Keywords
PCOS, LH-FSH ratio, total testosterone, Hyperandrogenemia, Sudan
PCOS, LH-FSH ratio, total testosterone, Hyperandrogenemia, Sudan
Polycystic ovary syndrome (PCOS) is a prevalent endocrine condition affecting women of reproductive age, with a reported frequency of 6 to 15%.1,2 PCOS is characterized by a female sex hormone imbalance and increased androgen production, which results in irregular or extended menstrual periods, obesity, and excessive hair growth.3 Genetic and epigenetic factors, as well as environmental variables have been identified as risk factors for intra-ovarian hyperandrogenism.3 PCOS is a challenging disorder to diagnose since it is a diverse condition with different characteristics. It is currently diagnosed using revised Rotterdam criteria, which has been recently approved by an international evidence-based PCOS guideline.4,5
According to certain studies, the brain is both a regulator and an impacted organ in PCOS (the brain phenotype). The brain, which contains multiple receptors, and neurons with their neurotransmitters, produces a higher pulse frequency of gonadotropin. As a result, people with PCOS have more luteinizing hormone (LH) than follicle-stimulating hormone (FSH) secretion.6 Increased ovarian androgen production is caused by a change in the LH-FSH ratio. Furthermore, hyperandrogenemia lowers estrogen's negative feedback to the hypothalamus, resulting in increased LH pulse frequency. As a result, a vicious cycle is set in motion, causing a variety of clinical symptoms of PCOS, including hyperandrogenism.7 The goal of this study was to see possible link between the LH-FSH ratio and total testosterone levels in Sudanese women with PCOS.
This was an observational study with a cross-sectional design. The study was conducted between October 2020 and September 2021 in Khartoum- Sudan. Various infertility centers clinics were visited to select the study sample, using a convenience sampling method. The inclusion criteria were women with confirmed PCOS based on Rotterdam criteria,5 where at least two of the following criteria were fulfilled: oligomenorrhoea/anovulation (delayed menses >35 days or <8 spontaneous hemorrhagic episodes/year), hyperandrogenism (clinical hirsutism using modified Ferriman-Gallwey score of ≥8 or biochemical) and morphology of polycystic ovaries on ultrasonography (12 or more follicles in each ovary measuring 2 to 9 mm in diameter, and/or increased ovarian volume>10 mL3). Women with systemic disease (cardiovascular disease and diabetes mellitus), on medication prior to the study (oral contraceptives, glucocorticoids, ovulation induction agents, and estrogenic or anti-androgenic) were excluded from the study.4,5
At the time of the study, there was no published data about the prevalence of PCOS in Sudan. For this, the prevalence of PCOS in unspecified populations 3–10%.8 The sample size was determined based on the following equation9:
where:n = sample size
Z1-α/2 = 1.96 for 95% confidence level
d = Desired margin of error, expressed as a decimal (0.05)
P = Prevalence of the disease (10.0%)
n = 138
The study protocol was approved by the Federal Ministry of Health at Khartoum- Sudan. This study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration. The study objective was explained to potential participants, and those who agreed to participate provided a signed consent before the start of the study.
After signing an informed consent form, the socio-demographic characteristics, medical and gynecological history were taken from each patient using a questionnaire. The detailed medical and gynecological history (menstrual pattern, fertility, hirsutism) were taken from all patients included in the study. Then full general and pelvic examinations were performed.
Following standard protocols, weight was measured twice. After calibration, OMRON BF508l Body Fat Scales (China) were utilized. Women were told to remove their bulky attire. The weights were calculated to within 0.1 kg. After calibration, ladies were requested to remove their shoes and a portable stadiometer (SECA-213 model, Germany) was used to measure their height twice. Quetelet's BMI was estimated using a conventional formula (weight in kilograms divided by height in meters2). Underweight (<18.5 kg/m2), normal (18.5-24.9 kg/m2), overweight (25.0-29.9 kg/m2), and obese (30 kg/m2) were the BMI categories used by the WHO.10 During the follicular phase, ultrasonography was performed by Mindray (model: DP-50, Germany) and vaginal transducer where presence or absence of ovarian cysts were conformed or excluded. If cysts were seen, then the volume as well as the number of small follicles were determined, in each ovary.
Women were asked to come back on days 2-5 after a spontaneous menstruation or on a convenient day if they had amenorrhea. In a fasting state, 5 mL venous blood was collected between the hours of 8 a.m. and 9 a.m. The blood was centrifuged using Hettich (D-78532 Tuttlingen: Germany), then plasma obtained, and kept at -20 °C until the assay. Enzymes linked immunosorbent assay (ELISA); ASYS (model: Expert Plus; type G020150; serial nr: 28382; Austria) was used to quantify serum LH, FSH by indirect method, and TT competitive methods.
Data was coded, and statistical analysis was performed with social package of statistical science version 26.0 (SPSS Inc., IBM, Chicago, IL, USA). The Kolmogorov–Smirnov was used for testing the normality of continuous data. All data was skewed. Continuous variables were presented as mean with standard deviation, and median with interquartile ranges. Whereas qualitative data was expressed with frequency (%). Mann-Whitney U test, and Chi-square test for qualitative variables were used to assess the relationship between LH-FSH ratio groups and androgen status. The association between the LH-FSH ratio, serum TT, and BMI was tested using Spearman's correlation test. The link between the LH-FSH ratio>1 and other factors was investigated using binary logistic regression analysis. The serum LH-FSH ratio was studied using a receiver-operating characteristic (ROC) curve to see if it might distinguish androgen status.
In total, 350 Women were screened; 300 of them fulfilled the inclusion criteria.11 Their mean (SD) age was 29.1 (5.8) years, and BMI 27.9 (4.6) kg/m2. Overall; 30.3% (n=91) of women were obese based on their BMI, and 59.0% (n=117) had positive family history to PCOS. More than half (52.3%, n=157) of women had menstrual cycle irregularity. More than two-thirds of them (71.0%, n= 213) had altered LH-FSH ratio with cut-off > 1.0. According to their serum TT (> 109.5 ng/dL), 58.3% (n= 175) had hyperandrogenemia.
Women with LH- FSH ratio >1 (based on their serum LH-FSH ratio) had significantly increased serum TT level (P = 0.000), and 70.0% of them had TT> 109.5 ng/dL. 55.4% (n= 118) of them had positive family history to PCOS, compared with counterpart. There were no significant differences in age, BMI, and menstrual cycle frequency irregularity between groups (Table 1).
| Variables | LH-FSH ratio (cut-off > 1.0) | Total | P- value | |
|---|---|---|---|---|
| Normal (n=87.0) | High (n=213.0) | |||
| Age/year | ||||
| Mean (SD)# | 29.5 (5.9) | 28.9 (5.9) | 29.1 (5.9) | 0.538* |
| Median (SEM)† | 29.0 (0.63) | 29.0 (0.40) | 29.0 (0.34) | |
| Q1-Q3 | 25.0-34.0 | 25.0-33.0 | 25.0-33.0 | |
| BMI kg/m2 | ||||
| Mean (SD)# | 29.2 (4.7) | 28.2 (4.5) | 28.5 (4.6) | 0.101* |
| Median (SEM)† | 29.0 (0.51) | 28.3 (0.31) | 28.4(0.3) | |
| Q1-Q3 | 25.91-32.03 | 24.98-30.67 | 25.3-30.8 | |
| Normal weight | 16.0 (18.5)‡ | 53.0 (24.9) | 69.0 (23.0) | |
| Overweight | 41.0 (47.1) | 99.0 (46.5) | 140.0 (46.7) | |
| Obese | 30.0 (34.5) | 61.0 (28.6) | 91.0 (30.3) | |
| F. C | ||||
| Yes | 59.0 (67.8)‡ | 118.0 (55.4) | 117.0 (59.0) | 0.047# |
| No | 28.0 (32.2) | 95.0 (44.6) | 123.0 (41.0) | |
| M.C (n, %)‡ | ||||
| Regular | 39.0 (44.8)‡ | 104.0 (48.8) | 143.0 (47.7) | 0.529# |
| Irregular | 48.0 (55.2) | 109.0 (51.2) | 157.0 (52.3) | |
| TT ng/dL | ||||
| Mean (SD)# | 118.3 (106.2) | 256.2(164.2) | 216.3 (162.1) | |
| Median (SEM)† | 86.0 (11.39) | 270.0 (11.25) | 184.0 (9.4) | 0.000* |
| Q1- Q3 | 48.0-134.0 | 86.4-400.0 | 70.0-390.0 | |
| ≤109.5ng/dL | 61.0 (70.1)‡ | 64.0 (30.0) | 125.0 (41.7) | 0.000# |
| > 109.5 ng/dL | 26.0 (29.9) | 149.0 (70.0) | 175.0 (58.3) | |
| AMH | ||||
| Mean (SD)# | 6.7 (2.5) | 7.4 (3.4) | 7.2 (3.2) | 0.345* |
| Median (SEM)† | 5.9 (0.27) | 6.2 (0.23) | 6.2 (0.2) | |
| Q1- Q3 | 5.1-7.6 | 5.1-8.35 | 5.1-7.8 | |
* P-value were obtained using Mann-Whitney test; and P# were obtain using Chi- Square test.
In comparison to their counterpart, women with hyperandrogenemia (serum TT > 109.5 ng/dL) had significant increase in LH-FSH ratio (P= 0.000). Furthermore; obesity was found in 30.9% (n= 54) of them based on their BMI (Table 2).
| Variables | Androgenemia (cut-off > 109.5 ng/dL) | P- value | |
|---|---|---|---|
| ≤ 109.5 (n=125) | >109.5 (n=175) | ||
| Age/year | |||
| Mean (SD)# | 29.3 (5.9) | 28.9 (5.8) | 0.434* |
| Median (SEM)† | 29.0 (0.5) | 29.0 (0.4) | |
| Q1-Q3 | 25.0-34.0 | 24.0-33.0 | |
| BMI kg/m2 | |||
| Mean (SD)# | 28.5 (4.7) | 28.4 (4.5) | 0.962* |
| Median (SEM)† | 28.5 (0.4) | 28.3 (0.3) | |
| Q1-Q3 | 25.1-30.4 | 25.3-30.8 | |
| Normal weight | 30.0 (24.0)‡ | 39.0 (22.3) | 0.935# |
| Overweight | 58.0 (46.4) | 82.0 (46.9) | |
| Obese | 37.0 (29.6) | 54.0 (30.9) | |
| Family history | |||
| Yes | 81.0 (64.8)‡ | 96.0 (54.9) | 0.084# |
| No | 44.0 (35.2) | 79.0 (45.1) | |
| Menstrual Cycle n (%)‡ | |||
| Regular | 57.0 (45.6)‡ | 86.0 (49.1) | 0.545# |
| Irregular | 68.0 (54.4) | 89.0 (50.9) | |
| LH- FSH ratio | |||
| Mean (SD)# | 1.32 (1.0) | 2.15 (1.2) | 0.000* |
| Median (SEM)† | 1.06 (0.09) | 1.91 (0.09) | |
| Q1- Q3 | 0.75-1.49 | 1.29-2.58 | |
| ≤1 | 61.0 (48.8)‡ | 26.0 (14.9) | 0.000# |
| >1 | 64.0 (51.2) | 149.0 (85.1) | |
| AMH | |||
| Mean (SD)# | 7.2 (3.3) | 7.1 (3.0) | 0.883* |
| Median (SEM)† | 5.9 (0.2) | 6.2 (0.2) | |
| Q1- Q3 | 5.1-7.8 | 5.0-7.6 | |
As shown in Figure 1, the Spearman’s correlation revealed that serum LH-FSH ratio was significantly correlated with serum TT (ng/dL) (r= 0.329, P= 0.000). However; both LH-FSH ratio and TT were insignificantly correlated with BMI among women with PCOS (results not shown on figure).
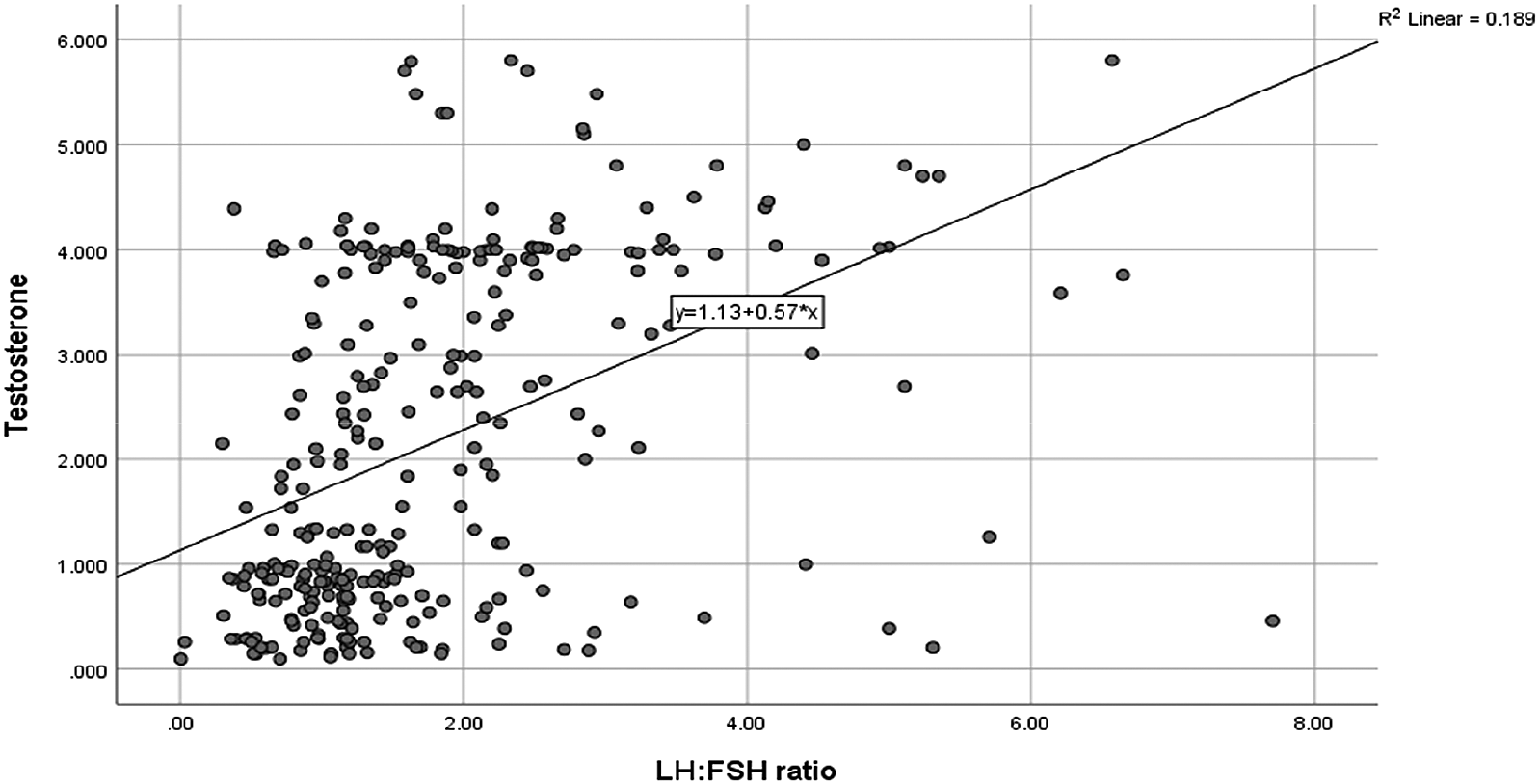
LH-FSH: luteinizing hormone to follicle-stimulating hormone, TT: total testosterone.
As shown in Table 3, the serum LH-FSH ratio (odds ratio (OR) (95% confidence interval (CI)): 2.308 (1.698-3.139, P = 0.000) was independently related to androgen status and can be used as a predictor in women with PCOS.
According to the ROC curve analysis; LH-FSH ratio>1 can distinguish hyperandrogenemia from normoandrogenemia in women with PCOS (AUC = 0.726, P=0.000, 95% CI: 0.668-0.785; sensitivity 70.0%, specificity 77.1%) at TT threshold 109.5 ng/dL (Figure 2).
In the present study, the link between serum LH-FSH ratio and total testosterone level were investigated in a cross-sectional analysis of 300 Sudanese women with PCOS. The study revealed that 30.3% of women were obese based on their BMI. Obesity was found in 50–80 % of women with PCOS, according to McCartney and Marshall.12 This may differ depending on race, ethnicity, location, and environmental factors (physical activity, diet, stress).
The most common clinical symptom of women diagnosed with PCOS, according to Malini and George,13 is a greater LH-FSH ratio. The LH-FSH ratio in healthy women is 1:1. In PCOS, the LH level is higher than the FSH level, resulting in increased ovarian androgen production and ovulatory failure. Various LH-FSH ratio cut-offs have been proposed. However, the cut-off more than 1.0 was found to be the most successful in diagnosing PCOS in terms of sensitivity and specificity.14,15
According to our findings, 71.0% of women with PCOS exhibited an abnormal LH-FSH ratio with a cut-off of >1. Morshed et al. reported that 71.0% PCOS patients (cut-off more than1.0) exhibited an aberrant LH-FSH ratio.16 According to Nath et al.,17 70.58% of women with PCOS have an increased LH-FSH ratio. As a result, the authors proposed that the LH-FSH ratio is one of the defining characteristics of PCOS women.17
Study revealed 58.3% of women with PCOS had hyperandrogenemia (cut-off TT more than 109.5 ng/dL). Furthermore, the findings showed a statistically significant difference in the frequency of hyperandrogenemia between the changed and normal LH-FSH ratio groups demonstrated that an altered LH-FSH ratio with a cut-off of 1.0 was associated with androgen status in women with PCOS. Moreover, the LH-FSH ratio was linked to a higher level of serum TT. Alexiou et al., reported hyperandrogenemia was present in 78.2% of PCOS.18 Livadas and his colleagues reported that prevalence of hyperandrogenemia was 58.8%.19 The imbalance in LH: FSH causes proliferation of ovarian theca cells leading to increased steroidogenesis, and ultimately leading to hyperandrogenemia in PCOS women.20
In the hyperandrogenic group LH-FSH ratio was higher and differed significantly from the value of the normoandrogenic group. This may suggest that LH-FSH ratio has an independent relation with TT, which is consistent with previous studies.13,21
In regression analysis our study observed that LH-FSH ratio>1 was significantly positive independently associated to hyperandrgenemia. Moreover, ROC analysis indicated that serum LH-FSH ratio>1 was significantly discriminate androgen statuses among PCOS women with cut-off to serum TT 109.5 ng/dL. The studies13,21 also demonstrate that an LH-FSH ratio greater than 1.0 is enough to generate significant testosterone production from the ovaries, resulting in hyperandrogenemia.
There are several solid points in the present research. A high sample size was used in this study. It looked at the relationship between the LH-FSH ratio>1 and TT in Sudanese women PCOS. It is the first study to use an LH-FSH ratio greater than 1 to distinguish hyperandrogenic status in PCOS Sudanese individuals. Our research can be used as a starting point for further research. To compute the free testosterone index, serum sex hormone binding globulin or free testosterone were not assessed. Furthermore, we lack population-specific data on serum TT reference ranges.
Sudanese women with PCOS are prone to androgenemia. Furthermore, among women with PCOS, the LH-FSH ratio was found to be substantially linked with total testosterone. The finding of this study may aid in a better understanding of the pathophysiology and management of hyperandrogenemia in PCOS women of Sudanese descent.
Figshare: PCOS in Sudan. https://doi.org/10.6084/m9.figshare.19102715.11
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
The authors are most grateful to all the women who agreed to take part in this study. Also, our gratitude to the physicians who assessed the participants.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
References
1. Szczuko M, Zapałowska-Chwyć M, Maciejewska D, Drozd A, et al.: High glycemic index diet in PCOS patients. The analysis of IGF I and TNF-α pathways in metabolic disorders.Med Hypotheses. 2016; 96: 42-47 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: I am an expert in the field of nutrition, including PCOS. I have written a total of over 10 papers on various aspects of PCOS
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 16 May 22 |
read | read |
|
Version 1 07 Feb 22 |
read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)