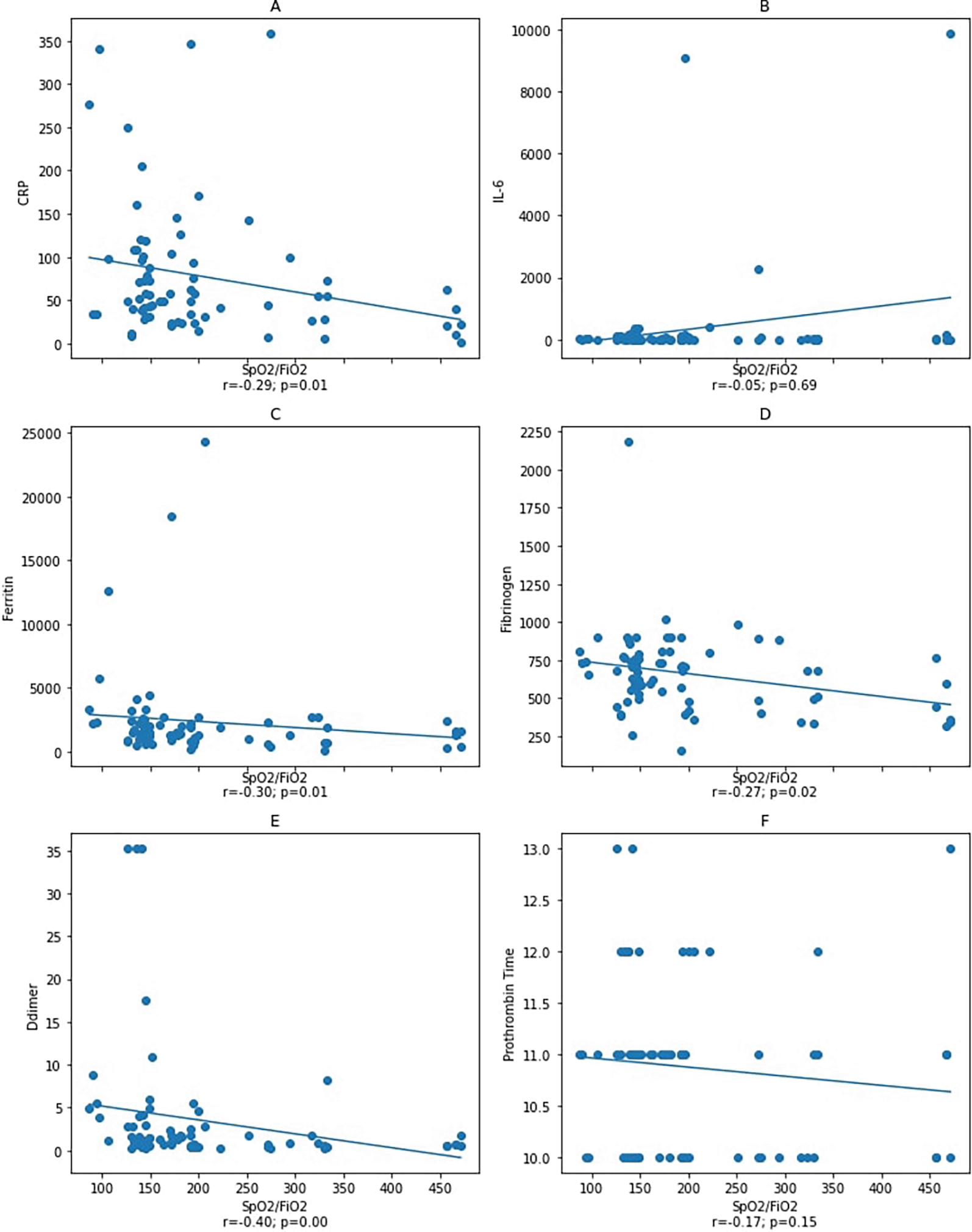
Spearman correlation test. CRP: C-reactive protein.
Notes: A–D: correlation between SpO2/FiO2 ratio and inflammatory markers CRP, IL-6, ferritin, and fibrinogen with a statistical significance was found between SpO2/FiO2 ratio and inflammatory markers CRP, ferritin, and fibrinogen, but not with IL-6. Figure E and F: correlation between SpO2/FiO2 ratio and coagulation markers D-dimer and prothrombin Time with a statistical significance was found between SpO2/FiO2 ratio and D-dimer, but not with prothrombin time.
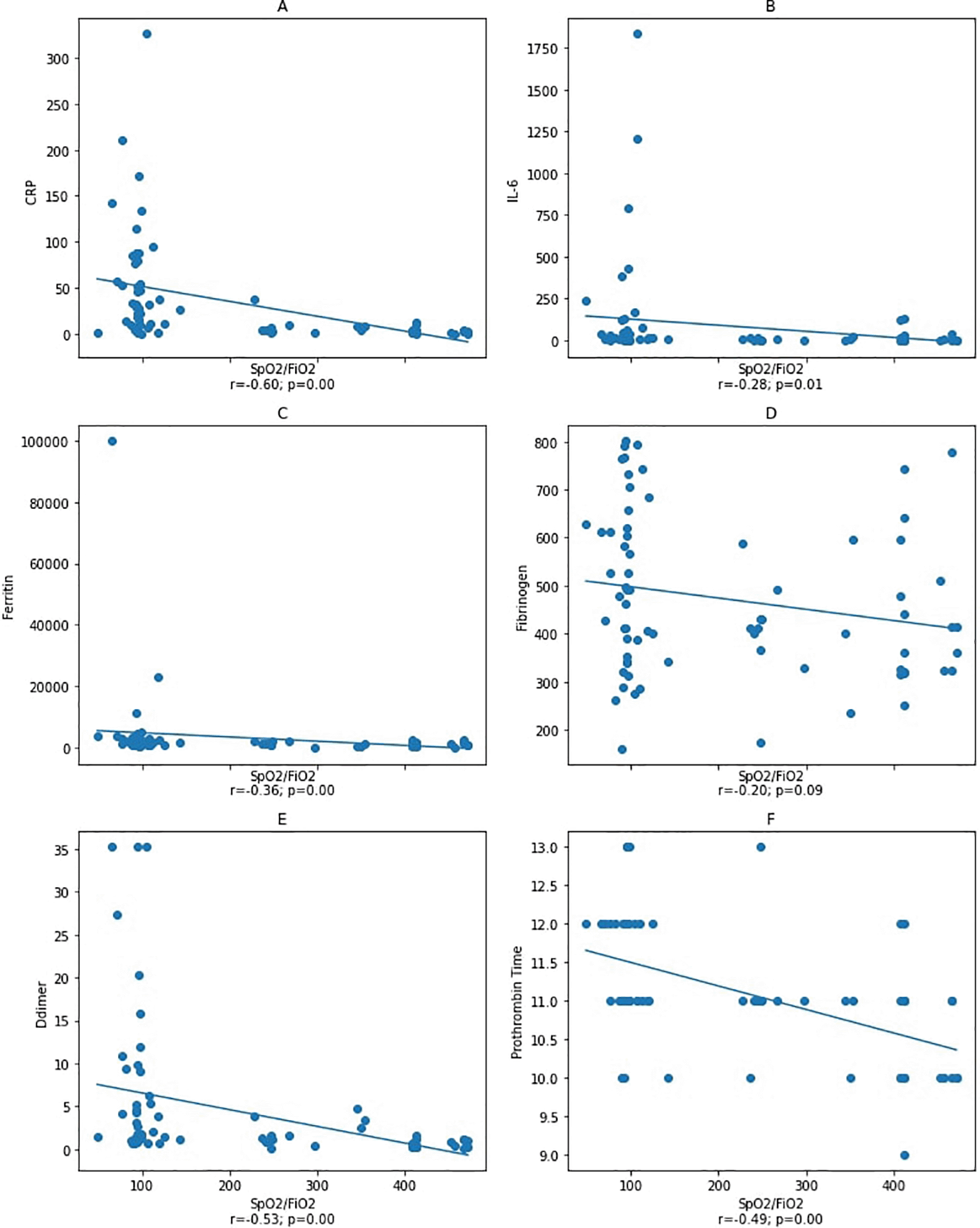
Spearman correlation test. CRP: C-reactive protein.
Notes: A–D: correlation between SpO2/FiO2 ratio and inflammatory markers CRP, IL-6, ferritin, and fibrinogen with a statistical significance was found between SpO2/FiO2 ratio and inflammatory markers CRP and ferritin, but not with IL-6 and fibrinogen. Figure E and F: correlation between SpO2/FiO2 ratio and coagulation markers with a statistical significance was found between SpO2/FiO2 ratio and D-dimer and prothrombin time.
Males were predominant in this study (64%) with the age range 52–68 years old.10,11 Hypertension (44.4%) and diabetes (31.8%) were the most common comorbidities, and similar results were also reported in other studies from China.12,13 This study found that the comorbidity was not associated with the patient's deterioration. Subjects with hypertension tend to experience worsening, as stated by Li Yongheng et al.13
In this study, most subjects with moderate disease experienced improvement. Despite the standard therapy, subjects with severe disease experienced worsening on day 14 of the illness. Meanwhile, all the subjects with moderate illness and 55.7% with severe illness survived (Table 2).
The courses of the COVID-19 disease have been reported comprehensively, with mild symptoms occurring mainly on the 5th day until the 10th day of illness (phase II disease).14,15 In this phase, most patients begin to feel shortness of breath accompanied by hypoxia. Our study subjects visited the emergency department during the mild symptom phase on the sixth day of hospitalization. Furthermore, they showed increased IL-6 and APR of ferritin and fibrinogen. Subjects who visited the emergency department for more than 6 days (mean 7.5 days) had almost three times greater risk of worsening than those who came for less than 6 days (mean 4 days).
Subjects with severe disease had higher levels of D-Dimer (Table 1), which is consistent with previous studies conducted by Chen et al, Zhou et al, and Guan et al.16–18 Additionally, levels of D-dimer were higher in the worsening compared with the improved group. This study reports that levels of D-Dimer tend to increase at the second sample collection (Table 2).
According to Chen et al.16 and Zhou et al.,17 non-survivor COVID-19 subjects had higher ferritin levels than the survivors. At admission, this study shows that subjects with moderate and severe conditions had higher ferritin levels (Table 1).
Levels of IL-6 at the first sample collection (Table 1) were similar to the previous study.17 In contrast to the study by Jin Zhang et al.10 and Awasthi et al.,19 study subjects did not show a significant difference in IL-6 levels at the first measurement between moderate to severe illness.
In acute phare response (APR), IL-6 is a pro-inflammatory cytokine that increases and determines infection-associated ferritin levels.20,21 However, we found no correlation between ferritin levels and IL-6 in the first and second sample collection (Figure 3).
Although subjects did not experience an inflammatory process at admission, we found an increase in ferritin and fibrinogen levels, indicating that inflammation occurred in most study subjects. Furthermore, other factors contributing to increased ferritin levels, such as epithelial damage, were also considered. Since a decrease in the SpO2/FiO2 ratio was due to an acute respiratory distress syndrome (ARDS) manifestation,13,22 the correlation between SpO2/FiO2 ratio with ferritin and fibrinogen was investigated to determine whether the epithelial damage affected IL-6 and other the inflammatory variables. Our result showed a statistically significant correlation between SpO2/FiO2 ratio with ferritin and fibrinogen (Figure 2). This correlation showed that inflammation affected the SpO2/FiO2 ratio from the onset of the illness. However, since the increase of IL-6 was not correlated with SpO2/FiO2 ratio, we assumed that inflammation and ferritin release was not affected by IL-6.7,23 According to a study by Zhi et al., several possibilities cause the increase of ferritin: 1) pro-inflammatory cytokines stimuli (i.e., IL-1β, tumour necrosis factor α (TNF-α), and IL-6) caused an inflammatory reaction that damaged the cells, 2) intracellular ferritin leakage due to cell damage and inflammation, and 3) the ferritin leakage from the injured cells further triggers the damage of other cells via Fenton and Haber–Weiss's reaction.24
Inflammation will trigger the coagulation system characterized by changes in the value of D-dimer, fibrinogen, and PT at the time of viral entry.25 Thus, we investigated the correlation between levels of IL-6 and markers of coagulopathy and inflammation.
Since there was no correlation found between IL-6, ferritin, and the incidence of coagulopathy, we concluded that coagulopathy in COVID-19 patients could occur without the role of IL-6 and ferritin. Such explanations for this finding are: 1) ARDS pathophysiology in the infected subjects with moderate and severe illness is related to a massive loss of the angiotensin-converting enzyme (ACE)-2 enzyme, causing damage to the alveolar epithelium and vascular endothelium,26 2) compartmentalization of the inflammatory cascade, as described by Chow and Tisoncik et al.,27,28 and 3) an acute phase response (APR) increase may be due to other pro-inflammatory cytokines, such as TNF-α or IL-1,23,25,29–31 and the aforementioned vicious cycle of cellular destruction.
An analysis was carried out on the second blood collection after patients had a worsening during treatment to determine whether the incidence of coagulopathy correlates with IL-6 levels, in which the effect of IL-6 and cytokine storm was the utmost. The data from the second blood collection showed a significant correlation between the SpO2/FiO2 ratio and the D-dimer, ferritin, PT, and IL-6 (Figure 3). Furthermore, the D-dimer, fibrinogen, and PT were correlated with ferritin, while there was no correlation with IL-6. Our result demonstrated that inflammation correlates with coagulopathy, but conceivably not via a direct IL-6 pathway. To explain this finding, according to a study described by Sinha et al. that the interaction of mediators and pathways involved is not always constantly linear or uniform.32
Worsening subjects showed significant changes in D-dimer, fibrinogen, and PT. Since there was no correlation between IL-6, ferritin and PT, fibrinogen, and D-Dimer, we determined the changes in the variables' mean values between the first and second blood collections. Furthermore, Wilcoxon's non-parametric test was performed since the data was not normally distributed (Figure 4).
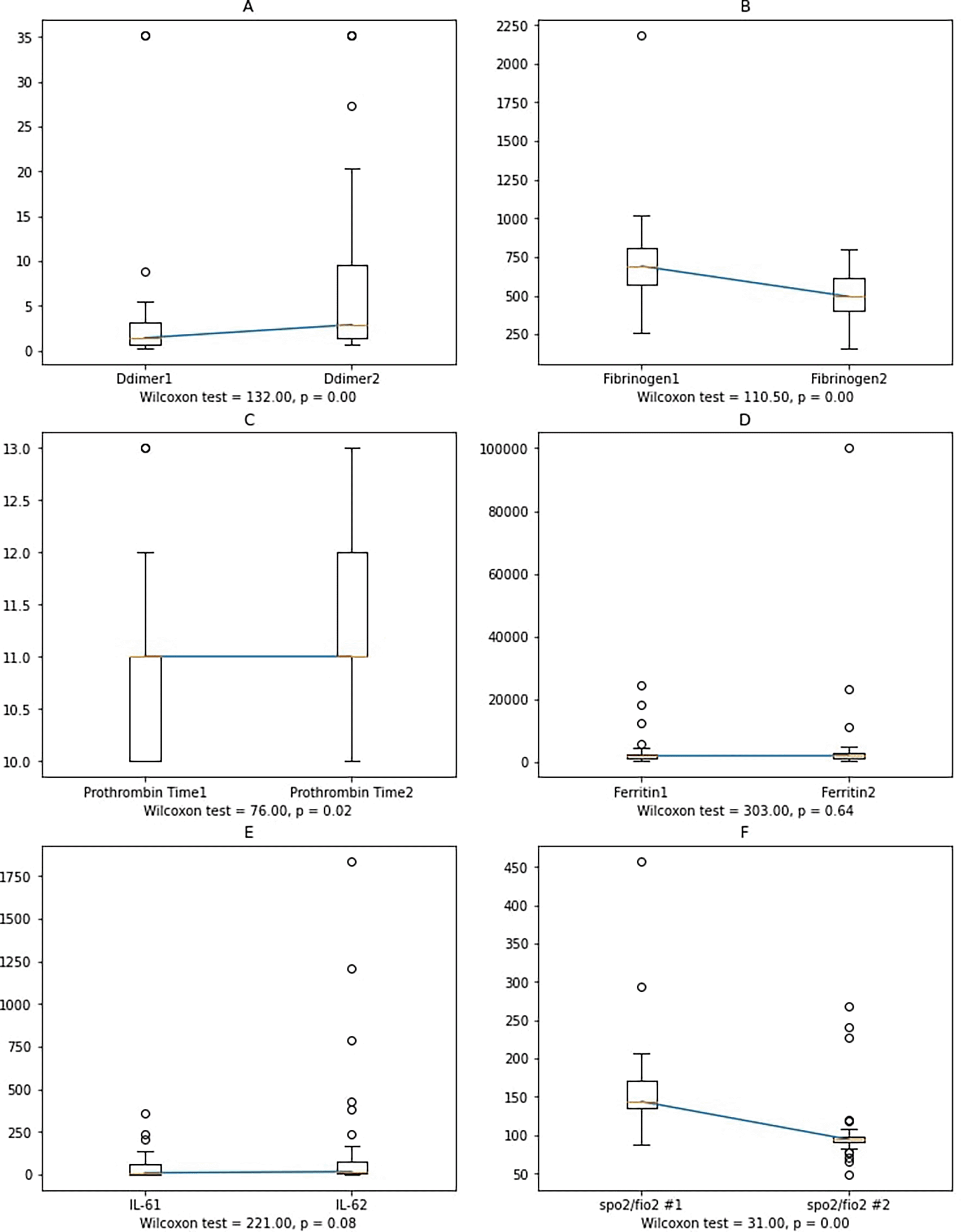
Notes: Ddimer1, Fibrinogen1, Prothrombin Time1, Ferritin1, IL-61: D-dimer, fibrinogen, prothrombin time, ferritin, and IL-6 at the first sample collection; Ddimer2, Fibrinogen2, Prothrombin Time2, Ferritin2, IL-62: D-Dimer, fibrinogen, prothrombin time, ferritin, and IL-6 at the second sample collection.
In worsening subjects, the second IL-6 blood collection had a higher value than the first one, albeit not statistically significant. Possible reasons include: 1) cytokines other than IL-6 affect the increase in D-dimer, fibrinogen, and ferritin levels, or 2) external factors, such as administration of anti-inflammatory corticosteroids and heparin, also have anti-inflammatory effects,33 resulting in an insignificant increase of IL-6 and ferritin. During the study period, corticosteroids and heparin were administrated following the standard therapy for moderate and severe COVID-19 patients, or 3) large amounts of soluble IL-6 receptors production binds free IL-6 to reduce the concentration, as described by Garbers et al.,34 or 4) the presence of other cytokines (IL-6 cytokine family), including IL-11,35 that also has a pro-inflammatory effect.36
The disease may be influenced by the period between the onset and the patient's admission to the hospital. However, we found no differences between severe and moderate illness subjects. This result demonstrated that the disease onset was not correlated with the disease severity.
The disease severity was determined using WHO criteria for oxygen saturation, respiratory rate, and radiological findings. Most subjects were admitted to the emergency department with severe illness (83%), with a median SpO2/FiO2 ratio was 97. In moderate illness, there were no differences in IL-6 at the baseline.
Our study found a negative correlation between D-dimer, fibrinogen, and PT levels with the SpO2/FiO2 ratio, reflecting the disease severity. This finding demonstrated that coagulopathy had a role in the patient's deterioration even though SpO2/FiO2 ratio showed no correlation with IL-6. The worsening lung function was due to pre-existing inflammation before the increase in IL-6 may explain the correlation with the coagulation system.
Sinha,32 and Leisman31 proposed that the pathophysiology of moderate and severe COVID-19 ARDS differs from typical ARDS, where the inflammatory system activation marked by increased IL-6 occurred only in a few COVID-19 ARDS patients. Our study showed that only 15.1% of subjects had IL-6 elevation at the initial examination, with an increase of 10-times the lower standard limit. Furthermore, if COVID-19 ARDS is considered a hyperinflammatory type, the IL-6 will be much lower than the value found in studies of the hyperinflammatory type that showed an increase of more than 100-times the lower standard limit.
The second blood collection sample showed that IL-6, ferritin, D-dimer, and PT had a statistically significant negative correlation with the SpO2/FiO2 ratio, suggesting that deteriorating lung function is also correlated with inflammation and coagulopathy. However, we found no statistically significant correlation between IL-6 with D-dimer, fibrinogen, PT, and ferritin. As mentioned previously, this phenomenon may be caused by administering anti-inflammatory drugs that can reduce APR levels.
Fibrinogen is a soluble glycoprotein synthesized by the liver, and the activation can produce insoluble fibrin in the plasma. This process occurs via intrinsic and extrinsic pathways, and the activity is assessed by measuring PT.
Fibrinogen level is elevated in inflammatory conditions.23 In patients who experienced worsening, we found that the fibrinogen levels at admission were higher than normal, with no changes in PT values. Thachil argued that the initial increase of fibrinogen would regulate inflammation. However, when the D-dimer levels continue to elevate with decreased fibrinogen levels, the protective role of fibrinogen ceases, and thrombus formation begins.37 In our finding, the initial fibrinogen levels in moderate and severe COVID-19 were increased but decreased at the second blood collection sample analysis, which showed a tendency towards normal compared to the worsening group. This result demonstrated that hypercoagulable conditions might have a role in the pathology of the worsening group, a condition that differs from the hypothesis as reported by Thachil.37 The hypercoagulable state can result from neutrophils’ subsequent pulmonary capillary endothelial activation, namely the formation of neutrophil extracellular traps (NETs).38–42
As part of APRs, ferritin was negatively correlated with the SpO2/FiO2 ratio (Figure 2). We assumed that the inflammation process had occurred prior to the patients' hospitalization, which explains the presence of a correlation between ferritin and the disease severity (moderate and severe). In the early phase, ferritin levels did not correlate with D-dimer, PT, and fibrinogen levels, which unlikely indicate that inflammation triggers coagulopathy.
On the initial observation, there was an elevation in ferritin levels in the subjects with moderate and severe illness (Table 1). In the second blood collection sample, ferritin levels increased in the worsening but decreased in the improved group. Finally, we found the most significant changes in the improved group of moderate illness subjects (Table 2), as also reported by Zhi et al.24 To explain this finding, we hypothesized that 1) pro-inflammatory cytokines such as IL-1β, TNF-α, and IL-6 can increase ferritin synthesis, 2) damage to the cellular level due to inflammation will release intracellular ferritin,30 and 3) in acidosis, increased production of reactive oxygen species induce the release of iron from the ferritin. In addition, Fenton and Haber–Weiss reaction increases the concentration of hydroxyl radicals and eventually damages the cells. Overall, this process will initiate a vicious cycle of cell damage by increasing ferritin concentration.
Rosario,43 explained that ferritin has immunosuppressant and pro-inflammatory effects. The immunosuppressant properties were found in the ferritin H fraction, supressing B lymphocyte antibodies' production. Additionally, ferritin also reduces granulocytes' phagocytosis, regulates the production of granulocytes-monocytes, and induces the production of IL-10 in lymphocytes. Moreover, it also inhibits the function of CXCR4 (chemokine receptor 4), an activator of the mitogen-activated protein kinase system that plays a role in the proliferation, differentiation, and migration of the cells. The role of ferritin in the inflammatory process has been described in hepatic stellate cells. For example, increased nuclear factor κB can promote the expression of inflammatory mediators, including IL-1β, and induce the production of nitric oxide synthase. This immunomodulatory process belongs to ferritin H and L properties. However, the role of ferritin as an inducer of inflammation or anti-inflammatory in COVID-19 infection has not been proven since the elevated ferritin type is still unknown.20,30,43
The correlation between ferritin with fibrinogen, D-dimer, and PT in COVID-19 patients is recognized as a damage-associated molecular pattern that can modulate IL-6 concentrations.30
There was no correlation between decreased SpO2/FiO2 ratio and IL-6 in the first blood collection sample, but SpO2/FiO2 ratio was correlated with acute-phase protein ferritin and fibrinogen. Albeit D-dimer, fibrinogen, and PT were correlated with the SpO2/FiO2 ratio, we found no correlation between ferritin and those. Based on this finding, we demonstrated that the increase in APR does not correlate with the incidence of coagulopathy in COVID-19 since the condition has different mechanisms, including epithelial damage due to the entry of the SARS-CoV-2 virus and the increased production of NETs by neutrophils, as previously described.
Theoretically, inflammatory (IL-6 and ferritin) and coagulopathy (D-dimer, fibrinogen, and PT) markers are correlated with patients' deterioration. Interleukin-6 regulates the inflammatory process, and the elevation is associated with the worsening of COVID-19 patients. This study found that increased levels of IL-6 can predict the deterioration of subjects, but it is insufficient to justify it as a routine examination. Additionally, uncorrelated IL-6 with coagulopathy markers, perhaps due to the ability of IL-6 to activate the inflammatory cascade in multiple body systems,32 inducing amplification of the cascade.
Inflammation due to COVID-19 can activate epithelium, endothelium, macrophages, and neutrophils. Massive recruitment of neutrophils to the lungs and release of NETs regulates coagulation and IL-6 activation.40,44 We found that alteration in D-dimer levels can predict the patients' deterioration. Therefore, the availability of D-dimer in hospitals can justify its function as a routine examination.
Although the alteration of ferritin and fibrinogen can not predict the patient's deterioration, these APR markers can be elevated in inflammatory conditions.20,23 Thus, administration of corticosteroid therapy or anti-inflammatory agents, such as heparin, can reduce the inflammation process,33,45 as marked in our study by the unaltered ferritin and decreased fibrinogen levels (Table 2).
Prothrombin time is the period required for plasma to clot after adding tissue factor. The reaction velocity is the result of coagulation factors activation that consist of coagulation factors XII to X. Furthermore, Yu Zhang et al. found that PT had a sensitivity of 83.54% and specificity of 65.22% for sepsis patients at the cut-off 20.46 Our study showed a 75% quartile of 12 in severe COVID-19 subjects, describing that levels of PT were insensitive to measure patient's deterioration. A similar finding was also reported in a retrospective study by Long et al.,47 where the initial measurement of PT was not correlated with the disease severity. This phenomenon may demonstrate the effect of hypercoagulation as the dominant pathophysiology in the early phase of the disease, in contrast to DIC in sepsis.48,49
Our study limitation is that the IL-6's diurnal variation cannot be eliminated since the use of a consecutive sampling method and the disease phase factor.
In moderate and severe COVID-19 patients, there was a correlation between elevated IL-6 and D-dimer levels with disease deterioration. There was no correlation between elevated IL-6 levels with ferritin, D-dimer, fibrinogen, and PT levels.
Written informed consent from the patient/patient’s family for the use and publication of the patient’s data was obtained from all subjects involved in the study. Informed consent was conceptualized according to local ethics committee, and hospital review committee.
Underlying data cannot be shared due to privacy concerns. Data will be made available to readers and reviewers on request from Alvin Tagor Harahap (alvinharahap@gmail.com).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Neuroendocrine disorders, Coagulation disorders in Neurosurgery, Neurospinal disorders, vascular Neurology
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Clinical hematology, malignant hematology, benign hematology, autologous hematopoietic stem cell transplant
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 22 Sep 23 |
read | read |
|
Version 1 10 Nov 22 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)