Keywords
Cocoa pod husk; Simian retrovirus-2; MCF-7; HeLa; phytochemical profile
Cocoa pod husk; Simian retrovirus-2; MCF-7; HeLa; phytochemical profile
Theobroma cocoa pod husk, a cacao by-product, has intrigued many researchers to study its potential as natural medicine. A review article has highlighted its bioactivities as an anticarcinogenic, antibacterial, antiviral, antidiabetic, neuroprotective, and anti-inflammatory agent.1 This is due to the fact that T. cocoa pod husk is rich in flavonoids and polyphenols which have been associated with a series of medicinal properties of plant extracts.2–4 Several methods to extract the active phytoconstituents have been carried out previously, including those using supercritical CO2 and microwave assistance.5,6 A simple maceration technique has also been carried out using different solvents namely methanol:acetone,7 ethyl acetate,8 and n-hexane solvents.9 In our recently published work, we extracted the T. cocoa pod husk using distilled water and found its potential as a source for dietary antioxidants.10 Nonetheless, the research on methanolic extract of T. cacao pod husk has been scarcely reported. Some studies suggest the methanolic extraction could attract a wide-range of bioactive compounds.11–13
In previous research, T. cacao was found to be active in inhibiting the growth of Caco-2 colon cancer, HL-60 leukemia, SH-SY5Y neuroblastoma, MCF-7 breast cancer, HepG2 hepatoma, and Int-407 intestinal cancer cell lines.2 Polyphenols from T. cacao were responsible for the downregulation of transcription factors nuclear factor-κB (NF-κB) and activator protein-1 (AP-1) that are involved in oncogenic process.14,15 Under the same light, the phytoconstituents of T. cacao may also have a role in immunomodulation. In fact, these biological activities have been utilized to reactivate latent human immunodeficiency virus type 1 (HIV-1).16 Herein, we investigated the methanolic extract from T. cacao pod husk and its partitions for their anticancer and antiretroviral activities. We used MCF-7 breast cancer and HeLa cervical cell lines to investigate the anticancer activities as these cells have been used for anticancer screening of natural products.17–20 Simian retrovirus-2 (SRV-2)-infected A549 cell line was used to study the antiretroviral activity as this method has been developed to culture the Indonesian strain.21 The active extract sample was fractionated for in-vitro antioxidant and cytotoxicity screenings and further sub-fractionated for phytoconstituents analysis.
Materials used in this research included technical methanol, technical n-hexane, technical ethyl acetate, silica gel G60 F254, He gas, 2,2-diphenyl-1-picrylhydrazyl (DPPH) powder, ascorbic acid, pro-analytical methanol, gallic acid, and quercetin. Reagents used for the phytochemical screening included Folin-Ciocalteu reagent, Na2CO3 10%, AlCl3, and potassium acetate. Dulbecco’s Modified Eagle Medium (DMEM) from Gibco (Thermo Fisher Scientific, Waltham, MA, USA) was used for cell culture. In addition, NaHCO3, fetal bovine serum (FBS) (Invitrogen, USA), Penicillin-Streptomycin (Invitrogen, USA), trypsin (Gibco, Thermo Fisher Scientific, Waltham, MA, USA), trypan blue (Sigma, USA), PBS (phosphate buffer saline) (Gibco, Thermo Fisher Scientific, Waltham, MA, USA), dimethyl sulfoxide (Sigma, St. Louis, MO, USA), CO2 incubator, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) (Sigma, St. Louis, MO, USA), and pro-analytical ethanol (Merck, Germany) were also used. Kimwipes® tissue (Kimberly-Clark, Jawa Barat, Indonesia), Direct-zolTM RNA PreWash, RNA Wash Buffer, RNase-Free Water, primers SRV-2 5737 U19 and SRV-2 5943 L20, SSoFast Evagreen Mastermix (Bio-Rad Laboratories, Hercules, CA, USA), and nuclease-free water (NFW) were used for the real-time polymerase chain reaction (RT-PCR) protocol.
Theobroma cacao L. specimen was collected from Desa Lawe Loning, Lawe Sigala-Gala District, Aceh Tenggara Regency, Aceh Province, Indonesia on 20 August 2021. The plant specimen was identified at the Department of Biology, Universitas Syiah Kuala, Indonesia with a voucher number of 795/UN11.1.28.1/DT/2008. The cocoa pod husk was washed with distilled water, cut into small pieces, and air-dried at 25±1°C for 7—10 days before crushed into powder.
The bioindicators MCF-7 cell line, HeLa cell, A549 cell, and Simian retrovirus-2 (SRV-2) were obtained from the Pusat Studi Satwa Primata-Lembaga Penelitian dan Pengabdian pada Masyarakat, Institut Pertanian Bogor (PSSP LPPM-IPB), Indonesia.
Previously prepared cacao pod husk powder (5 kg) was macerated using methanol for 24 h, performed repeatedly until the filtrate became clear. The methanolic filtrate was concentrated via rotary evaporation to produce the methanolic extract which was then labeled as TCM. Thereafter, the TCM was partitioned sequentially using n-hexane and ethyl acetate, where each sample was labeled as TCH and TCEA, respectively.
MCF-7 and HeLa cancer cell lines (5x103 cells/well) were respectively grown in 96 well plate for 24 h (37°C; 5%CO2) with DMEM medium which had been priorly added with FBS 5% and Penicillin-Streptomycin 1% antibiotic. On the following day, each well was added with DMSO-dissolved extracts (TCM, TCH, and TCEA) with concentrations of 500, 250, 125, 62.5, and 31.25 μg/mL. The cell cultures were then incubated for 48 h before being added with 10 μL MTT and re-incubated for 4 h. Subsequently, as much as 100 μL ethanol 96% was added and optical density (OD) reading was carried out on a microplate reader (ELISA reader) at 595 nm. The procedure was performed in triplicate for each sample.
The concentration range for anti-SRV-2 studies was determined by MTT assay (as explained previously), but the non-infected A549 lung cancer cell line was used instead. For the antiretroviral study, SRV-2-infected A549 cell culture (50x103) was firstly sub-cultured in a 12-well plate and incubated for 24 h at 37°C, 5% CO2. Afterward, extract samples (2 mL) with a concentrations range that was non-cytotoxic against A549 cells (inhibition percentage < 50%) were added to each well (performed in duplicate). Cell receiving no treatment was taken as control. For the positive control, we used lamivudine at 25 μg/mL. Thereafter, the cells were incubated until day-7, where the supernatant was harvested on day-1, -3, -5, and -7. In each harvesting, the supernatant was taken as much as 500 μL, and into the cell culture, 500 μL sample solution was re-added. The harvested supernatant was stored at -80°C.
The viral RNA was extracted from the supernatant using QIAamp Viral RNA Mini Kits (Qiagen, Hilden, Germany) which was then reverse transcripted using SuperscriptTM III First-Strand Synthesis System for RT-PCR (Invitrogen) to obtain the viral cDNA. Primers used in the RT-PCR were SRV-2 5737U19 and SRV-2 5943L20 (collections of PSSP LPPM-IPB) which had been amplified with envelope gene gp70. As much as 1 μL of each primer was added to the master mix (PCR reaction mixture) containing 10 μL Sofast Evagreen Master Mix, 6 μL Nuclease Free Water dan 2 μL cDNA template. The PCR was performed in an iCycler Thermal cycler with iQ5 multicolor real time PCR detection system at 95°C for 30 seconds, followed by 40 cycles consisting of: denaturation (95°C; 30 second), annealing (47°C; 20 seconds), and DNA extension (72°C; 20 seconds). Standard used for the quantification of SRV-2 was SRV-2 plasmids with a concentration range of 101—106. This protocol was performed in duplicate, following the suggestion from a previously published study.22
Extracts with anticancer and antiretroviral activities were further isolated using gravitational column chromatography with silica gel G60 F254. The extract was treated with gradient elution starting with non-polar solvent n-hexane (100%) up to semi-polar solvent ethyl acetate:methanol (1:1). Fractions with the similar patterns from the thin layer chromatography were combined into subfraction, and then tested for antioxidant and cytotoxic activities.
The subfraction was re-chromatographed using a column with silica gel G60 F254 based on gravitational force. The obtained subfraction was tested on thin layer chromatography and sprayed with vanillin sulfate and FeCl3 5% to identify the polyphenol contents. Subfraction with the most dominant polyphenol content was investigated on gas chromatography-mass spectroscopy (GC-MS) for phytoconstituents profiling.
Antioxidant activities were based on the inhibition of DPPH radical (molecular weight=394.32 g/mol). As much as 11.82 mg DPPH powder was dissolved in methanol using 75-mL volumetric flask. The extract sample and positive control (ascorbic acid) were prepared in concentrations of 3, 6, 9, 12, and 15 μg/mL. Into each solution, DPPH 0.4 mM was drop-wised (1 mL), and the volume was increased with methanol until it reached 5 mL. The container was then sealed with aluminum foil to avoid the light exposure. Thereafter, the mixture was homogenized with vortex mixer and incubated for 30 minutes at 37°C. The mixture solution was measured for its absorbance at 517 nm using UV-vis spectrophotometer.
The cytotoxicity was determined by brine shrimp lethality test (BSLT) using Artemia salina larvae. The larvae (±50-100 mg) were firstly incubated in a covered container with saline water for 48 h. On the other hand, the extract sample (30 mg) was diluted with 3 drops of DMSO 5% and added with 30 mL saline water, and subsequently sonicated until homogenous. The concentrations of the prepared extract sample ranged from 1000 to 1 μg/mL, and a combination of saline water and DMSO 5% was taken as the negative control. A glass container was filled with the prepared extract sample and then added with 10 brine shrimps before incubated (24 h; room temperature). Dead brine shrimps were then counted in each glass container.
TCH, TCEA, and TCM were screened for their anticancer activities against MCF-7 breast cancer and HeLa cervical cancer cell lines, where the results have been presented in Figure 1. At 31.25 μg/mL, TCH has a higher inhibition than TCEA (33.29% versus 18.93%). Meanwhile, no inhibition was observed in sample treated with TCM 31.25—62.5 μg/mL until its concentration increased to 125 μg/mL (inhibition=28.35%). Interestingly, at 250 μg/mL, TCM appeared to yield the highest inhibition (97.62%) as compared to TCEA (95.66%) and TCH (93.85%). Concentration-dependent increment of the MCF-7 inhibitions were observed when the extract concentrations ranged from 31.25 to 250 μg/mL. Hence, the IC50 values were calculated based on the polynomial regression on the concentration range of 31.25—250 μg/mL (Table 1). The calculation of the IC50 against MCF-7 revealed TCH as the most potent extract (IC50=45.36 μg/mL) among others (IC50=53.91 and 161.53 μg/mL for TCEA and TCM, respectively) (Table 1).
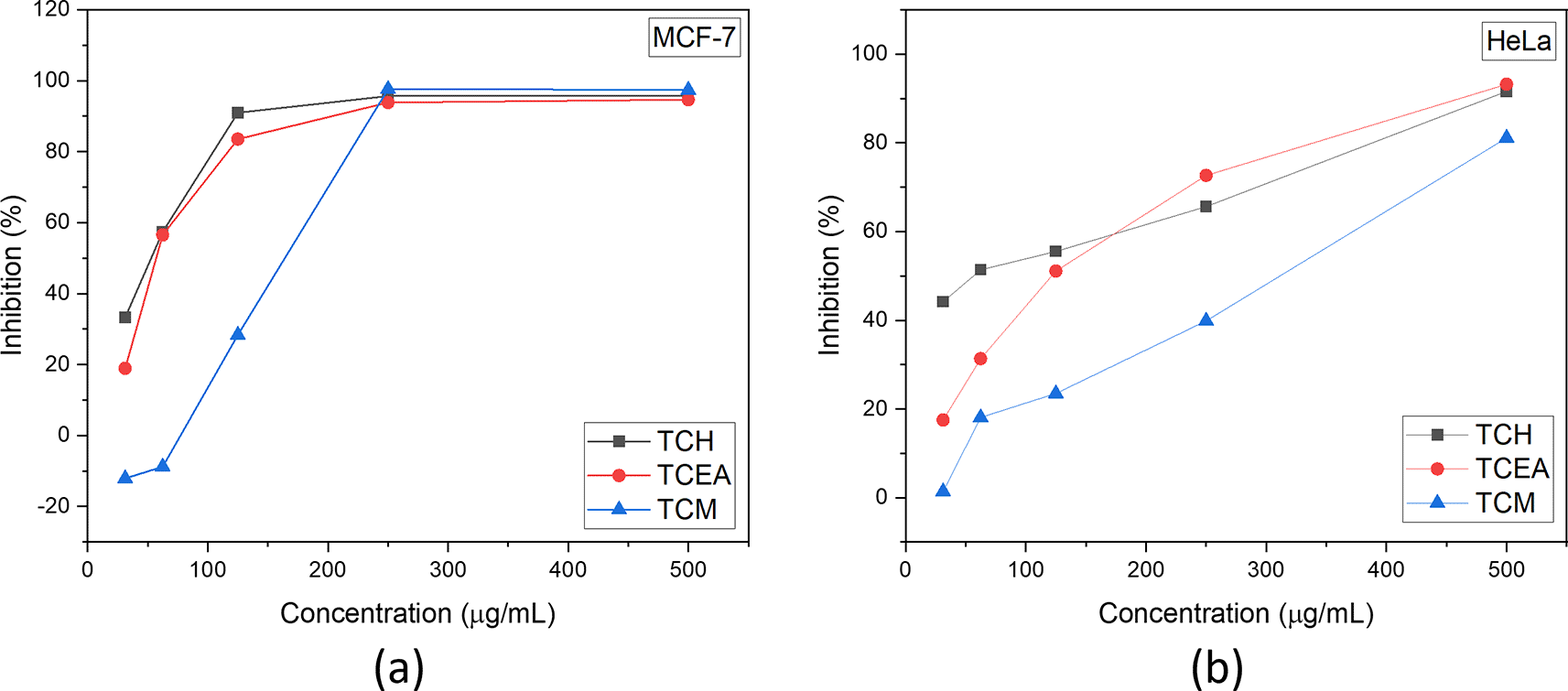
A similar trend of anticancer activities was also observed for the HeLa cancer cell line, where highest to the lowest, the order was as follows: TCH, TCEA, and TCM with inhibition percentages of 44.15, 17.5 and 1.39%, respectively. For TCH and TCM, the increase of concentration from 31.25 to 62.5 caused a rapid elevation of inhibition. As for TCEA, the rapid increasing trend of inhibition percentage was observed until 125 μg/mL. Due to this nature of the curves, we employed polynomial regression for the IC50 calculation so that R2>0.90 could be obtained (Table 1). The lowest IC50 was obtained by TCH (82.44 μg/mL). Meanwhile, TCEA and TCM have IC50 against HeLa cervical cancer cell line of 120.71 and 272.58 μg/mL, respectively.
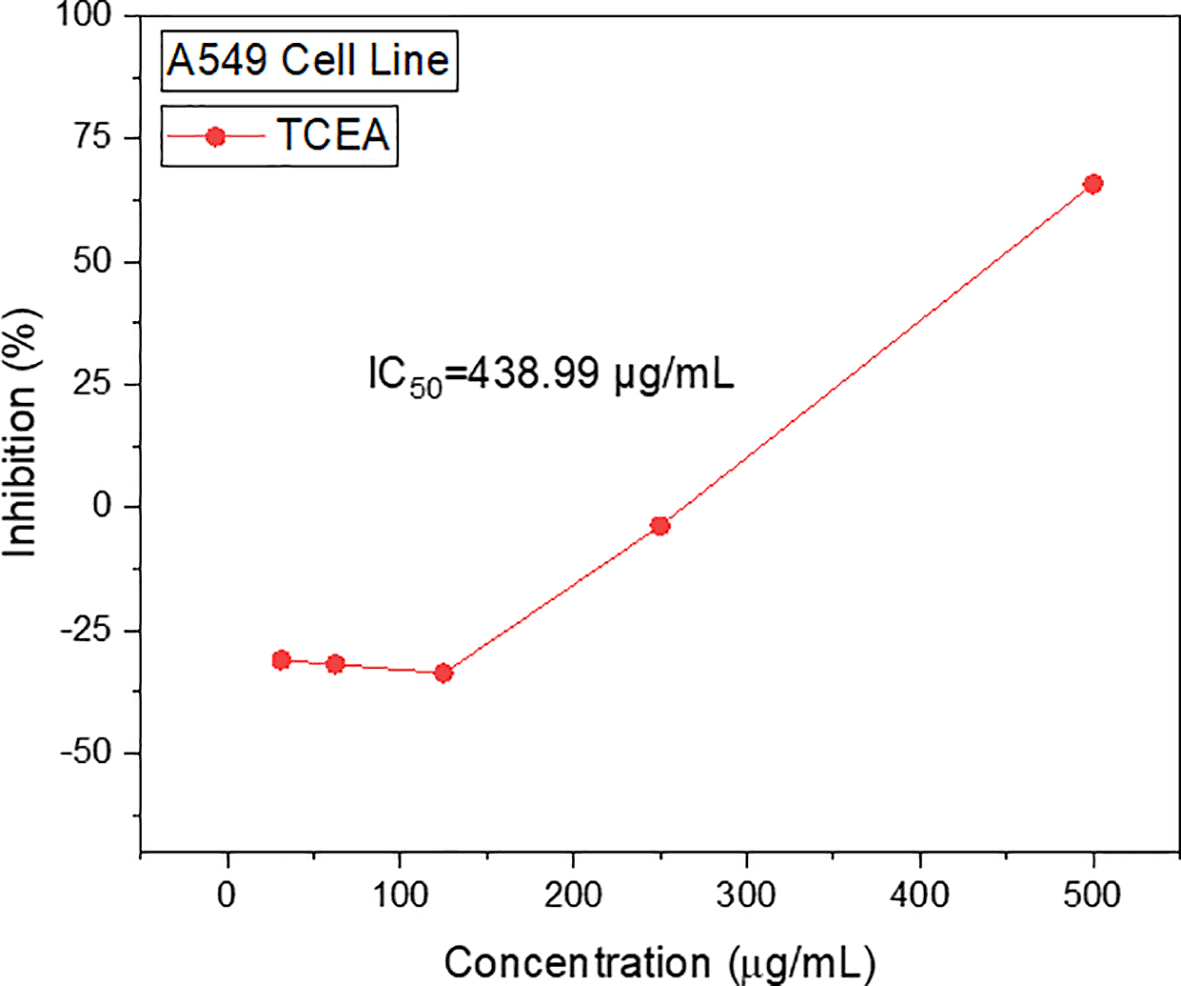
Screening of anticancer activities revealed that TCEA and TCH fractions had higher activities as compared to TCM. Herein, we tested the antiretroviral activity of TCEA and further continued to analyze its subfractions along with antioxidant, cytotoxicity, and phytochemical screenings. The investigation on TCH fraction is currently being carried out and will be published separately later. For the antiretroviral investigation, we calculate the non-cytotoxic concentration of TCEA against A549 cell line since it was used for the viral replication (Figure 2). From the study, we obtained 438.99 μg/mL as the IC50 value which was then set as the cytotoxic concentration.
The antiretroviral activity of TCEA against SRV-2 with concentration range of 31.25—125 μg/mL has been presented in Table 2. On Day-1 the SRV-2-infected cells receiving TCEA 125 μg/mL and positive control experienced replication inhibitions, but not in TCEA 62.5 and 31.25 μg/mL groups. Viral replications of lower than that of the negative control (indicating the inhibitory activity) were significantly observed in TCEA 62.5 and 31.25 μg/mL only after 5 days of incubation. TCEA with a concentration of 125 μg/mL was considered as having more potent antiretroviral activity compared to lamivudine (positive control).
As many as 581 fractions were obtained from the column chromatography of TCEA, where those with the same stain patterns were combined one into a single subfraction. As many as 9 subfractions were obtained from the procedure and each of the subfractions was labeled with TCEA 1 to 9. The nine subfractions were analyzed with thin layer chromatography, and the results have been presented in Figure 3. Observation using vanillin sulfate revealed that the TCEA 1—4, TCEA 4—7, and TCEA 7—9 were predominated by terpenoids (purplish blue), flavonoids (yellow), and tannins (brownish color), respectively. Meanwhile, the FeCl3 5% confirmed the presence of polyphenols in all samples as indicated by the dark green color.
Antioxidant activities of TCEA 1—9 based on the radical DPPH inhibition have been presented in Figure 4. TCEA was found to have the weakest antioxidant power with IC50 of the DPPH inhibition only reaching 137.511 μg/mL. TCEA 6 has the lowest 7.64 IC50 of 6.49 μg/mL, hence the most potent antioxidant among others. TCEA 5 was only seconds after TCEA 6 with IC50 of 7.64 μg/mL. It is worth noting that both TCEA 5 and 6 were obtained from column chromatography using n-hexane:ethyl acetate (3:7) eluent. Meanwhile, as a comparison, the IC50 obtained from the ascorbic acid was almost twice lower than TCEA 6 (3.25 μg/mL versus 6.49 μg/mL).
The cytotoxicity profiles of TCEA 1—9 based on the brine shrimp mortality are presented in Table 3. TCEA 8, 9, and 1 had the lowest LC50 with the values being 411, 394, and 252, respectively. Similar to the antioxidant test, we found TCEA 5 and 6 being the two most cytotoxic subfractions (LC50=5 and 2 μg/mL, respectively). The lethal cytotoxicity of these two subfractions could be observed even when the concentration was as low as 1 μg/mL (3 and 4% mortality for TCEA 5 and 6, respectively). TCEA 6 reached 100% mortality when the concentration was 500 μg/mL. Meanwhile, the same level of mortality was reached by TCEA 5 when its concentration was 1000 μg/mL. Other than TCEA 5 and 6, none of the subfractions could reach 100% mortality even when the concentration was as high as 1000 μg/mL.
Due to its high antioxidant and cytotoxicity activities, TCEA 6 was selected for re-chromatography to obtain sample with higher purity. The re-chromatography was carried out using gradient elution of n-hexane and ethyl acetate combination resulting in another four subfractions (TCEA 6.1—6.4). The phytoconsituents of each subfraction were qualitatively analyzed using thin layer chromatography as presented in Figure 5. When sprayed using vanillin sulfate, the presence of flavonoids (yellow) was observed and more pronounced in TCEA 6.3 and 6.4. It was confirmed by FeCl3 5% test, where a dark green color was observed indicating the high content of polyphenols. We were interested in further analyzing TCEA 6.3 through GC-MS for phytoconstituents profiling due its green color being the most intense among others.
A list of phytoconsituents identified by the GC-MS analysis from sample TCEA 6.3 has been presented in Table 4. As many as 39 compounds were identified from the 40 peaks in the chromatogram where benzoic acid, 4-hydroxy-3,5-dimethoxy- has the widest peak area (21.56%). Lupeol was identified twice, assigned for peaks 4 and 5, but the former had lower similarity than the latter (75.3 and 93.9%). Bioactive compounds which had been recognized for their anticancer and/or antioxidant activities were identified, namely lupeol, syringaresinol, catechol, and squalene. Among the four aforementioned compounds, catechol had the highest quantity (peak area = 9.29%) with similarity over 93.9%.
Based on the IC50 obtained herein, TCH was moderately active against MCF-7 and HeLa cancer line, whilst TCEA was only moderately active against MCF-7. Moderate activity could be ascribed to the presence of non-anticancer compounds in the extract, as suggested by a previous study investigating Calotropis gigantea extract against P388 leukemia cell lines.23 In our previous study, a moderate anticancer activity against MCF-7 cell lines was also obtained from n-hexane extract of Myristica fragrans Houtt roots, where a lignan compound was isolated and identified.20 The anticancer activity increased when the analysis was carried out on a fraction of the foregoing extract,19 indicating the activity is dependent on the bioactive compound concentrations. This is also the reason why TCEA and TCH were more active against the cancer cell lines than TCM because TCEA and TCH had higher purities as they were obtained from the fractionation of TCM.
In the case of T. cacao, its anticancer activities have been anticipated by researchers due to its rich content of dietary polyphenols.2 A review article has summarized these phenolic compounds that include catechin, 3′-O-methyl epicatechin, epicatechin, procyanidin B2, and polymer procyanidins.3 Our data is in an agreement with that from a previously published study, where the methanolic extract from T. cacao pod husk had lower IC50 on MCF-7 as compared to HeLa cell lines.24 Previously, BSLT cytotoxic activities of n-hexane extract from T. cacao pod husk have been documented to yield LC50<10 μg/mL.9
In this present study, the TCEA had a significantly higher inhibitory effect even when compared to lamivudine. Previous studies have reported that the NaOH extract from T. cacao pod husk mainly worked as antiviral by preventing the infection of human immunodeficiency virus (HIV) to the host immune cell including the syncytium formation.25 The lignin-carbohydrate complex derived from NaOH extraction in combination with acid precipitation of T. cacao pod husk had anti-HIV activity and immunomodulating activities.26 Moreover, the flavonoid compound from T. cacao could help the efficacy of antiretroviral therapy through the reactivation of latent HIV in human T-cells which was found correlated to NF-κB and MAPK signaling pathways.16 A review on the antiviral potential of T. cacao pod husk highlighted the inhibitory activity of its lignin fractions against cytopathic effects induced by influenza virus, in which this action could be synergized with ascorbic acid.1
Following the investigation of the anticancer and antiretroviral activities, the TCEA was fractionated producing TCEA 1—9 fractions. The TCEA was revealed as the most potent antioxidant (IC50=6.49 μg/mL) and cytotoxic (LC50=2 μg/mL) fraction among others. This value is lower in comparison to that obtained from our previous study using aqueous extraction, where the highest antioxidant was IC50=9.61 μg/mL and the highest cytotoxicity – 74 LC50=μg/mL.10 However, more cytotoxically active compounds were obtained in the n-hexane extract of T. cacao pod husk with LC50 as low as 0.29 μg/mL.9 The bioactivities of T. cacao pod husk could be associated with the phenolic compound contained in the extract as suggested previously.10
Further purification of fraction TCEA 6 in this present study produced 4 subfractions (TCEA6.1—6.4), with TCEA 6.3 being the most predominated with polyphenol (based on FeCl3 5% test). The GC-MS analysis on subfraction TCEA 6.3 revealed its phytoconsituents consisted of bioactive compounds lupeol, syringaresinol, catechol, and squalene among many other compounds. The structures of the foregoing compounds are presented in Figure 6. Lupeol itself has been named as a novel anti-inflammatory and anti-cancer agent by a review article in 2009.27 Interestingly, lupeol has been found to specifically attack cell cancer in which a study reported its high toxicity against 451Lu and WM35 melanoma cells but not in normal human melanocyte cells.28 As an antiviral, lupeol has been found active against herpes simplex virus type 1 (HSV-1) and even its acyclovir-resistant strains.29 Syringaresinol isolated from Rhus javanica var. roxburghiana could inhibit the replication of tobacco mosaic virus.30 The ability of syringaresinol to induce cancer cell death has been documented in a study using HL-60 leukemia cells.31 However, a recent study revealed that syringaresinol was not cytotoxic towards HepG2 and HT29 cells.32
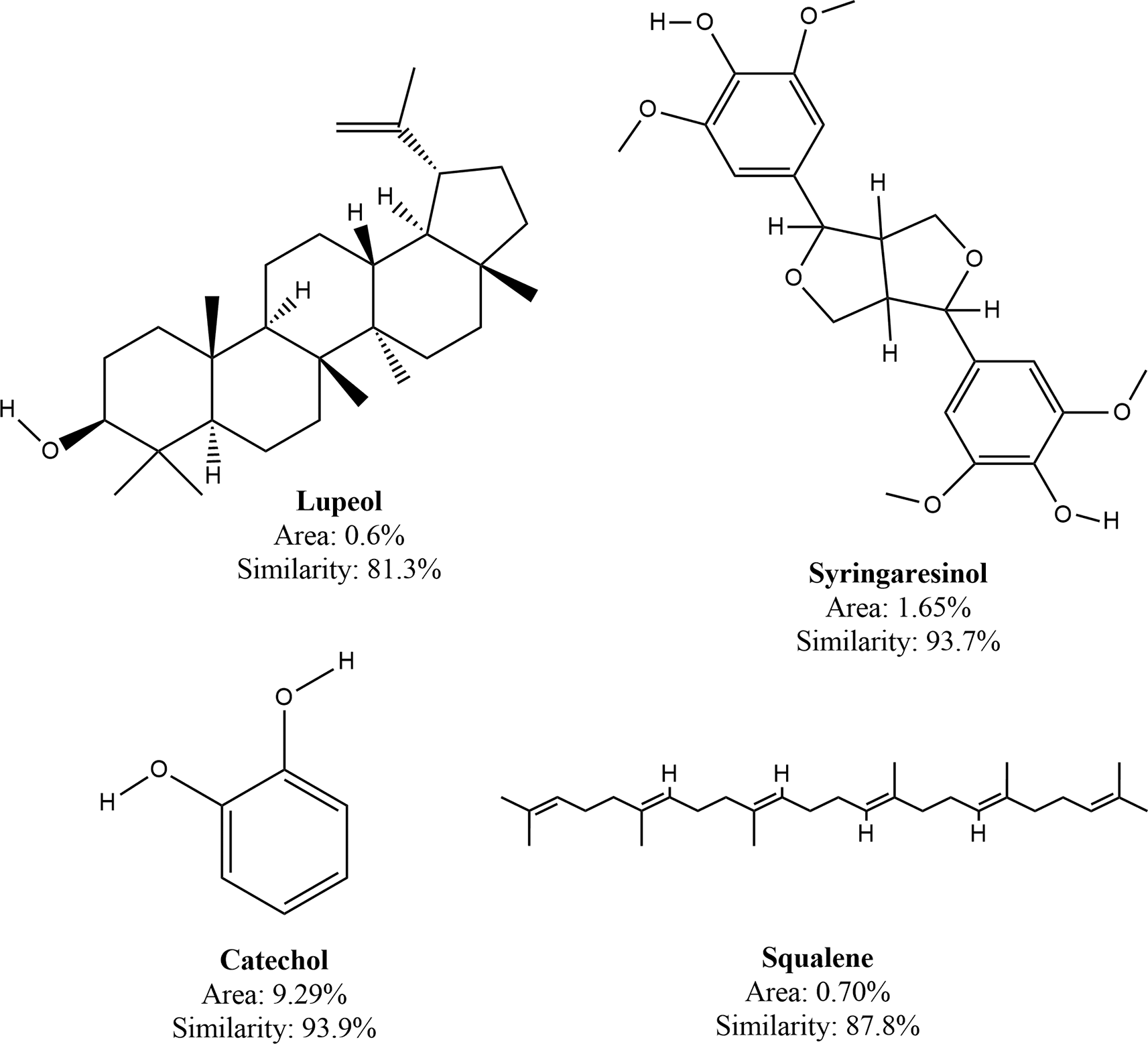
Catechol and its derivatives have been recognized as broad-spectrum antiviral and anticancer agents.33,34 A study suggested that the antiviral and immunomodulating activities of catechol are dependent on the structure and position of the substituent group located on its aromatic skeleton.35 A reputable natural antioxidant, squalene has long been associated with anticancer activities especially in the case of the absence of cancer in sharks.36 Squalene has been witnessed to not only increase the toxicity of several commercial anti-cancers but also provide cytoprotective effect against normal cells.37 A recent report revealed that intravenous squalene BID could significantly improve COVID-19 conditions without any adverse effect.38,39 Taken altogether, the foregoing phytocompounds contained in TCEA 6.3 had a potential to act as anti-cancer and anti-retroviral agent.
TCEA derived from the methanolic extract of T. cacao pod husk had moderate activity against MCF-7 cells and weak activity against HeLa cells. Antiretroviral study suggests that TCEA 125 μg/mL had higher inhibitory activity against SRV-2 replication as compared to lamivudine 25 μg/mL. Further fractionation using gravitational column chromatography yielded TCEA fractions with relatively high DPPH antioxidant and BSLT cytotoxic activities. The phytoconsituents of the subfraction revealed the presence of lupeol, syringaresinol, catechol, and squalene which have been reported previously as anti-cancer and anti-viral. The investigation on TCH for its anti-cancer and anti-retroviral activities is warranted.
Conceptualization, M.Y. and B.G.; methodology, N.S.; validation, N.S.; formal analysis, M.Y.; investigation, B.G.; resources, M.Y.; data curation, B.G.; writing—original draft preparation, M.Y.; writing—review and editing, B.G. and N.S.; visualization, N.S.; supervision, M.Y.; project administration, B.G.; funding acquisition, M.Y. All authors have read and agreed to the published version of the manuscript.
figshare: ‘Anticancer and Antiretroviral Activities of Methanolic Extract from Theobroma cacao L Pod Husk: Focusing on the Ethyl Acetate Partition’. https://doi.org/10.6084/m9.figshare.21483096.v4 40
This project contains the following underlying data:
- 1. MTT Test Design IC50 Calculation.xls [Table containing the raw data of the study].
- 2. OD Value of MCF-7, HeLa dan A549 Cells MTT Test.xlsx
- 3. Bar and Line Graphs represented MCF-7, HeLa & A549 Cells.xlsx
- 4. Data qRT PCR SRV.xlsx
- 5. TCEA Antioxidant Subfraction.xlsx
- 6. TCEA Cytotoxic Subfraction.xlsx
- 7. Supplementary Figures.zip [file containing supplementary figures of the study].
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
The authors appreciate the assistance from the Head of Lembaga Penelitian dan Pengabdian Kepada Masyarakat, Universitas Syiah Kuala and the Dean of Faculty of Mathematics and Natural Sciences, Universitas Syiah Kuala. A special acknowledgment is granted to Reka Nurmania, Ayu Rahmadani who, at the moment of this manuscript preparation, were the students of the Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Syiah Kuala.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
No
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Biomass valorisation, Bioactive extraction, Microwave extraction
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Phytotherapeutics, Clinical Biolchemistry, Medical Genetics
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 28 Nov 22 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)