Keywords
Placental malaria, Plasmodium vivax, LBW, HIF1-α, angiogenesis factors
Placental malaria, Plasmodium vivax, LBW, HIF1-α, angiogenesis factors
Malaria is still a major public health problem and the main cause of disease and death in developing countries1. Young children and pregnant women, especially in the 1st and 2nd trimesters, are the population most vulnerable to malaria infection1. In malaria-endemic areas, at least 25% of pregnant women are infected with malaria, which accounts for 20% of maternal deaths2,3. In 2019, for 33 countries in the World Health Organisation (WHO) African Region with moderate to high malaria transmission it was estimated that 12 million (35%) of 33 million pregnancies were exposed to Plasmodium falciparum malaria4. Rijken et al. reported that around 75 million pregnant women in the Asia-Pacific region were exposed to Plasmodium vivax in 20072. Globally, malaria causes more than 10,000 maternal deaths and 200,000 neonatal deaths annually, most of which are due to low birth weight (LBW)4–6. Pregnant women living in stable transmission areas are at risk for placental malaria, and pregnant women living in unstable areas have three times the risk of developing severe malaria than non-pregnant adult women living in the same areas2,3.
Indonesia is one of the countries in Southeast Asia that has a fairly high malaria case. In 2016 there were 218,480 positive cases of malaria, this condition decreased by almost half of the positive cases of malaria in 20127. The malaria parasites found were Plasmodium falciparum (62%) and Plasmodium vivax (37%). Reports by health facilities in Indonesia (2016) stated that there were 218,450 confirmed cases of malaria with a death rate of 161 cases8. The three high malaria-endemic provinces are Papua, West Papua, and East Nusa Tenggara (NTT). Three regencies in NTT which are high endemic areas for malaria are Sikka Regency, Lembata Regency, and Ngada Regency. Sikka Regency is located on Flores Island, bordered by East Flores Regency and Ende Regency, has an area of 7,436.10 km2 and a population based on a survey population in 2010 was 315,477 people9. In 2008, 87,622 clinical malaria cases were reported in Sikka Regency10. A previous study conducted on 92 babies born with low birth weight in T.C. Hillers Regional Hospital, Maumere, Sikka Regency between December 2012 to December 2013, reported that 39 (42.4%) infants had congenital malaria of which 19 (48.7%) infants were asymptomatic, while the rest had sepsis, jaundice, and prematurity11.
Placental malaria is characterized by the accumulation of parasite-infected erythrocytes, mononuclear cells and malaria pigment (hemozoin) in the placental blood vessels12. Most reports of placental malaria are caused by infection with Plasmodium falciparum, which sequesters in the syncytiotrophoblast. This sequestration occurs by expressing the surface protein Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) which specifically mediates the cytoadhesion of Plasmodium falciparum infected erythrocytes to placental chondroitin sulfate A (CSA) located in the syncytiotrophoblast6,13,14. This cytoadhesion and sequestration prevent the escape of circulating adult parasites thereby avoiding the mechanism of clearance by complement and spleen. During the occurrence of placenta malaria, the Th1/Th2 balance shifts to the Th1 pathway and there is an increase in the production of the pro-inflammatory cytokines, interferon-ϒ (IFN-ϒ) and tumour necrosis factor-α (TNF-α). This inflammatory response causes changes in the structure and function of the placenta which are associated with poor pregnancy outcomes such as maternal anemia, prematurity, stunted fetal growth and LBW15. Placental malaria is a major cause of stunted fetal growth with poor pregnancy outcomes in the form of babies born with LBW16.
Placenta malaria will evoke complement activation, both systemically and at the maternal-fetal interface in the placenta17. There is an increase in complement of anaphylatoxin C5a in circulating blood and in placental tissue in mothers with placental malaria5. Previous in vitro studies showed that Plasmodium falciparum glycosylphosphatidylinositol (PfGPI) together with C5a increased pro-inflammatory milieu at the maternal-fetal interface, in the form of increased cytokines and chemokines derived from monocytes18. One of the consequences is the occurrence of dysregulation of angiogenic factors19 which causes stunted fetal growth5,6,20,21. The local oxygen environment during pregnancy is one of the important regulatory factors of angiogenesis. The main pathway of oxygen regulation of gene expression is hypoxia inducible factor-1α (HIF-1α). Under hypoxic conditions, HIF-1α accumulation upregulates VEGF which is a major proangiogenic factor directly. VEGF activity will induce the expression of vascular endothelial growth factor receptor-1 (VEGFR-1/Flt-1) and vascular endothelial growth factor receptor-2 (VEGFR-2/KDR). During hypoxia, VEGF will bind to receptors and stimulate capillary growth. Hypoxia inducible factor-1α (HIF-1α) also induces an increase in the soluble anti-angiogenic factor Flt-1 (sFlt-1)22,23. High placental HIF-1α expression after the 1st trimester of pregnancy is an abnormal condition that describes the occurrence of placental hypoxia due to inadequate placentation. Increased placental HIF-1α expression will cause an increase in placental sFlt-1 levels which will be released into the maternal circulation. Soluble Flt-1 (sFlt-1) strongly binds free VEGF and free PlGF, decreased placental angiogenic activity. This condition is found in pregnancies with preeclampsia and IUGR24–26, and in placental malaria27. Increased placental HIF-1α expression is also found in maternal anemia28.
Carvalho et al. for the first time demonstrated that Plasmodium vivax-infected erythrocytes (Pv-iEs) can perform ex vivo cytoadhesion on human lung endothelial cells (HLECs), Saimiri brain endothelial cells (SBECs), and placental cryosections both under static and flow conditions. This cytoadhesion is smaller than that of Plasmodium falciparum-infected erythrocytes (Pf-iEs), and is partly mediated by the VIR protein encoded by the Plasmodium vivax variant (vir) gene29. Fernandez-Becerra et al. showed in his study that spleen-dependent Plasmodium vivax genes encode immunogenic proteins during the course of infection, so it can be said that the spleen is an organ that plays a major role in expressing parasitic proteins involved in cytoadhesion30. Pv-iEs binding to endothelial cells is mediated by glycosaminoglycans, namely chondroitin sulfate A (CSA) and hyaluronic acid (HA)29,31. Although all stages of the Plasmodium vivax were found in peripheral blood, only a few of the mature forms of schizonts were found. It is not yet possible to establish whether the disproportionate Plasmodium vivax parasitaemia results from its sequestration in specific organs32.
Data on the number of pregnancies infected with Plasmodium vivax have not been widely published. The mechanism and clinical implications of Plasmodium vivax infection in pregnancy are still not clearly known, possibly because it is considered to cause a milder clinical effect than Plasmodium falciparum infection33. However, many studies have reported that vivax malaria in pregnancy is more associated with low birth weight and maternal anemia34,35 and may not be affected by parity because women living in areas with low malaria endemicity have low immunity to malaria36. In this study, an in vivo study was conducted on pregnant women infected with malaria, with an emphasis on placental malaria, to determine the effect of placental angiogenesis factors and maternal plasma angiogenesis factors on the pathomechanism of LBW through HIF-1α regulation. This study is the first study of placental malaria angiogenesis factors in Indonesia using human placenta samples.
This research was conducted according to the guidelines of the Declaration of Helsinki and approved by the Faculty of Medicine Universitas Brawijaya Ethics Committee number 307/EC/KEPK/11/2018. Written informed consent, including for the baby's data to be used, was obtained from all subjects before participating in this study.
This study used a cross-sectional research design and took place in T.C. Hillers Regional Hospital, Maumere, Sikka Regency, East Nusa Tenggara Province; a malaria-endemic area in Indonesia. This study used a purposive sampling technique in managing research participants. Thus, all of the subjects which met the inclusion criteria were included. The study subjects were pregnant women who came to T.C. Hillers Regional Hospital during 2018 and met the inclusion criteria. The inclusion criteria were pregnant women who gave birth to babies with low birth weight (LBW) and small for gestational age (SGA). Subjects were divided into two groups, they were (i) case group, pregnant women who met the inclusion criteria and the malaria diagnosis criteria and (ii) control group, pregnant women who met the inclusion criteria and did not meet the malaria diagnosis criteria. Malaria cases were determined based on the discovery of Plasmodium parasites on examination of thin or thick blood smears and or the discovery of Plasmodium DNA in maternal blood and or umbilical cord blood by polymerase chain reaction (PCR) examination, and or the discovery of Plasmodium parasites or hemozoin in the placenta.
The diagnosis of malaria case in this study was confirmed by discovery of either the asexual form of Plasmodium on examination of thick or thin blood smears derived from maternal blood and or umbilical cord blood, and/or Plasmodium DNA obtained by polymerase chain reaction (PCR) examination, and/ finding of placental malaria. Hemozoin is a brown pigment crystal that forms in the digestive vacuole of Plasmodium as a product of hemoglobin catabolism37. Hemozoin was seen as greenish-black or yellowish-brown granules using a light microscope and sequestered intra or extra erythrocytes. Observations were made using a light microscope with 1000x magnification in 100 fields of view. Identification of infected erythrocytes was carried out by observing 100 fields of view. Identification of hemozoin sequestration was carried out by observing 100 fields, then the total number of hemozoin was calculated by dividing all fields by 100. The count includes hemozoin found in and outside infected (free) erythrocytes, attached to connective tissue, on white blood cells in the intervillous space, or macrophages covered by fibrin in the intervillous space.
Low birth weight is defined as a birth weight of less than 2500 g38. In this study, what is meant by low birth weight is the difference between birth weight and the 10th percentile of the mean birth weight curve for boys and girls by gestational age from the reference curves of birth weight, length, and head circumference for gestational ages in Yogyakarta, Indonesia39.
In total, 10 milliliters of maternal venous blood were drawn from the median vein and put into a vacutainer containing anticoagulant (BD Vacutainer EDTA Tubes, 366643) for making blood smear preparations, plasma and dried blood spots on filter paper. Microscopic examination was carried out on blood smear preparations. Dried blood spots then were used for PCR examination to confirm the diagnosis of malaria. Plasma was stored at 20°C and sent to Malang city (in collaboration with the Prodia Laboratory) in less than 48 hours. These samples were then used to examine plasma levels of VEGF, PlGF, and VEGFR-1 (sFlt-1), VEGFR-2, and HIF-1α using ELISA method.
In total, five milliliters of blood were taken from the umbilical artery of the newborns and put into a blood collection tube containing anticoagulant (BD Vacutainer EDTA Tubes, 367863) to make blood smears for malaria parasites identification and make dried blood spots on filter paper for PCR examination, and for hemoglobin and leucocyte test.
The blood sample was dropped on an object-glass where a thin smear was made and dried. Furthermore, the smears were fixed evenly using absolute methanol and dried. The smears were stained with Giemsa solution (a mixture of Giemsa stain (Merck, HX612241) and Giemsa buffer (Bioanalytica, Indonesia) with a ratio of 1:9), then rinsed and dried. The thin smear slides were examined microscopically at 100 times magnification under the objective lens with immersion oil, on at least 100 visual fields for each examination. To calculate the degree of parasitemia, 1000x magnification was carried out using a light microscope (Olympus Biological Microscope, CX23), counting the number of erythrocytes infected with malaria parasites per 1000 erythrocytes40.
Samples obtained from the central part of the gross placental anatomy with a size of 2 cm and fixation with 10% neutral buffer formalin, were then embedded into paraffin for immunofluorescence examination to determine the expression of VEGF, PlGF, VEGFR-1, VEGFR-2, and HIF-1α, the sequestration of infected erythrocytes and or hemozoin, as well as placental parasitemia. Placental histology preparations were made by cutting tissue that had previously been fixed with 10% neutral buffer formalin and inserted into a tissue cassette. Then, the samples were put into a basket and processed using tissue processor tool (Thermo Scientific Microm STP 120 Spin Tissue Processor). The process of casting into paraffin blocks was done by using tissue embedding tool (Sakura Tissue-Tek TEC 5 Embedding Station). The paraffin blocks were cooled in a freezer before cutting with a microtome (Leica, RM2245). Then, the tissue was put into an incubator (Memmert, UN30) for 30 minutes in a 70-80oC temperature setting to maximize further deparaffination. Furthermore, after the tissue was removed from the incubator, deparaffination and Hematoxylin-Eosin staining were performed using the Tissue Tex DRS 2000 Multiple Slide Stainer tool.
Immunofluorescence examination procedure on placental tissue was used to measure placental expression of variables VEGF (anti-VEGFA antibody, SC 7269 FITC), PlGF (anti-PLGF antibody, SC 518003), VEGFR-1 (anti VEGFR-1 antibody, SC 271789 PE), VEGFR-2 (anti VEGFR-2 antibody, SC 6251 FITC), and HIF-1α (anti HIF-1α antibody (SC 13515 PE). The immunofluorescence procedure was performed by single staining for HIF-1α and double staining for other variables. The results of the immunofluorescence images were then quantified using ImageJ 1.52p Fiji software.
DNA samples were obtained from blood sample extraction using PureLinkTM Genomic DNA Kits (Invitrogen, Carlsbad, California, USA) according to the manufacturer's instructions. After purification, all samples were stored at -20◦C till ready for the nested PCR. For the amplification of Plasmodium genus sequences, outer primer pairs (rPLU1 and rPLU5) were used. In the second reaction, two pairs of inner primers were used to detect P. falciparum (rFal1-rFal2) and P. vivax (rVIV1-rVIV2). PCR was performed using a Go Tag® Green Master Mix (Promega, Madison, Wisconsin, USA). 1 l template was treated with the following conditions: 1 l of each primer (10 M), 12.5 l of PCR master mix, and 9.5 l of double-distilled H2O2. The second nest reactions were carried out in a similar way using different primers. Sterile distilled water was used as a control11.
ELISA examination was performed to measure plasma levels of VEGF (human VEGF ELISA kit, Biolegend, Catalog No. 446507), PlGF (human PGF ELISA kit, bt-Lab, Catalog No. EO138Hu), VEGFR-1(sFlit1) (human VEGFR-1 ELISA kit, Invitrogen, REF#BMS268-3), VEGFR-2 (human VEGFR-1 ELISA kit, Invitrogen, REF#BMS2019), and HIF-1α (human HIF-1α ELISA kit, Invitrogen, REF#EHIF1A). The technique used was a quantitative sandwich enzyme immunoassay with the following procedure. The ELISA's standard was pipetted into a microplate that had been pre-coated with a specific antibody to the antigen according to the manufactured ELISA Kit. Each sample was pipetted into each well so the antigen would be bound to the immobilized antibody. Unbound materials were discarded. Then biotin-conjugated antibody specific for antigen was added into the wells, then washed. Afterward, avidin-conjugated Horseradish Peroxidase (HRP) was added, then washed to remove any unbound materials. Staining was done by adding a solution substrate, incubated for 30 minutes, and added a stop solution to stop the reaction. The preparations were read with a microplate reader (ZENIX-320).
Newborns were examined by the obstetrician for the birth weight, length, head circumference, mid-upper arm circumference, chest circumference, Apgar score, placental size, and placental weight. The birth weight was measured immediately after birth using calibrated weighing scales. The birth length, head circumference, mid-upper arm circumference, and chest circumference were measured using a measuring tape. Apgar score was noted at the first minute of birth. The placental size was measured using a ruler, and the weight was measured using calibrated weighing scales.
All statistical analyses were conducted using SPSS 23 Statistic Program for Windows. The techniques for analyzing data include the normality test using the Shapiro Wilk test to determine the normal data distribution. Comparative analysis between the case and control groups was carried out using a one-way ANOVA test for normally distributed data and the Kruskal Wallis test for non-normally distributed data to assess dysregulation of angiogenesis in the pathomechanism of low birth weight due to malaria-inhibited fetal growth in the placenta. The correlation test used was a Pearson test for normally distributed data and the Spearman's rho test for non-normally distributed data to assess the correlation between the variables observed in the study. Path analysis was used to assess the conceptual research framework, i.e. whether the authors' concepts are relevant to the research findings. Data analysis was carried out with a confidence level of 95% and a degree of significance by p≤0.05.
A total of 33 subjects met the inclusion criteria of this study. Then laboratory tests were carried out to identify the control group and the case group, as shown in Table 141.
Plasmodium vivax was identified in all subjects of the case group, and no Plasmodium falciparum nor mixed infection was found. Histopathological examination of placental tissue from the case group showed sequestration of infected erythrocytes and/or hemozoin, followed by monocytes infiltration in the intervillous space in all samples. Two of the 19 malaria cases were identified from cord blood PCR. Examination of maternal peripheral blood smear can at least identify parasites compared to PCR and histopathological examination of the placenta, which is about 36.84%. The blood smears from the malaria case group showed in Figure 1.
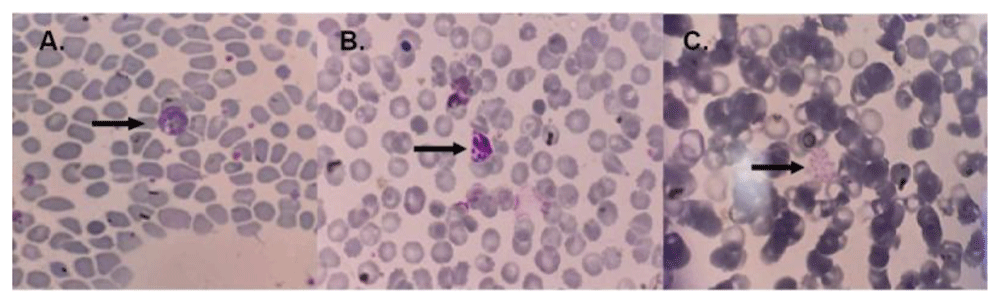
In panel A, the arrow indicates the amoeboid form of Plasmodium vivax, panel B shows young schizonts of Plasmodium vivax, and panel C displays the mature schizont of Plasmodium vivax. The image size is the only modification made; no changes to brightness or contrast have been done.
This study showed no statistically significant differences in maternal age, gestational age, and maternal leukocyte count between the case and control groups. It can be seen that in the case group, the age distribution of pregnant women is slightly lower than the age distribution of pregnant women in the control group, but this is not statistically different. Statistically significant differences were only found in maternal hemoglobin levels in the case group (p=0,000<α), lower than in the control group. The subject characteristics and distributions showed in Table 2.
The characteristics of the babies in this study were recorded as shown in Table 3. There was a significant difference (p=0,000<α) in the mean Δ 10th percentile growth curve of birth weight between the case group (413.16±460,33 g) and the control group (-42.86±129.88 g). However, there was no significant difference (p=0.325>α) in the mean birth weight (g) between the case group (2189.47 ±182.06 g) and the control group (2239.29 ±190.85 g). It appears that the mean value of birth weight in the case group is significantly smaller than the mean in the control group. It can be said that pregnant women with malaria are likely to give birth to a baby with a smaller birth weight than a baby born to a non-malaria pregnant woman.
| Variables | Case Mean ± deviation | Control Mean ± deviation | p-value |
|---|---|---|---|
| Δ 10th percentile growth curve (g) | 413.16±460.33 | -42.86±129.88 | 0.000** |
| Baby birthweight (g) | 2189.47 ±182.06 | 2239.29 ±190.85 | 0.325** |
| Body length (cm) | 42.95±3.58 | 41.86±2.91 | 0.452** |
| Head circumference (cm) | 27.47±1.26 | 27.21±1.05 | 0.495** |
| Upper arm circumference (cm) | 6.05±0.71 | 6.21±0.70 | 0.509** |
| Chest circumference (cm) | 21.00±1.89 | 20.71±1.33 | 0.528** |
| APGAR score | 9.16±0.60 | 9.14±0.36 | 0.852** |
| Placental size (cm3) | 392.16±40.08 | 404.86±44.84 | 0.415* |
| Placental weight (g) | 450.00±57.73 | 457.14±43.22 | 0.519** |
| Hemoglobin level (g dL-1) | 14.96±2.49 | 16.35±2.30 | 0.113* |
| Leukocyte level (103 µL-1) | 12.70±2.81 | 10.24±2.11 | 0.010* |
VEGF in the placenta of the case group was lower (64,404±28,942) than the control group (226,693±39,025). In both the case and control groups, VEGF expression was more abundant in the trophoblast cells of the placental villi than in the intervillous space. PlGF expression in the placenta of the case group was lower (22,814,440±9,497,663) than the control group (84,693,238±29,981,727). PlGF expression in the case group was abundant in trophoblast cells of the placental villi, whereas it was mostly found in the intervillous space in the control group. PlGF expression in the placenta of the case group was significantly lower than that of the control group.
The expression of VEGFR-1 in the placenta of the case group was lower (107,444±46,696) than the control group (213,410±35,251). VEGFR-1 expression was more abundant in trophoblast cells in the placental villi than in the intervillous space in the case and control groups. The expression of VEGFR-1 in the case group's placenta was significantly lower than that of the control group. VEGFR-2 expression in the placenta of the case group was lower (96,968,870±24,300,623) than the control group (167,673,566±90,824,566). VEGFR-2 expression was abundant in trophoblast cells in the placental villi in the case and control groups. The expression of VEGFR-2 in the case group's placenta was significantly lower than that of the control group. The expression of HIF-1α in the placenta of the case group was higher (80,375,094±40,647,360) than the control group (25,286,646±27,238,953). In the case and control groups, HIF-1α expression was more commonly found in the trophoblast cells of the placental villi. HIF-1α expression in the placenta of the case group was significantly higher than that of the control group, as seen in the Figure 2. The quantification of angiogenic factor expression in the case and control groups are presented in Figure 3.
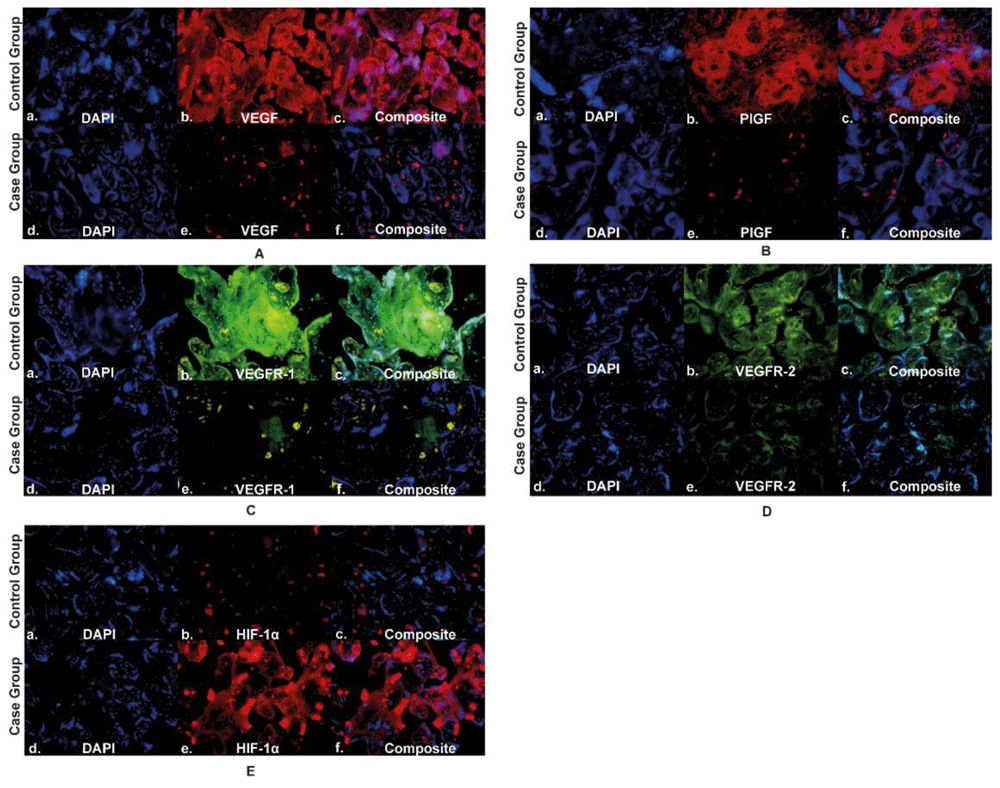
Panel A to E shows the angiogenic expression in the placenta using immunofluorescence staining of VEGF, PlGF, VEGFR-1, VEGFR-2, and HIF-1α, respectively; VEGF: vascular endothelial growth factor; PlGF: placental growth factor; VEGFR-1: vascular endothelial growth factor receptor-1; VEGFR-2: vascular endothelial growth factor receptor-2; HIF-1α: hypoxia-inducible factor-1α.
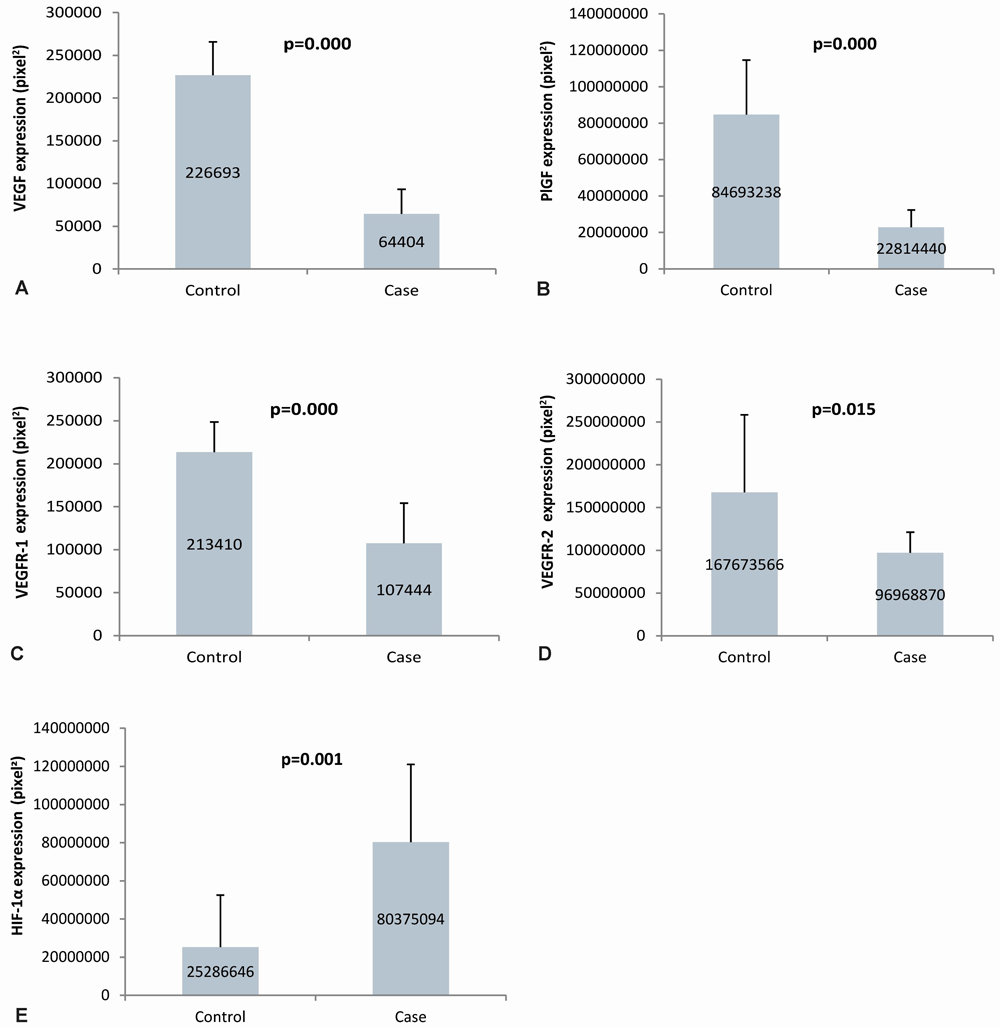
Panel A to E shows semiquantitative analysis of VEGF, PlGF, VEGFR-1, VEGFR-2, and HIF-1α expression in placenta, respectively; VEGF: vascular endothelial growth factor; PlGF: placental growth factor; VEGFR-1: vascular endothelial growth factor receptor-1; VEGFR-2: vascular endothelial growth factor receptor-2; HIF-1α: hypoxia-inducible factor-1α.
The results of the path analysis test showed that there was a significant direct effect (p=0.035) HIF-1α expression on VEGF expression with an effect coefficient of -0.545. Likewise, there was a significant direct effect (p=0.026) of HIF-1α expression on PlGF expression with an effect coefficient of -0.603. VEGF expression had no direct effect on VEGFR-1 expression (p=0.886), but had a direct effect on VEGFR-2 expression (p=0.000) with a coefficient of effect of 0.748. There was a significant direct effect (p=0.000) PlGF expression on VEGFR-1 expression with an effect coefficient of 0.937 shown. VEGFR-1 expression had no direct effect on birth weight (p=0.464), but VEGFR-2 expression had a significant direct effect (p=0.020) on birth weight with an effect coefficient of 0.743.
The mean maternal plasma VEGF levels in the case and control groups were almost the same; statistical results showed no statistically significant difference (p=0.769). The mean maternal plasma PlGF, VEGFR-1, VEGFR-2 level in the case group was lower than those in the control group. Statistically, there is a significant difference (p=0.026; 0.044; 0.042, respectively). Similar to VEGF plasma level, the HIF-1α plasma levels in the case and control groups had values that were not much different, which showed no significant difference (p=0.402). The angiogenic factor level in the case and control group are presented in Figure 4.
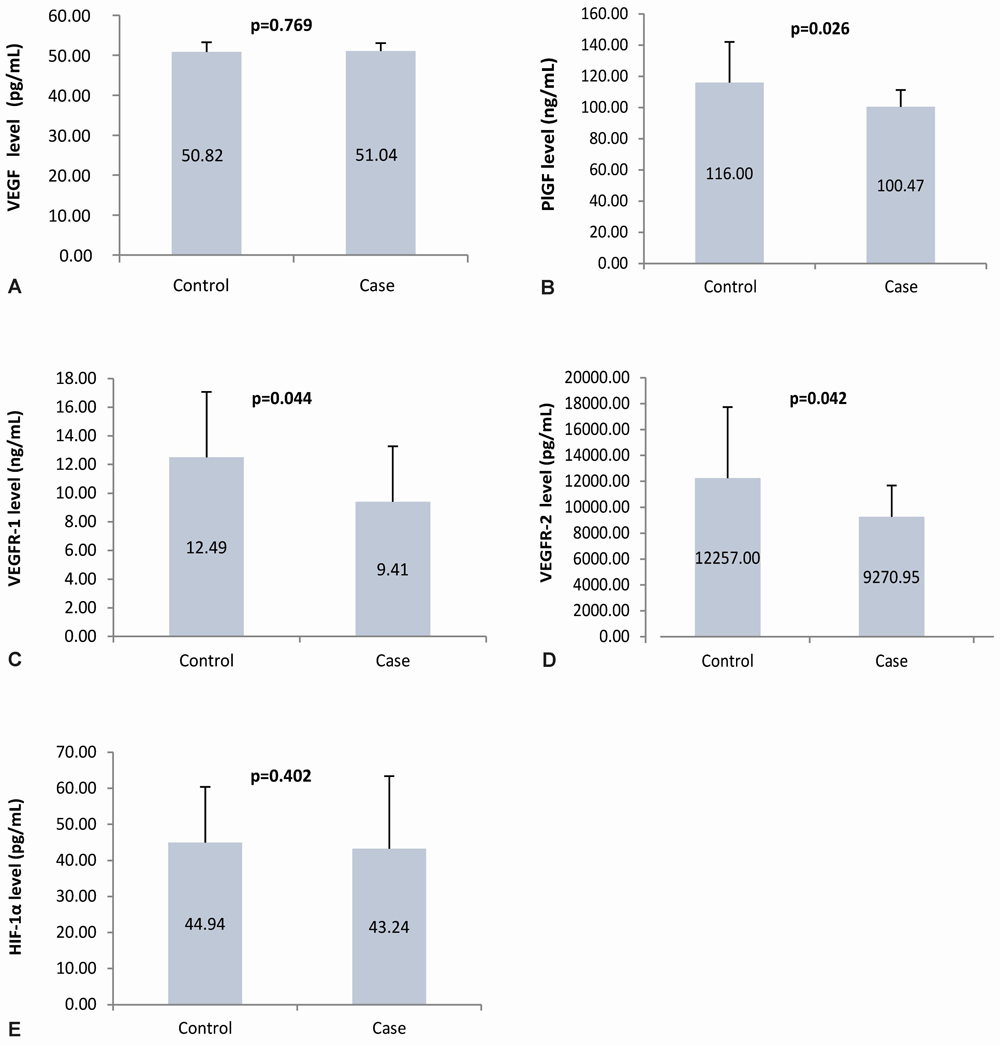
Panel A to E shows the angiogenic factors level in the maternal plasma using ELISA of VEGF, PlGF, VEGFR-1, VEGFR-2, and HIF-1α, respectively; VEGF: vascular endothelial growth factor; PlGF: placental growth factor; VEGFR-1: vascular endothelial growth factor receptor-1; VEGFR-2: vascular endothelial growth factor receptor-2; HIF-1α: hypoxia-inducible factor-1α.
There was a significant difference in the mean expression of VEGF, PlGF, VEGFR-1, and VEGFR-2 placenta between the case and control groups. The mean value of placental VEGF, PlGF, VEGFR-1 and VEGFR-2 expression in the case group was lower than the mean in the control group. In addition, there was a significant difference in the mean placental HIF-1α expression between the case and the control groups. However, in contrast to other angiogenic factors, the mean placental HIF-1α expression in the case group was higher than the mean placental HIF-1α expression in the control group. The comparison of angiogenic factor expression in the placenta is shown in Table 4.
| Variable | Case group (n=19) Mean ± SD | Control group (n=14) Mean ± SD | p-value | |||
|---|---|---|---|---|---|---|
| Placenta (pixel2) | Plasma | Placenta (pixel2) | Plasma | Placenta | Plasma | |
| VEGF | 64,404 ± 28,942 | 51.04 ± 1.99 (pg/mL) | 226,693 ± 39,025 | 50.82 ± 2.41 (pg/mL) | 0.000* | 0.769* |
| PlGF | 22,814,440 ± 9,497,663 | 100.47 ± 10.72 (ng/mL) | 84,693,238 ± 29,981,727 | 116.00 ± 26.19 (ng/mL) | 0.000* | 0.026* |
| VEGFR-1 | 107,444 ± 46,695 | 9.41 ± 3.86 (ng/mL) | 213,410 ± 35,251 | 12.49 ± 4.57 (ng/mL) | 0.000* | 0.044* |
| VEGFR-2 | 96,968,870 ± 24,300,623 | 9271 ± 2419 (pg/mL) | 167,673,566 ± 90,824,566 | 12257 ± 5482 (pg/mL) | 0.015** | 0.042* |
| HIF-1α | 80,375,094 ± 40,647,360 | 43.24 ± 20.19 (pg/mL) | 25,286,646.1±27,238,953 | 44.94 ± 15.49 (pg/mL) | 0.001** | 0.402** |
There was no significant difference in the mean VEGF plasma levels between the case group and the control group. The mean value of VEGF plasma levels in the case group was slightly higher than the average VEGF level in the control group. However, this increase was not statistically significant. Similar to VEGF, the mean plasma levels of HIF-1α between case and control groups did not show a significant difference. However, HIF-1α in the case group was slightly lower than the mean HIF-1α expression in the control group. The plasma levels of PlGF, VEGFR-1, and VEGFR-2 between the case group and the control group showed significant differences. The mean levels of PlGF, VEGFR-1, and VEGFR-2 in the case group were lower than in the control group. The comparison of maternal plasma angiogenic factor levels is shown in Table 4.
All malaria-positive samples in this study were identified either using maternal peripheral blood smear examination or PCR examination caused by Plasmodium vivax (Table 1). Microscopic examination of blood smears only detected 7 of the 19 positive samples (36.84%). On the other hand, 12 blood samples were detected positively by PCR examination (63.16%). The results showed that PCR is more sensitive than blood smear examination. Malaria detection using microscopic examination was only able to detect Plasmodium at levels of 20 parasites/μL and was influenced by the experience of the observer and the quality of staining blood smears. PCR is the most sensitive method because it can detect 2-6 parasites/μL42–45. It was surprising that all the histopathological preparations of 19 samples showed pigment/hemozoin depositions with or without infected erythrocytes or monocytes accumulation, indicated that all of the samples had already been infected as active acute, active chronic, and past infection46–49. In this study, all positive samples did not show signs and symptoms of malaria, so all positive samples were subclinical infections.
Investigation of acute infection through maternal peripheral blood smear examination cannot fully describe the history of maternal exposure to malaria during pregnancy. The absence of parasitemia on peripheral blood smear examination does not always represent a malaria-free placental infection48. In this study, acute, chronic, and past malaria infection in pregnancy may be related to placental histopathological changes' adverse consequences. In cases of subclinical malaria, placental histopathological changes might be found due to most of the sub-populations of parasites sequestered in several organs that serve as reservoirs of the parasite, such as the spleen and placenta, cause other inflammatory reactions43,48,50,51.
Characteristics of mothers. In this study, there were significant differences in the mean hemoglobin levels in women infected with Plasmodium vivax during pregnancy compared to the group of women who were not infected. At the same time, gestational age, maternal age, and leukocyte count showed no significant difference. The average hemoglobin level of pregnant women infected with malaria was 1.22 g/dL lower than the control group (mean ± standard deviation [SD]; 9.88±0.62 vs. 11.10±1.20, p=0.000). This finding aligned with several previous studies, which also shown that vivax malaria infection is associated with maternal anemia, abortion, premature birth, congenital malaria, and other severe complications36,52–56. Single Plasmodium vivax infection during pregnancy had a significant association with a twofold increased risk of maternal anemia compared to the group of mothers who did not experience malaria infection36,57. The occurrence of maternal anemia is closely correlated with nutritional status36. However, one of the other risk factors that can increase anemia in pregnant women with Plasmodium vivax infection is young age pregnancy58. In this study, the mean age of pregnant women in the case group was lower than the control group (mean ± SD; 27.68 ± 7.21 vs. 32.21 ± 6.25, p = 0.076); there was no significant difference. This factor may be considered one of the critical risk factors in the subsequent study involving many subjects.
The parity distribution in the case group was mainly primiparity (11/19; 57.9%), while in the control group, the highest number was mothers with third parity (6/14; 42.9%). This result has a distribution pattern similar to Bardaji et al. In their study, most malaria pregnant women were primigravida, and there was a tendency to decrease malaria prevalence as gravidity increased35. In a study of Karen women in Thailand involving a comparable number of participants, Plasmodium vivax was more common in primigravidas than in multigravida, and this was associated with anemia and an increased risk of low birth weight/LBW53.
Both case and control groups showed no significant difference (mean ± SD: 39.47±1.39 vs. 38.64±0.84, p=0.078) in gestational age, but all participants gave birth at term. In the study of Bardaji et al., it was found that the average gestational age was 38.6 weeks, with 12.6% of all participants giving birth prematurely (<37 weeks)35. From the results of their study, it cannot be concluded that there is a related relationship because it requires a joint analysis with other factors such as the severity of malaria infection, the onset of malaria infection, and pregnancy outcomes, which in this case includes the baby's birth weight. As in this study, all infants were born at term but had a significant effect of small for gestational age with malaria infection status in the form of the chronic active or previous history of malaria.
Characteristics of babies. This study showed a significant difference in the mean Δ 10th percentile growth curve of birth weight between the case group. However, there were no significant differences in the average birth weight of the baby, the length of the baby's birth weight, head circumference, upper arm circumference, chest circumference, Apgar score, placental weight, placenta size, and hemoglobin levels. However, the baby's mean hemoglobin level of the case group was 1.39 g/dL lower than the control group. The result was consistent with a previous study, which reported that single-episode of P. vivax infection during pregnancy was associated with a significant reduction in neonatal birth weight and maternal hemoglobin levels33. The results of the meta-analysis study also stated that there was an increased risk of 1-2 times the occurrence of LBW in pregnant women infected with malaria compared to those who were not infected59.
Meanwhile, babies in both the case and control groups were included in LBW criteria, so it is not yet known whether the leading cause of LBW in the case group is influenced by the main factor of Plasmodium vivax infection, as in the previous studies33,58. While according to Bardaji et al., no direct relationship between Plasmodium vivax infection and an increased risk of LBW but stated that Plasmodium vivax infection during pregnancy is closely associated with maternal anemia, where this condition may harm the health of the newborn35. In addition, maternal anemia is a risk factor for the occurrence of LBW with an odds ratio (OR) value of 1.23 (95% confidence interval [CI]: 1.06–1.43)60.
The results showed significant differences in the LBW leukocyte count parameters in the case and control groups (mean ± SD: 12.70 x 103±2.81/μL vs. 10.24 x 103±2.11/μL, p<0.05). In general, the range of leukocyte count values in newborns can range from 10,000-26,000/μL, which is also highly dependent on the time of sampling61. Meanwhile, the results of the leukocyte count in newborns at Bantul Hospital, Indonesia, in 195 infants showed an average leukocyte count of 14.62 x 103±3.49/μL62. The leukocyte count in newborns in the case and control groups showed values within normal limits, with the leukocyte count in the case group significantly higher than the control group. Several factors can increase the leukocyte count in newborns, including infection, inflammation, medication history, and stress63–65.
Sequestration is the attachment of infected erythrocytes to host cells associated with severe malaria, such as cerebral malaria and malaria in pregnancy. Sequestration occurs in the capillaries and post-capillary venules of specific organs such as the brain, lungs, and placenta. Sequestration correlates with mechanical obstruction of blood flow in the microvasculature and the activation of vascular endothelial cells, leading to pathological outcomes. Some parasitic and host proteins as ligands and receptors for sequestration have been identified and explored further66.
Heretofore, the sequestration phenomenon only occurred in Plasmodium falciparum infection46,67,68. Interestingly, none of the subjects in this study were infected with Plasmodium falciparum, but instead, hemozoin depositions were found in all 19 positive samples, while Plasmodium vivax infected erythrocyte sequestrations were found in some samples. The result indicates that the Plasmodium vivax could cause sequestration of infected erythrocytes. Submicroscopic placental infection of Plasmodium vivax confirmed by placental histopathology indicates that the Plasmodium vivax can be selectively sequestered in the placenta even at low densities in peripheral blood69. The finding of Plasmodium vivax-infected erythrocytes sequestration was also mentioned by Carvalho et al. through an ex vivo study, which found that Plasmodium vivax-infected erythrocytes could adhere to the placental tissue. This study also demonstrated the cytoadherence of Plasmodium vivax-infected erythrocytes on endothelial cells by in vitro approach and was known to have the same strength as cytoadherence of Plasmodium falciparum-infected erythrocytes29. Toda et al. found that Plasmodium vivax-infected reticulocytes could bind to human spleen fibroblasts (hSF). The binding involves the NF-kB signaling pathway transcription factor by plasma-derived extracellular vesicles that act as cargo for the Plasmodium vivax parasite. This process facilitates the expression of ICAM-1 on the surface of hSF as a receptor for Plasmodium vivax sequestration51. Other evidence supporting placental malaria due to other non-falciparum Plasmodium species is a submicroscopic infection of Plasmodium malariae and Plasmodium ovale in the placenta confirmed by PCR of placental blood samples. Detection of Plasmodium malariae infection was higher in placental blood samples than in peripheral blood samples, indicating a possibility of Plasmodium malariae binding affinity to the placenta. However, the study of non-falciparum placental infection was not supported by histopathological examination of the placenta70.
Malaria infection causes changes in the placenta structure in the form of hemozoin deposition and an increase in monocyte infiltration in the intervillous space, which interferes with maternal-fetal circulation48. Sequestration of infected erythrocytes, hemozoin deposition, and monocyte infiltration in placental histopathology are the main signs of placental malaria12,71,72. The pathomechanism of sequestration of infected erythrocytes in the placenta is clearly described in Plasmodium falciparum infection. However, Plasmodium vivax, which has been considered to cause a mild latent infection than Plasmodium falciparum, has also been reported to cause placental malaria. Histopathological changes of placental malaria due to Plasmodium falciparum can show a different representation of placental malaria due to Plasmodium vivax. However, these differences are not delineated yet48. The histopathological appearance of the placenta due to Plasmodium vivax infection is similar to that of Plasmodium falciparum. Plasmodium vivax had also demonstrated the sequestration mechanism of infected erythrocytes in placental tissue structures49,73. These findings are consistent with the results of this study.
Several mechanisms can explain the Plasmodium vivax-infected erythrocytes sequestration phenomenon. First, the Plasmodium vivax-infected erythrocytes sequestration can occur under low shear stress conditions in the placental intervillous space and causes a local inflammatory process of the placenta31. Second, Plasmodium vivax lacks surface proteins, such as Plasmodium falciparum Erythrocyte Membrane Protein-1 (PfEMP-1) called Variant Surface Antigen-2-CSA (VAR2CSA) for adhesion and sequestration of CSA in the placental intervillous space74,75. However, the Plasmodium vivax genome contains a subtelomeric multigene family called VIR proteins. VIR proteins can mediate the adhesion of infected erythrocytes to ICAM-1 by VIR14 and the CSA by VIR2 and VIR24. The bindings are speculative and require further research73,74,76. The discovery of VIR binding in ICAM-1 and CSA proved the presence of Plasmodium vivax sequestration in the previous study29. The third mechanism that can explain the sequestration of Plasmodium vivax is the formation of rosettes by interacting with the Glycophorin C receptor present on normal erythrocytes, which has the adhesive power as in Plasmodium falciparum. However, further research is needed to determine the specific mechanism of rosette formation73,77,78.
On histopathological examination of the placenta, it was found that pigment/hemozoin depositions were trapped in the fibrin or freely circulating in the intervillous space and monocyte infiltration in all 19 positive samples. This finding indicates that the mother had experienced malaria infection during her pregnancy46,79. The presence of pigment/hemozoin and monocyte depositions in the placenta indicates that malaria infection does not occur in late pregnancy. The infection process has occurred long enough to cause deposition and accumulation of inflammatory cells in the placenta acquired early in pregnancy or before pregnancy69,70. However, the time required for the appearance of placental malaria is not known and requires further research.
Vascular endothelial growth factor (VEGF). In this study, VEGF expression in the placenta of the case group was lower than in the control group. In the case and control groups, VEGF expression was more abundant in trophoblast cells in the placental villi than in the intervillous space. VEGF expression in the case group placenta was significantly lower than in the control group (p=0.00). Expression of VEGF placental IUGR was inconsistent between several studies that have been carried out. The results of this study are consistent with several studies, which reported a decrease in VEGF expression accompanied by increased placental PlGF expression of IUGR80,81. Several studies reported an increase in the expression of VEGF, VEGFA, bFGF, and eNOS in the IUGR placenta due to placental hypoxia82–84. However, another study concluded that there was no decrease in VEGF expression of IUGR compared to normal placentas85,86. In addition, VEGF expression and plasma VEGF levels could not predict late-onset preeclampsia, small gestational age (SGA), or premature delivery87.
Plasma VEGF levels in the case group were lower and not significantly different from the control group in this study (p=0.769). The findings were consistent with several studies, which found that maternal plasma VEGF levels of IUGR and SGA did not show significant differences compared to preeclampsia88 or normal pregnancy89. Moreover, Tang et al. reported that maternal plasma VEGF levels in pregnancies with preeclampsia were lower than normal pregnancies and were associated with impaired fetal growth90. However, Boras et al. found that maternal free plasma levels of VEGF and sFlt-1 were higher in IUGR than in normal pregnancies91.
Besides being involved in the regulation of development and vascular remodeling during placentation, VEGF, PlGF, and Angiopoietin are also involved in trophoblast invasion. Failure of trophoblast invasion and remodeling of the spiral arteries causes placental hypoxia, which involves pregnancy complications such as preeclampsia and IUGR92, causing the fetus to experience hypoxia. At low oxygen concentrations, there should be an increase in VEGF expression. The mechanism that can explain the decrease in placental VEGF expression in this study is increased soluble VEGFR-1 (sFlt-1) in the placental intervillous space. sFlt-1 is a form of the VEGFR-1 gene that is physiologically secreted by the placenta. sFlt-1 physiologically binds to free VEGF and PlGF with strong affinity and inhibits their binding to VEGFR-1 and VEGFR-2 receptors, thereby decreasing placental VEGF and PlGF expression. HIF-1α regulates placental sFlt-1 expression; under conditions of placental hypoxia, there will be an increase in placental sFlt-1 expression93,94. The sFlt-1 expression was also reported to increase in IUGR placentas95,96. An in vivo study in transgenic mice by Vogtmann et al. found that an increase in placental sFlt1 would disrupt the vascular endothelial growth factor signalling pathway, resulting in decreased expression of VEGFA, VEGFB, PlGF, and increased the expression of band and mRNA of Caspase-997. Placental hypoxia will increase placental VEGF expression92,93, but the source of circulating VEGF is not only from the placenta but also from another organs98, then those can explain why the plasma levels of VEGF in the case group and the control group were not different.
The finding of decreased placental VEGF expression in this study can describe early-onset IUGR because of placental hypoxia, correlated with placental HIF-1α expression. However, histo-morphological studies of placental villi were not performed to confirm the dominance of branching angiogenesis in placental villi. Early-onset IUGR in this study can be caused by malaria infection before pregnancy or early pregnancy46, as evidenced in this study.
Placental growth factor (PlGF). The placenta is the only organ that produces PlGF98. PlGF has a significant function in the activation, proliferation, and migration of endothelial cells and trophoblast invasion into the maternal spiral arteries99. In this study, the expression of PlGF in the placenta in the case group was lower than the control group (p=0.00). In the case group, PlGF expression was more abundant in trophoblast cells in the placental villi, whereas in the control group, it was primarily found in the intervillous space. Studies on PlGF expression in placental IUGR had also shown inconsistent results. The results of this study support previous studies which reported that placental PlGF expression in severe IUGR was significantly lower than in controls and inhibition of PlGF/Flt-1 signalling will interfere with trophoblast proliferation and migration99–101. However, Alahakoon et al. found an increase in PlGF and KDR expression in IUGR and preeclampsia placentas86. It explained that hyperoxia conditions in IUGR due to impaired oxygen extraction to the fetus increase PlGF expression and decrease placental VEGF expression, with the consequence of reduced capillary branching and terminal villi resulting in placental dysfunction92,93,102,103.
Placental hypoxia conditions that occur early in pregnancy result in a decrease in placental PlGF production by the syncytiotrophoblast, so PlGF expression and PlGF levels in maternal circulation decreases92,93,100. In this study, the plasma levels of PlGF in the case group were significantly lower than in the control group (p=0.026). Previous studies have also shown that plasma or plasma PlGF levels are lower in pregnancies with IUGR95,104,105 in pregnancies with preeclampsia97,106–108 and pregnancies with preeclampsia and IUGR88,90,96,109–115. Decreased maternal plasma PlGF levels in pregnancy complications due to defective placentation are associated with increased production of sFlt-1 in the ischemic placenta, which is then secreted into the maternal circulation104,106. Furthermore, significantly lower PlGF levels in the case group indicate that the causes of IUGR in the two groups may be different. Malaria infection in the placenta causes the activation of complement C5a, which will stimulate the accumulation of monocytes to release sFlt-118, explaining that the plasma levels of PlGF in the case group were significantly lower than the control group.
Vascular endothelial growth factor receptor (VEGFR)-1 and VEGFR-2. This study showed that VEGFR-1 expression in the placenta was lower in the case group than in the control group (p=0.00). VEGFR-1 expression was more abundant in trophoblast cells in the placental villi than in the intervillous space, both in the case and control group. VEGFR-1 is a receptor for VEGF and PlGF. Helske et al. found that VEGFR-1 expression was increased in preeclamptic and IUGR placentas compared with normal placentas. The up-regulated expression is not exactly found on all preeclamptic samples but may be associated with hypoxia and abnormal function of the placenta116. However, down-regulation of both receptors was also reported during mid-pregnancy with IUGR117.
In line with VEGFR-1, the expression of VEGFR-2 in the placenta of the case group was lower than the control group (p=0.015). In the case group and the control group, VEGFR-2 expression was more abundant in trophoblast cells in the placental villi. VEGFR-2 is a significant receptor for VEGF, plays a role in endothelial proliferation and normal vascular formation. In the placenta, the role of VEGFR-2 is to change trophoblasts into intravascular trophoblasts and form spiral arteries. VEGFR-2 is expressed in low amounts under hypoxic conditions because oxygen levels regulate VEGFR-2 expression. Placental VEGFR-2 and sFlt-1 expression was also reported to decrease in cases of preeclampsia95,118.
In this study, plasma levels of VEGFR-1 in the case group were lower and significantly different with the control group (p=0.044) Anna et al. found that serum VEGFR-1 concentrations in women with IUGR were decreased, along with a decrease in PlGF. At low oxygen concentrations, PlGF and VEGFR-1 levels decrease119. Besides that, serum levels of VEGFR-2 in the case group were lower and significantly different with the control group (p=0.042). Chaiworapongsa et al. found soluble VEGFR-2 (sVEGFR2) concentration increased in women with preeclamptic pregnancies and interpreted that sVEGFR-2 in maternal plasma could reflect endothelial cell function120.
Hypoxia-inducible factor-1α (HIF-1α). This study revealed that the expression of HIF-1α in the placenta of the case group was higher than the control group (p=0.001). In the case group and the control group, HIF-1α expression was more commonly found in trophoblast cells in the placental villi. There are not many studies in humans examining HIF-1α expression in the placenta of pregnancies with complications such as preeclampsia, IUGR, and SGA. The results of this study support an in vivo study on the IUGR mice model conducted by Robb et al., who found an increase in HIF-1α expression in the placenta121.
In this study, plasma levels of HIF-1α in the case group were lower and not significantly different with the control group (p=0.402). So far, studies of HIF-1α have been mainly carried out in placental tissue, as in the discussion on placental HIF-1α expression above. Azhur-Fabian et al. found elevated HIF-1α and p21 mRNA expression in all pregnancy plasma samples with hypoxia and IUGR and recommended them as markers for hypoxic pregnancy and/or IUGR122. In pregnancy, HIF-1α is expressed in the placenta early in normal pregnancy and throughout pregnancy under hypoxic conditions123. Those explain no statistically significant difference in plasma HIF-1α levels in the case and control groups.
Under normoxic conditions, HIF-1α will undergo rapid degradation so that it is considered inactive. At low oxygen levels, HIF-1α will regulate genes that control cell growth, differentiation, and metabolism. The active form of HIF-1α that was continuously exposed to cultured cells was shown to inhibit trophoblast differentiation. In placental IUGR, impaired invasion and remodelling of the spiral arteries result in placental ischemia throughout pregnancy because of the increased expression of HIF-1α. The increase in placental HIF-1α expression in this study represents early-onset IUGR. However, this study has not determined whether placental hypoperfusion causes hypoxia as the main factor causing the increase in HIF-1α because other factors such as reactive oxygen species (ROS) and inflammation induced by nuclear factor kappa (NF-κB) can also modulate HIF-1α accumulation.
Path analysis revealed the effect of placental angiogenesis and HIF-1α transcription factors on LBW. From this analysis, it was seen that the signalling pathway from HIF-1α to placental VEGF expression, then from placental VEGF expression to placental VEGFR-2 expression, and from placental VEGFR-2 expression to LBW showed statistically significant results. In contrast, HIF-1α could not cause a direct effect on LBW (p=0.881). Placental VEGF expression significantly affected placental VEGFR-1 expression. The signalling pathway of HIF-1α significantly affected placental PlGF expression, then placental PlGF expression significantly affected placental VEGFR-1 expression, but this pathway did not significantly affect the incidence of LBW (p=0.504).
VEGF and VEGFR regulate vasculogenesis during early embryogenesis and angiogenesis in later stages of pregnancy. VEGF bind to VEGFR-1/Flt-1 and VEGFR-2/KDR, playing an essential role in physiological and pathological angiogenesis124. PlGF binds to the VEGFR-1 receptor, plays a role in angiogenesis in the later stages of pregnancy125. VEGFR-2 is the primary receptor mediating the pro-angiogenic effects of VEGF126. sFlt1, the free form of Flt-1, has a strong ability to bind to VEGFA, PlGF, and VEGFB. Because the trophoblast lies between the maternal and fetal vascular systems, it is thought that sFlt1 functions as a separator between the maternal and fetal circulations in the placenta by suppressing both excessive angiogenesis and abnormal vascular permeability. Therefore, under physiological conditions, sFlt1 levels are maintained in the suitable range. The increased expression of sFLT-1, triggered by hypoxic conditions, will bind to VEGF, causing disturbances in vasculogenesis and angiogenesis, leading to obstetric complications. Several studies have reported overexpression of sFlt1 and decreased expression of VEGFA in preeclamptic patients127.
In placental malaria infection, there is an accumulation of Plasmodium-infected erythrocytes in the intervillous space of the placenta. Several histopathological characteristics of placental malaria, including thickening of the basement membrane and infiltration of monocytes in the intervillous space of the placenta (intervillositis), can increase the resistance to oxygen transport across the placenta. Accumulation of inflammatory cells and infected erythrocytes can cause placental hypoxia due to oxygen consumption by these cells, in addition to decreased blood perfusion due to reduced effective surface area for feto-maternal exchange. Such placental dysfunction can result in stunted fetal growth, characterized by low birth weight babies27.
Increased expression of HIF-1α is a marker of placental hypoxia, which should increase the expression of VEGF, VEGFR-1, and VEGFR-2. Decreased expression of VEGFR, VEGFR-1, and VEGFR-2 is possible through increased sFlt-1 binding to VEGF with strong affinity. sFlt-1 of the placenta increases its expression under hypoxic conditions93,94. Further studies are needed to prove this.
The pathway analysis results indicate a dysregulation of the angiogenic factor VEGF and its receptor VEGFR-2 due to the regulation of HIF-1α in placental malaria on the incidence of LBW. It can be interpreted that the occurrence of LBW in placental malaria due to the influence of angiogenesis factors VEGF and VEGFR-2, which are regulated by HIF-1α and are more significant than LBW in non-malaria cases.
The effect of plasma angiogenesis and HIF-1α transcription factors on the incidence of LBW was observed using binary logistic regression as bivariate analysis. Based on the results of the observational test of data in maternal plasma, four parameters were significantly different between the case group and the control group, namely: haemoglobin, PlGF levels, VEGFR-1 levels, and VEGFR-2 levels. It showed that haemoglobin levels were the most significant (p=0.010) in influencing the incidence of LBW, followed by plasma levels of PlGF (p=0.021) and plasma levels of VEGFR-1 (p=0.056). The plasma levels of VEGFR-2 did not affect the incidence of LBW (p=0.131).
The haematological effect of Plasmodium vivax malaria is anaemia with its consequent increased morbidity and mortality and more frequent in blood transfusions. Although the parasitemia of Plasmodium vivax malaria is lower than that of Plasmodium falciparum malaria, it can cause severe anaemia, as in Plasmodium falciparum malaria. Anaemia in Plasmodium vivax malaria can be explained by two mechanisms. First, production/transfer of infected and uninfected erythrocytes are more in Plasmodium vivax malaria, 34 uninfected erythrocytes for one infected erythrocyte (in Plasmodium falciparum malaria, the ratio is 8:1). Second, Plasmodium vivax-infected erythrocytes undergo more rapid deformity limiting erythrocytes that are expelled through the microvessels of the spleen during the 'spleen clearance' phase. In addition to these two mechanisms, activation of the immune system due to Plasmodium vivax infection increases the detection and removal of abnormal infected and uninfected erythrocytes57.
In this study, maternal anaemia significantly affected the incidence of LBW. Several publications also reported the same thing. Plasmodium vivax malaria infection in pregnant women in Bolivia was more at risk of anaemia and giving birth to low birth weight babies than pregnant women who were not infected36 as well as in a multicenter study in an area of low malaria transmission35 and Mangaluru, India128.
Based on the above discussion, in this study, it can be concluded that angiogenesis factors and HIF-1α transcription factors in placenta or in local environment play a more important role in the incidence of low birth weight than those in systemic. Those indicated that there is dysregulation of placental angiogenesis factors VEGF, PlGF, and their receptors VEGFR-1 and VEGFR-2, which is triggered by placental hypoxia conditions (marked by increased placental HIF-1α expression) during placental Plasmodium vivax infection.
Figshare: HIF-1α REGULATED PATHOMECHANISM OF LOW BIRTH WEIGHT THROUGH ANGIOGENESIS FACTORS IN PLACENTAL Plasmodium vivax INFECTION. https://doi.org/10.6084/m9.figshare.1657746841.
This project contains the following underlying data:
- Raw Data.xlsx (ELISA Graph and Raw Data, Immunofluorescence Graph and Raw Data, Subject Characteristics, and Baby’s Characteristics Data)
- Figure 1A (Blood Smear Figure 1A)
- Figure 1B (Blood Smear Figure 1B)
- Figure 1C (Blood Smear Figure 1C)
- Figure 2A (VEGF immunofluorescence)
- Figure 2B (PlGF immunofluorescence)
- Figure 2C (VEGFR-1 immunofluorescence)
- Figure 2D (VEGFR-2 immunofluorescence)
- Figure 2E (HIF-α immunofluorescence)
- Figure 3 (Angiogenic Factor Expression Graph)
- Figure 4 (Angiogenic Factor Level in Maternal Plasma Graph)
- PCR Images 1-4 (Raw Gel Images of PCR test from maternal (1-3) and cord (4) blood samples)
Data are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
The authors would like to acknowledge and thank the Chairman of dr. T.C. Hiller Regional Hospital, Public Health Office of Sikka Regency, and Public Health Office of Nusa Tenggara Timur (NTT), Indonesia, for the permission and substantial support to this research. We also thanks to Tarina Widaningrum S.Si., MP. Ami Maghfironi, S.Si., and Wahyuda Ngatiril Lady S.Si., for their assistance in carrying out laboratory work.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Immunology and molecular epidemiology of malaria
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Malaria Clinical and epidemiological aspects. Malaria immunology and molecular diagnosis.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 3 (revision) 05 Jun 24 |
read | ||
|
Version 2 (revision) 27 Feb 24 |
read | ||
|
Version 1 01 Feb 22 |
read | read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)