Keywords
synergistic effect, poly-herbal combinations, Curcuma longa, curcumin, antioxidant
synergistic effect, poly-herbal combinations, Curcuma longa, curcumin, antioxidant
The reviewer comments were meaningful for improving the quality of our manuscript. All comments were addressed thoroughly. The differences between the last version are the detail of procedures in the sample preparation and the figure captions.
See the authors' detailed response to the review by Andrzej L. Dawidowicz
Antioxidant activity plays a fundamental role in treatments for several diseases, particularly degenerative and metabolic diseases. Therefore, there has been an exponential surge of studies on antioxidant activity in the last decade (Dumore and Mukhopadhyay 2020). The antioxidant compound is beneficial for reducing oxidative stress. It is associated with an imbalance between reactive oxygen species (ROS) and the systems. ROS in our body generates several oxidation mechanisms that promote cell damage and imbalance of the metabolic equilibrium, particularly in the long term. Therefore, it should be minimalized (Amin and Bano 2018). Antioxidant compounds offer a simple step to reduce and inhibit ROS generation in the body. These compounds reduce radical biological generation by stabilizing the unpaired electron in ROS and the distribution in their structure. Furthermore, it reduces cell damage and minimizes the prevalence of chronic degenerative diseases (Amin and Bano 2018; Maya-Cano, Arango-Varela, and Santa-Gonzalez 2021).
Generally, plants contain several compounds that can serve as antioxidant sources, for example, polyphenols and flavonoids. The multi-compounds contained in plants have better antioxidant activity compared to single compounds. This is due to the interaction between compounds in plants. Herbal therapists administer a concoction of herbal medicines to treat several diseases. However, there is no scientific basis for combining two or more plants in herbal medicine, particularly to achieve higher activity due to their interaction (Salaj et al. 2021). The synergistic effect refers to increasing the activity of combined substances over the additive effect, whereby they interact with compounds through poly-herbal combinations (Du et al. 2021; Zonyane, Van Vuuren, and Makunga 2013). The synergistic effect enhances the antioxidant activity through a combination of several potent herbal plants with high antioxidant activity. The synergistic effect can be assessed using a statistical technique that examines the interaction between two compounds and poly-interaction simultaneously (Liang et al. 2021). The experimental design can offer a statistical evaluation of factors on response in an integrated assessment based on determining factors. The mixture design is a simple technique that can assess the mixture interaction between herbal plants to provide scientific justification for its effects.
Indonesia has diverse herbal plants that are traditionally used to reduce the risk of degenerative diseases and cancer and are applied as immunomodulators (Illian et al. 2021; Liu 2021). Curcuma longa L. and Curcuma xanthorrhiza Roxb have been reported to contain curcumin, which has several known benefits (Azeez and Lunghar 2021; Sultana et al. 2021). It is used for not only preventive treatment but also curative treatment for particular diseases (Catanzaro et al. 2018; Răducanu et al. 2021). Centella asiatica L. has been reported to have neuroprotective effects, whereby it affects antioxidant activity in the brain and surrounding tissues. In addition, traditionally, it has been applied to promote healthy aging (Sabaragamuwa, Perera, and Fedrizzi 2018). Another frequently applied Indonesian herbal medicine, Phyllanthus niruri L., is also known to have potential immunomodulatory and antioxidant properties due to its containing polyphenol and lignin compounds (Colpo et al. 2014; Nhu et al. 2020). Moreover, Moringa oleifera contains high polyphenol compounds, flavonoids (Wang et al. 2020), and peptides (Avilés-Gaxiola et al. 2021). Therefore, it has much potential for promoting antioxidant activity and for use in degenerative disease treatments. The aforementioned plants have been reported and explored extensively. Indonesian herbal therapists widely apply them for their preventive or curative benefits for degenerative diseases. In addition, a study reported that self-medication using herbal medicine is a common practice, particularly in rural areas (Rahayu, Araki, and Rosleine 2020). Therefore, it is worth studying this combination, particularly assessing the synergistic effect between two mixtures and their multi-component poly-interaction. To the best of our knowledge, no studies have assessed the antioxidant activity of these five traditional Indonesian plants using an experimental design. Therefore, the present study aimed to assess the poly-interaction of a multi-component mixture of five traditional Indonesian plants and their synergistic effects on antioxidant activity using a mixture design based on a simplex centroid design model.
Dried plant material of Curcuma xanthorrhiza (root), Curcuma longa (root), Centella asiatica (herb), Phyllanthus niruri (herb), and Moringa oleifera (leaves) was obtained from the Center for Research and Development of Medicinal Plants and Traditional Medicine (B2P2TOOT), Ministry of Health, Republic of Indonesia (Tawangmangu, Indonesia). All plants were cultivated in the Tawangmangu district under controlled monitoring by B2P2TOOT from September to December 2020. Sample plants were authenticated and identified by a plant taxonomist at B2PO2TOOT, and it was deposited as herbarium at B2P2TOOT. The dried plants were processed by following an efficient traditional herbal medicine preparation process, which included sorting, drying, and storing under close monitoring by B2P2TOOT, to ensure the quality of the plants.
Ethanol, n-hexane, and potassium persulfate were imported from Merck (Darmstadt, Germany). 1,1-Diphenyl-2-picryl hydrazyl (DPPH) and 2,2-azinobis(3-ethylbenzothiazoline-6-sulfonate) (ABTS) were purchased from Sigma Aldrich (St. Louis, MO).
The dried plants were cut into small pieces and grinding and passed through a 250 μm mesh sieve. A maceration process was carried out to extract plant material using a solvent ratio of 1:10 (w/v). Previously, the dried plants were purified using n-hexane to eliminate resin and chlorophyll and plant material to n-hexane ratio of 1:5 (w/v) for 24h. After that, the residue was collected and extracted using ethanol 95%. The macerate was filtered and concentrated using a Stuart RE300P rotary evaporator (Staffordshire, UK) at temperature and vacuum pressure of 50°C and 630 mmHg, respectively. The obtained extract was stored in a refrigerator (4–6°C) for further analyses.
The total phenolic content (TPC) in each extract was measured using the Folin-Ciocalteu assay, based on Ainsworth and Gillespie (2007), after making minor modifications to the process. An accurate predetermined concentration of each extract dissolved in ethanol was used as the sample. In addition, gallic acid was used as a standard, in a range of 10–160 μg/mL. A 0.30 mL sample was mixed homogeneously with 1.50 mL of 10% Folin-Ciocalteu reagent (diluted 1:10 with deionized water) for 5 min. The mixture was neutralized with 1.20 mL of 7.5% sodium carbonate solution and incubated along with shaking for 30 min according to the optimized operating time. The sample absorbance was recorded spectrophotometrically using Genesis 150 spectrophotometer (Thermo; Waltham, MA) at 764 nm. The TPC of each extract was calculated according to the gallic acid standard calibration curve (y = 0.0116x + 0.2110, where x is gallic acid [μg/mL], and y is absorbance; R2 0.9968) and presented as the percentage of gallic acid equivalent (%w GAE).
A simplex centroid design model was employed to assess the main effects of and poly-interactions among the five traditional Indonesian plant extracts. A 41-run design was applied to assess the variables of antioxidant activity. The design consisted of 31 runs for simplex point, five for augmenting design, and 5 for replication using a quadratic model. All design points are presented in Table S1 (data set). A multiple linear regression analysis was performed to construct the model, and the data were fitted to Equation 1. A linear, quadratic, or special cubic was selected according to the best goodness of fit parameters at a 95% confidence level.
Where a, b, c, d, and e are the coefficient regression of the linear model, known as the main effect. The interaction between two factors was determined by coefficient regression of ab, ac, ad, …, and cd. Meanwhile, the poly-interaction between three factors was assigned by abc, abd, …, and cde. The interaction contributions were calculated according to the percentage of interaction regression coefficient and the total of the regression coefficient. The model was considered significant at p<0.05. In addition, the fitting was straightforward in terms of error, and accordingly, the lack of fit indicated non-significance (p>0.05). A high coefficient determination (R2) was required to obtain an adequate model. It was also followed by a low gap between adjusted R2 and R2. In addition, the model was validated by a leave-one-out technique to achieve the predicted R2. Therefore, the difference between predicted and adjusted R2 was not more than 20%.
Each run was prepared by mixing of each component based on the design points in the Table S2 (data set). The purified extracts were dissolved separately in ethanol to obtain an accurate predetermined concentration. The multi-component mixtures between plant extracts namely, binary, ternary, quaternary and quintenary mixtures, were prepared by mixing of the predetermined extract solution to obtain the desired concentration according to the design point proportion. The sample solution for each run was applied for further evaluations.
In-vitro antioxidant activity was assessed by a radical scavenging technique using DPPH and ABTS, given their reproducibility and feasibility.
All samples and combinations were dissolved and diluted using ethanol concentrations ranging from 0 to 200 μg/mL. A 1.0 mL sample was mixed with 1.0 mL DPPH (0.4 mM in methanol) and diluted with 3.0 mL of methanol. The reaction was incubated under ambient (25±1°C, RH 50±10%) and dark conditions for 30 min according to the optimized operating time. The control solution was a sample concentration of 0 μg/mL. The absorbance of the sample (As) and control solution (Ac) was recorded at 517 nm. The percentage of inhibition was calculated using Equation 2.
An ABTS assay was performed according to the described method after making minor modifications. The ABTS radical was achieved by reacting 7.0 mM ABTS solution with 2.45 mM potassium persulfate. The mixture was stored in a dark room overnight. A 0.5 mL sample (0-200 μg/mL) was added to 1.0 mL ABTS+ radical and incubated for 15 min in a dark room according to the optimized operating time. The control solution was prepared by mixing the ABTS+ radical 1.0 mL with 0.5 mL double distilled water. The absorbance of the sample (As) and control (Ac) was calculated using Equation 2.
The antioxidant profile was constructed according to the concentration and inhibition. The IC50 of each run was calculated according to the extrapolation of the inhibition of 50% to the curve by an appropriate statistical method and a 95% confidence level (p=0.05). Linear, log-linear, log non-linear, Weibull, non-linear and logistic models were fitted to the observed inhibition data. The best-fitting model was selected according to the best goodness of fit parameters (Sridhar and Charles 2019).
Prior to antioxidant evaluation, an extraction process was carried out. The extracts of the five traditional plants ranged from 2 to 6% of the dried weight. These results present the yield of the extraction process and indicate a relatively low yield due to the purification process to obtain higher antioxidant activity. The TPC of each extract was 10.13, 17.21, 1.03, 5.07, and 3.23 %w GAE for C. xanthorrhiza, C. longa, C. asiatica, P. niruri, and M. oleifera, respectively. In addition, the distribution of TPC in the extract combination is depicted in Figure 1. Due to abundant curcuminoid, C. longa had the highest phenolic content. In addition, C. xanthorriza had a high curcuminoid content. Meanwhile, the C. asiatica had the lowest phenolic content. Therefore, the TPC in the combination (Figure 1) increased gradually with the increment of C. longa or C. xanthorriza. However, C. asiatica and M. oleifera reduced the TPC content in the combination.
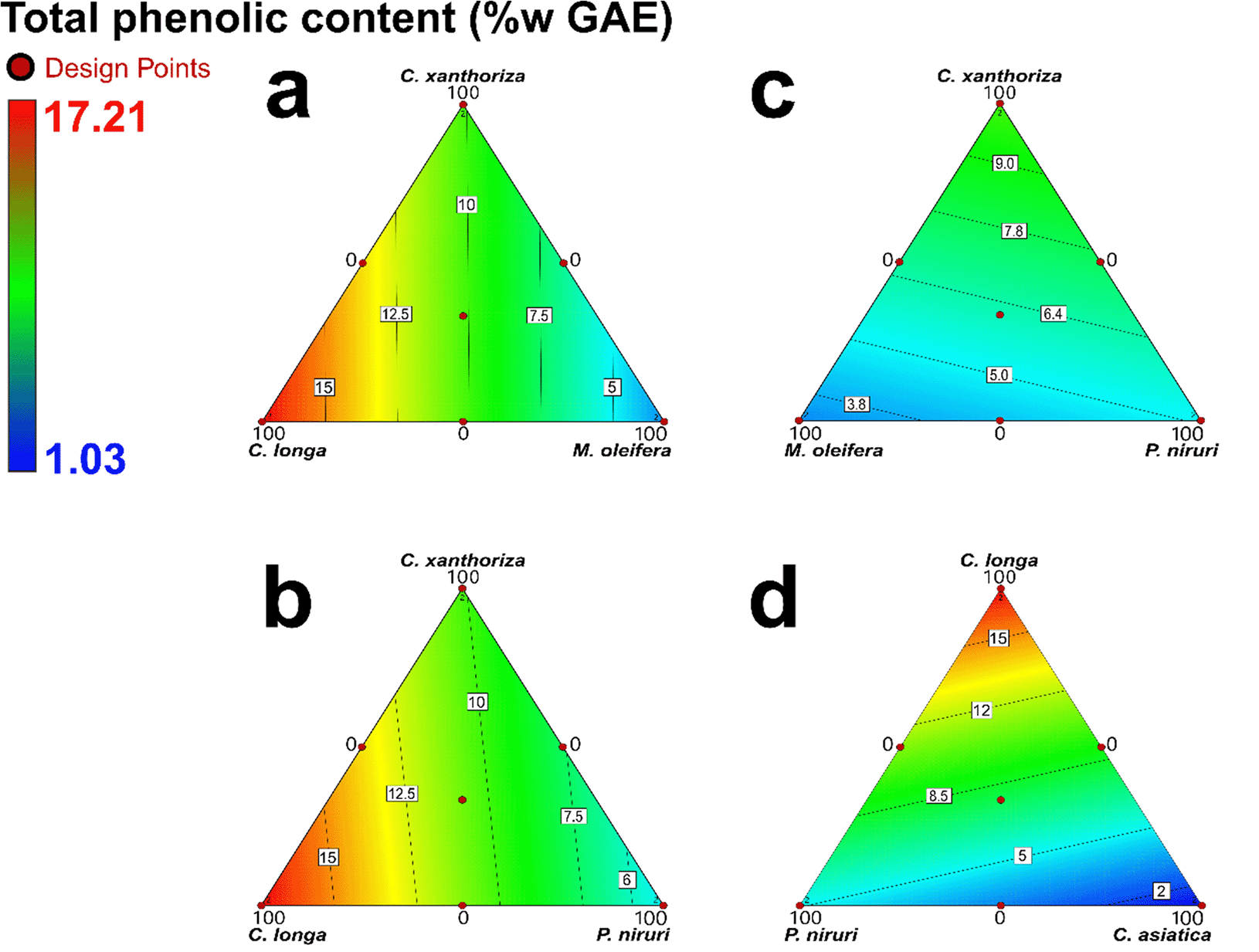
Each contour plot consists of three extracts combination and the others at 0%.
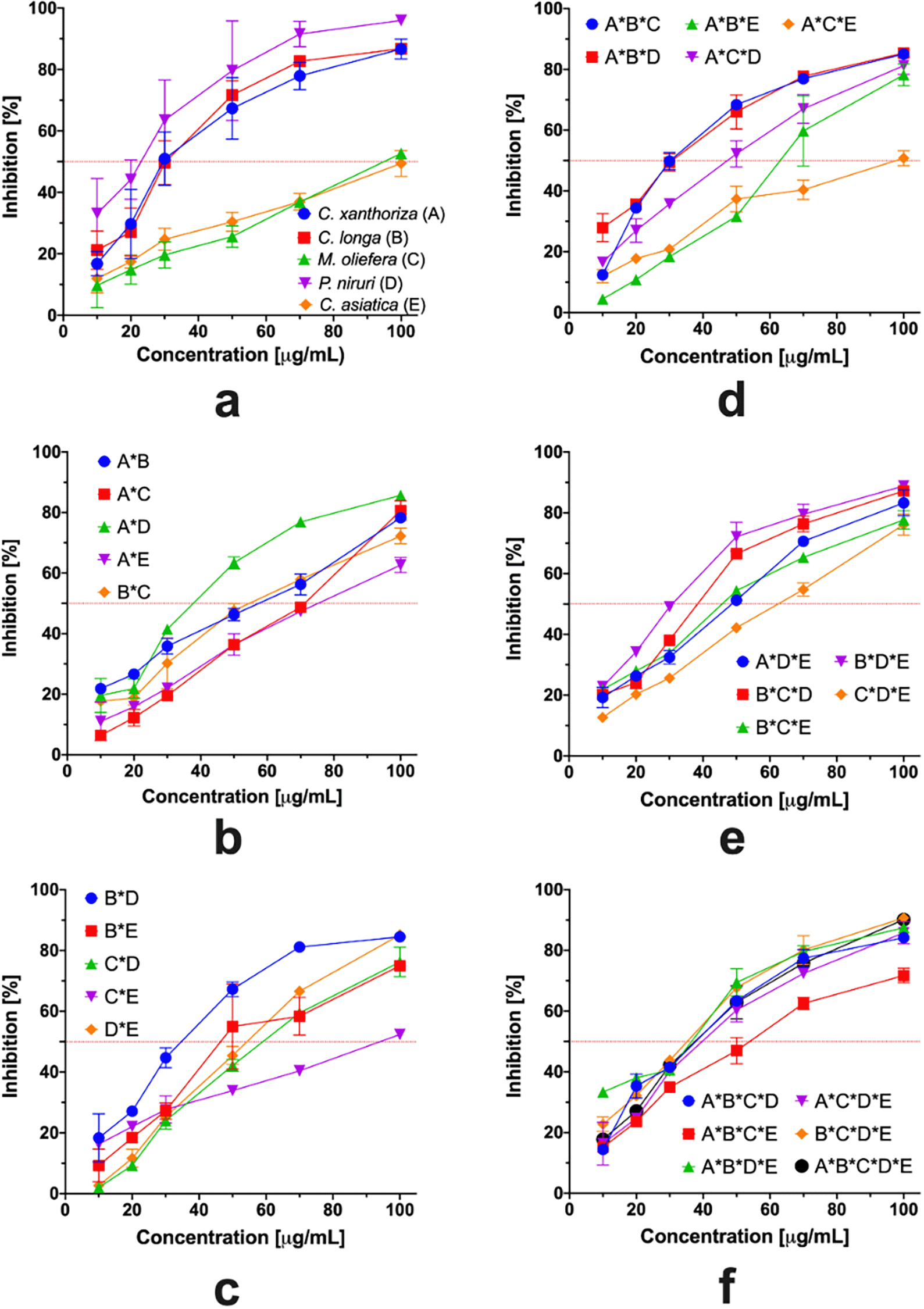
The radical scavenging profiles of DPPH are presented in Figure 2. A single component of the extract showed a different pattern of antioxidant profiles (Figure 2a). P. niruri had the best antioxidant activity and followed a similar pattern to that of C. xanthorrhiza and C. longa. A sigmoidal antioxidant profile was observed in these antioxidant profiles. However, a linear profile of antioxidants was observed in both M. oleifera and C. asiatica purified extracts. A linear pattern under 50% inhibition showed that both extracts had lower antioxidant activity than the previously mentioned extracts. The combination of two extracts altered all antioxidant profiles to be the linear model except for the interaction between P. niruri and C. xanthorrhiza or C. longa. The combination of three extracts was wholly altered to be the linear model. However, the model had a different IC50 value. The presence of P. niruri extract in the poly-combination had no significant effect on the IC50 value and produced similar patterns (Figure 2f). These results indicate that the presence of an interaction between the components in the extract altered the antioxidant profile and antioxidant capacity.
The antioxidant activity was expressed by Trolox equivalent (TE) per gram extract. The antioxidant capacity ranged from 0.30 to 1.29 mmol TE/g. To evaluate the main effect of and poly interaction among the five plants and their combination, the antioxidant capacity was fit to the MLRA equation. The antioxidant capacity using the DPPH assay was transformed into an inverse model to achieve a higher goodness of fit. Therefore, the response was also inversed along with the direction of value. The lower the response value, the higher the main effects and interactions. The model was significant (p<0.05), and the lack of fit test was not significant (p>0.05). The model showed that factors ~97% affected antioxidant capacity by the DPPH assay. Therefore, it has adequate power for supporting the main effect and interaction. According to the coefficient regression, P. niruri had the best antioxidant capacity. P. niruri, C. xanthorrhiza, C. longa, M. oleifera, and C. asiatica demonstrated the lowest to most potent antioxidant capacity.
Equation 3 shows the extracted plants' main effect, interaction, and poly-interaction. The main effect was significant (p<0.05), reducing the antioxidant capacity of M. oleifera and C. asiatica extract compared to the others. To quantify the main effect and interaction, their contribution was calculated, and it is presented in Figure 3. It also presents a different perspective regarding the main effect and interaction. The main effect was always positive in increase the antioxidant capacity. However, the lower the main effect, the better the contribution to the antioxidant capacity. Meanwhile, antagonism and synergistic effects showed positive and negative outcomes, respectively. Therefore, the interaction between C. xanthorrhiza and other extracts reduced the antioxidant capacity. In addition, other interactions increased the antioxidant capacity except for the interaction between C. longa and P. niruri. There was only one significant interaction (p<0.05) in improving the antioxidant capacity, i.e., the interaction between C. longa and C. asiatica. Both extracts could enhance the antioxidant capacity if they were combined, indicating a synergistic interaction. However, the other combinations showed no significant effects, altering the antioxidant capacity (p>0.05).
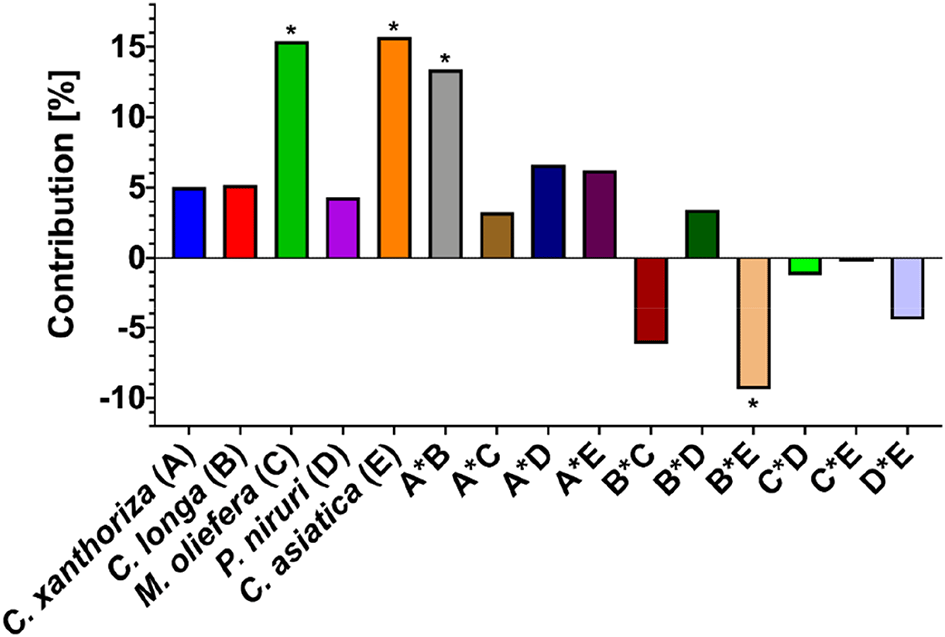
* = p<0.05.
By evaluating the contour plot (Figure 4), more specific information could be obtained regarding the interaction between the components. The highest antioxidant capacity was shown at a high proportion of P. niruri extract or binary component between C. longa and P. niruri. Meanwhile, the lowest antioxidant capacity was obtained at a high proportion of M. oleifera and C. asiatica. The interaction was depicted in parallel lines between each contour line. The interaction mainly occurred in the ternary mixture of each component. C. longa has the most significant effect on the interaction between the extracts.
Figure 5 presents the antioxidant profile determined using the ABTS assay. The single-component extract had a similar pattern to the DPPH assay. However, the gap between low and high potential antioxidant profiles was large. The IC50 value of all binary mixtures was 5–10 μg/mL, except for M. oleifera and C. asiatica binary mixture. In addition, the presence of both extracts reduced the antioxidant activity. All ternary extract combinations had similar patterns, and the IC50 was 5–13 μg/mL, except for the combination of M. oleifera, P. niruri, and C. asiatica. However, the quarternary mixtures and multi-component combination had nearly similar antioxidant profiles and activities.
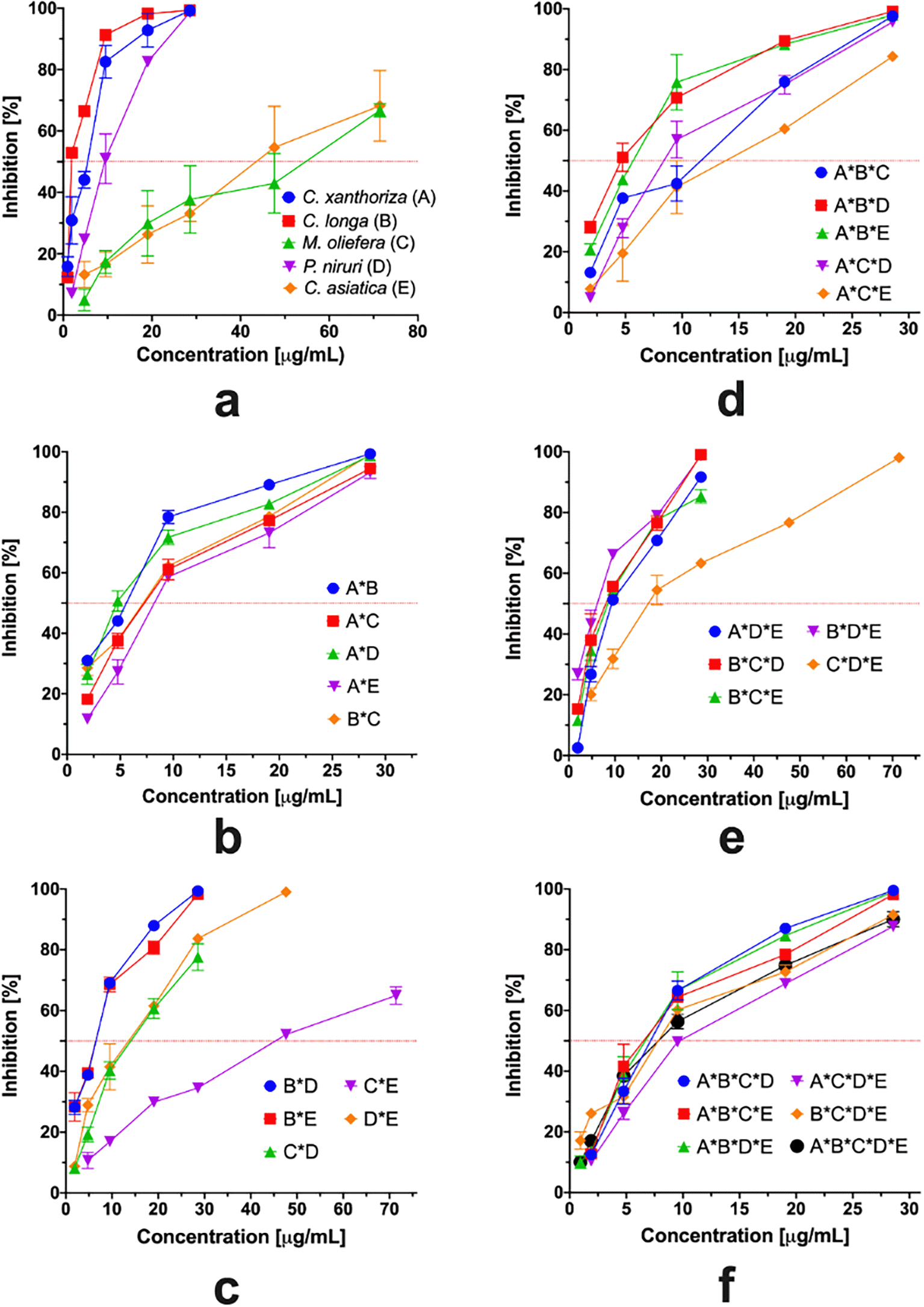
The antioxidant capacity of five plant extracts and combinations was also expressed using Trolox equivalent per gram extract. The antioxidant capacity of all combinations ranged from 0.18 to 3.71 mmol TE/g, suggesting that the antioxidant capacity using ABTS had more potential than when using the DPPH assay. To understand the effect of a single component and binary mixture of extracts, MLRA was applied to the antioxidant capacity using the ABTS assay. The data were fitted to a quadratic equation (Equation 4). By transforming the data to the square root model, a higher goodness of fit value could be achieved. In addition, the response was in a linear term along with the value. The model showed a significant effect on the response by 95.47% (p<0.05), and the lack of fit test was not significant (p>0.05); there was also a small gap between predicted R2 (0.8601) and adjusted R2 (0.9303).
According to Equation 4, C. longa had the highest antioxidant capacity, followed by C. xanthorrhiza, P. niruri, C. asiatica, and M. oleifera. The highest interaction was observed for the combination of C. longa and C. asiatica. The lowest interaction was achieved for the combination of C. longa and P. niruri. Based on the results of the coefficient regression in the model, the contribution of the main effect and interaction was calculated (Figure 6). Only C. xanthorrhiza, C. longa, and P. niruri significantly increased the antioxidant capacity (p<0.05).
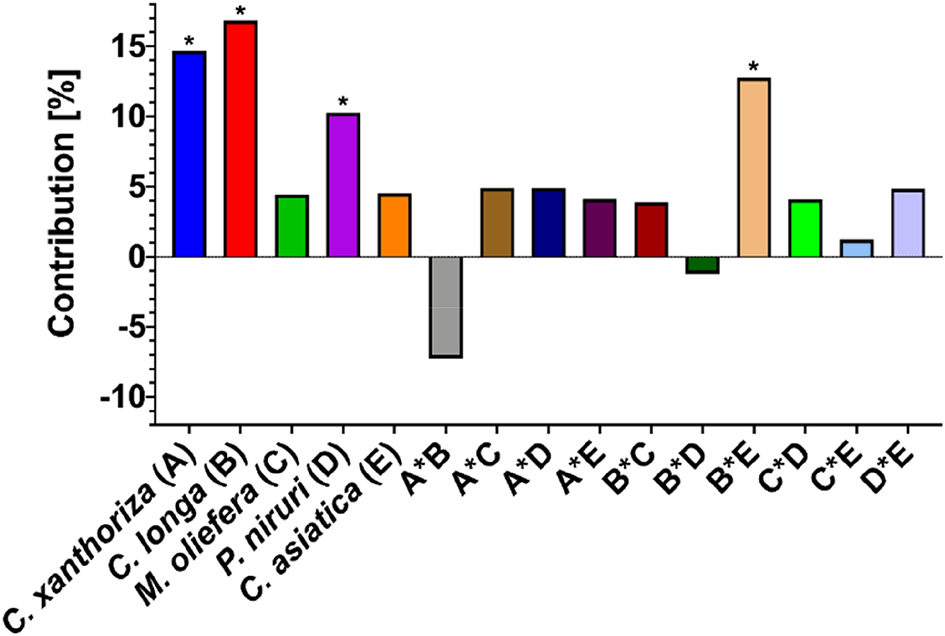
* = p<0.05.
Meanwhile, the interaction between C. longa and C. asiatica showed a significant synergistic mechanism (p<0.05). However, it should be considered that the interaction between C. xanthorrhiza and C. longa was antagonistic, which promoted a reduction in the antioxidant capacity. Contour plots are required to provide better insight into the interaction effects of the combination of extracts on antioxidant capacity (Figure 7) by monitoring the color pattern alteration. C. longa and M. oleifera showed the highest and the lowest antioxidant activity, respectively (Figure 7a). The poly-interaction between extracts was dominated by the presence of C. longa, P. niruri, and C. xanthorrhiza (Figure 7b). There was no interaction between the extracts in the presence of P. niruri, C. asiatica, and C. xanthorrhiza due to similar slopes and parallel and no intersection lines (Figure 7c). There was very little interaction between C. longa, C. asiatica, and M. oleifera (Figure 7d). In addition, there was no interaction between P. niruri, C. asiatica, and M. oleifera (Figure 7e).
The correlation between TPC and antioxidant activity is presented in Figure 8. The correlation depicts the relationship between phenolic compounds contained in the extract combination and the antioxidant activity. Figure 8a shows the correlation with antioxidant activity, analyzed using the DPPH assay. TPC and antioxidant activity had a medium correlation (r = 0.5502; 30.27%). Weak correlations were due to the non-linear effect, particularly for C. longa and P. niruri in single or high proportions in the extract combination. This suggests that the interaction between radical and phenolic compounds was mediated by the interaction between metabolite compounds in combinations. Meanwhile, the ABTS assay (Figure 8b) showed a strong correlation between phenolic compounds and antioxidant activity (r = 0.9094; 82.7%). However, the outlier data (outside the confidence interval of the regression model) may be attributed to a single or dominant proportion of C. longa and P. niruri extract. Both antioxidant assays proved a similar pattern in that the combination of extract had adequate correlation with antioxidant, but ABTS assays revealed a stronger correlation.
The purification process in this study was intended to obtain better antioxidant activity by eliminating ballast compounds. Better antioxidant activity was reported in low chlorophyll (Rajan et al. 2020) and resin (Wan et al. 2014) levels. Therefore, low yield of extraction was due to the absence of both ballast compounds. Moreover, it enhanced metabolic contents and antioxidant activity considerably.
Radical scavenging assays, DPPH and ABTS, were used to assess the antioxidant capacity of a combination of five traditional Indonesian plants. The reliability and stability of assay method mainly guided the selection of this assay and simple methods to assess the antioxidant activity (Mareček et al. 2017; Zhang, Yang, and Zhou 2018). Moreover, they are also applied extensively for more variation in plant sources (Sridhar and Charles 2019). The bioactive compound primarily determined the antioxidant activity in the extract. P. niruri contains a bioactive marker compound, phyllanthin, a lignin compound with potent antioxidant and immunomodulatory activities (Colpo et al. 2014; Naidu et al. 2004). Meanwhile, both C. longa and C. xanthorrhiza contain curcuminoid. It is also reported to have antioxidant and other pharmacological activities (Yang et al. 2020). The most potent antioxidant activity showed a sigmoidal curve. Thus, it assumed that the antioxidant profiles were covered from initial, inflection, and steady concentrations. It is a native characteristic of the interaction between radical and antioxidant molecules that involves radical stabilization. However, the linear pattern of antioxidants was observed in M. oleifera and C. asiatica due to the linear correlation between active compounds and radical scavenging activity. Both extracts contain major constituents, that is, flavonoid compounds, which governed the antioxidant activity (Zhang, Yang, and Zhou 2018). However, C. asiatica contains a specific biological marker, namely asiaticoside (Loc and Nhat 2013). The antioxidant profiles of binary, ternary, and poly-mixture were altered due to the contribution and interaction of the active compounds. Several studies have shown that the interaction of herbal mixture or poly-herbal mixture enhances antioxidant activity (Bhargavi and Madhan Shankar 2021; Salaj et al. 2021; Senol Deniz, Orhan, and Duman 2021). Therefore, the quantification of the interaction can be assessed by an experimental design model. Moreover, the requirement for model prediction should follow the best goodness of fit parameters (Choiri, Sulaiman, and Rohman 2020). Both antioxidant models had an adequate equation for predicting the response of the antioxidant activity. There was an antagonist interaction between C. xanthorrhiza and other plants due to the non-curcuminoid compound in C. xanthorrhiza. In addition, the antioxidant activity of C. xanthorrhiza was not a linear function, along with phenolics and flavonoids (Akter et al. 2019). Due to its containing sesquiterpene (e.g., xanthorrhizole and curcumene), the curcumin content was lower than C. longa (Losso et al. 2022; Awin et al. 2019). Therefore, it reduced the antioxidant activity when combined with other plants. However, another curcuminoid-contained plant, C. longa, had synergistic interaction. These data indicate that the non-curcuminoid compound in plants played a fundamental role in reducing the antioxidant effect. Curcumin in C. longa and C. xanthorrhiza has a scavenging ability, mainly through hydrogen atom transfer reactivity with •OH and •OOH radicals (Purushothaman et al. 2021). The synergistic interaction was affected by an interaction between specific components in both extracts. Future studies may explain this phenomenon more comprehensively.
The ABTS assay was also applied to assess the antioxidant activity of the five plants and their combination using the HET approach, which is similar to the DPPH assay. However, it differs from the DPPH assay. It uses a water-based solution for evaluating the antioxidant activity. The results indicated a different pattern of antioxidant activity, particularly at the gap between the low and high potential antioxidant profiles. As discussed above, the antioxidant properties of single-component and poly-combination were affected by the biological activity and interaction of components in the extract (Abbas et al. 2021). The interaction between C. longa and C. asiatica showed the synergistic effect through interaction between primary marker compounds—curcumin and asiaticoside. According to the results, C. longa made the most outstanding contribution to the antioxidant capacity. The use of a contour plot can be applied for a visual evaluation of the effect of each combination and interaction between two extracts simultaneously.
The correlation between the TPC and antioxidant activity involved the main responsibility of the antioxidant activity, particularly phenolic compounds (Cirak et al. 2022; Maya-Cano, Arango-Varela, and Santa-Gonzalez 2021). The major mechanism of radical scavenging involves the hydrogen electron transfer between radical ion and OH groups in phenolic compounds, followed by stabilization (Mareček et al. 2017; Schaich, Tian, and Xie 2015). Therefore, the higher the phenolic compound, the greater antioxidant activity, and thus, it had a linear correlation. However, a unique correlation between single extract and the poly-herbal combinations was observed. The outlier results (against the regression model) were observed particularly at single extract components or the dominant proportion of a particular extract. Meanwhile, the poly-combination showed a linear model and higher antioxidant activity. Hence, it was affected by a synergistic interaction between polyphenolic compounds or polyphenols with another compound in the extract (Cianciosi et al. 2022).
A combination of five traditional Indonesian plants was assessed using the simplex lattice design model. C. longa, P. niruri, and C. xanthorrhiza demonstrated more potent antioxidant activities than M. oleifera and C. asiatica. In addition, the phyto-components contained in C. longa governed the synergistic interaction during poly-herbal interaction. Meanwhile, non-curcuminoids in C. xanthorrhiza modulated the reduction of antioxidant activity during poly-herbal interaction. The polyphenol contents demonstrated a synergistic effect on the antioxidant activity. The synergistic interaction could be helpful to enhance antioxidant activity through multi-component interaction in the herbal combination.
Conceptualisation, S. Choiri, and A. Ainurofiq; methodology, N. Wiyono.; software, S.Choiri.; validation, All authors.; formal analysis, S. Choiri; investigation, R. Warni, N. Wiyono.; resources, N. Wiyono.; data curation, R. Warni; writing—original draft preparation, A. Ainurofiq.; writing—review and editing, S. Choiri; visualization, R. Warni; supervision, S. Choiri.; project administration, A. Ainurofiq.; funding acquisition, A. Ainurofiq.
OSF. Assessment of the synergistic effect of a poly-herbals combination on the antioxidant activity through a statistical approach. DOI: https://doi.org/10.17605/OSF.IO/2GFMN (Choiri, 2022).
This project contains the underlying data:
Data are available under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY Attribution 4.0).
The authors would like to thank the Ministry of Education, Culture, Research, and Technology, Republic of Indonesia & Universitas Sebelas Maret for funding and supporting this research.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: In pharmaceutical chemistry and bioorganic
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Analytical chemistry (chromatography); antioxidant properties of plant extracts
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Analytical chemistry (chromatography); antioxidant properties of plant extracts
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 20 Feb 23 |
read | read |
|
Version 1 16 Nov 22 |
read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)