Keywords
BOTOX injections, Pyridostigmine, neurotoxin, clostridium botulinum type-A
BOTOX injections, Pyridostigmine, neurotoxin, clostridium botulinum type-A
Acetylcholinesterase inhibitors, such as pyridostigmine, are important in clinical practice, i.e., they can be used to treat the symptoms associated with myasthenia gravis, Alzheimer’s disease, and multiple sclerosis.1–3 Acute or prolonged exposure to reversible acetylcholinesterase inhibitors in healthy individuals, however, can have positive or negative effects on neuromuscular transmission and muscle ultrastructure.4,5 Classically, the theory of receptor control suggests that chronic agonist stimulation of any receptor can lead to receptor desensitization and even down-regulation in a concentration- and time-dependent manner.6–9
Clostridium botulinum is a rod-shaped, gram-positive anaerobic bacterium. It is a ubiquitous soil microorganism that can remain dormant as a spore in favorable environments. Its spores can germinate and produce a neurotoxin known as botulinum toxin.10 In 1897, Van Ermengem discovered this bacterium when Belgian patients presented with botulism. After that, several strains of the bacterium that produced serologically distinct toxin types were identified and classified into seven serotypes, A, B, C1, D, E, F, and G.11 In 1928, Dr. Herman Sommer isolated the most potent serotype, botulinum neurotoxin (BoNT) type A.12 Each serotype has its own distinct pharmacological properties,13,14 and only materials containing BoNT type A or type B have been approved for human use. These neurotoxins act on the peripheral nervous system, where they inhibit the release of acetylcholine (ACh) from the synaptic terminal of neuromuscular junctions.15,16 Currently, BoNT type A injections are a popular non-surgical and non-invasive treatment to optimize and change an individual’s facial appearance and achieve rejuvenation.17
In this study, we aimed to demonstrate the efficacy of anticholinesterase inhibitor pyridostigmine in accelerating spontaneous recovery following botulinum toxin injections in a rabbit model.
An experimental interventional study was conducted on 40 rabbits to evaluate the antidote effect of pyridostigmine against BoNT. This study was one-way one-period (28 days) from 12th May to 11th June 2022. Rabbits were randomly divided into four groups, and each group consisted of 10 rabbits. Control group received distal water, pyridostigmine-treated group received oral pyridostigmine, BoNT only group received injections intramuscularly with a single dose of BoNT and BoNT+pyridostigmine treated group received injections with BoNT and oral pyridostigmine, as shown in the flow chart (Figure 1).
The drugs were bought from private pharmacies in Basrah city, Iraq. Tablets were crushed using a mortar and pestle, and then dissolved in distilled water to obtain a solution for administration to the rabbits. The doses of each drug administered were prepared as follows.
One 60 mg tablet of pyridostigmine was dissolved in 50 mL distilled water before each administration to obtain a suspension of concentration 1.2 mg/mL. The powder was completely dissolved to give a homogenous solution in a sealed glass container. A dose of 4 mL/kg from the prepared stock solution was orally administered to each rabbit according to their body weight for an accurate dose (5 mg/kg), and then the dose was increased to 7 mg/kg after three days to avoid any unwanted cholinergic effects.
Using an appropriate-sized needle and syringe, 2.5 mL of 0.9% non-preserved sterile saline was drawn up. The needle was inserted and the saline was slowly injected into the vial. A vacuum was present in the vial, which demonstrated that it was sterile. The syringe was then disconnected from the needle and the BoNT was gently mixed with the saline by rotating the vial. The date and time of reconstitution were recorded on the label. After that, a new sterile syringe was attached and the reconstituted fluid was drawn into the syringe by angling the needle into the bottom corner of the vial to enable complete extraction. Any air bubbles in the syringe were expelled. The syringe was then disconnected from the needle used for reconstitution and a 30-gauge needle was attached for the injection. The doses of the drugs used in this study were selected according to a literature review and a pilot study,18 as follows: Pyridostigmine19 and BoNT.20
The sample size calculation of this study is based on availability of rabbit, effect of group size on the findings, and significant of this number on the experimental level.
Inclusion criteria
Forty rabbits (Oryctolagus cuniculus) (skeletally mature males) were included in the study aged from 2–4 years. They were obtained from a local market in Basrah city, and their body weights were between 1000 and 2000 g. One animal died during the study period. The rabbits were locally bred and sexually mature. Animals were housed under a controlled animal house conditioned atmosphere at a constant temperature (25°C±3) and relative humidity (50±5%) and supplied with food and water ad libitum during the study period. Animals were kept under observation for one month and fasted for three hours at the time of dosing.
Exclusion criteria
Rabbits were excluded from the study when dying, and when any adverse effect seen after administration of drugs like bleeding at site of injection, vomiting and cramping.
Rabbits were acclimatised for seven days before the study. They were allowed normal cage (65 × 345 × 330 cm3) activity and fed a standard diet (refoil and lettuce). To reduce stress, rough handling and overcrowding were avoided. During the four weeks of treatment, the body weight of each animal was recorded weekly. All authors were aware of groups during allocation, at a period of conducting the experiment, at the time of the outcomes assessment and at the time of analysis of data.
This study was approved by the Ethical Committee of the Council of College of Medicine at the University of Basrah (No. 4502).
The animals were randomly divided into four groups (10 rabbits in each group). Each group was treated for four weeks as follows (Table 2):
Rabbits in this group were treated with 4 mL/kg distilled water orally for three days. Then the dose was increased to 6 mL/kg and they were injected with distilled water (10 U/kg) in the right quadriceps muscle as a single dose and euthanized on day 30 of the study.
Rabbits in this group were orally administered pyridostigmine (4 ml/kg). Then the dose was increased to 6 mL/kg for the remaining 30 days. The rabbits were euthanized at the end of the study.
Rabbits in this group were intramuscularly injected with a single dose of BoNT (10 U/kg) in the right quadriceps muscle (targeting the rectus femoris, vastus lateralis, vastus intermedius, and the vastus medialis) and euthanized on day 30 of the study.
Rabbits in this group were intramuscularly injected with BoNT (10 U/kg) into the right quadriceps muscle (single dose) and orally administered pyridostigmine (4 ml/kg) for three days. The dose was then increased to 6 mL/kg for the remaining 30 days before the rabbits were euthanized at the end of the study.
The following items were assessed: rabbits weight (kg) at the beginning of the study and at end of each of subsequent week, and weight of rabbit quadriceps muscle (g) after the end of the study.
The statistical analysis was performed using SPSS version 20 (IBM Inc., Chicago, IL, USA), Resource Identification Portal (RRID:SCR_004098).Descriptive statistics, consisting of numbers and percentages, are provided. The mean, median, range, minimum, maximum, and standard deviation (SD) were calculated for categorical data. Associations between groups were assessed by an unpaired t-test. Associations between variables were assessed by a chi-squared test. An ANOVA was used to describe associations between groups. A two-sided P-value of less than 0.05 was considered statistically significant. Details can be viewed at https://scicrunch.org/resources/data/record/nlx_144509-1/SCR_004098/resolver?q=SPSS%20version%2020&l=SPSS%20version%2020&i=rrid:scr_004098.
For the control group, the rabbits’ weight during one month of the experiment is shown in Table 3 and Figure 2. There was a statistically significant difference of weights during four weeks in the control group (P=0.03), which was more being in the third week (mean±SD=1.5±0.122 kg).
The mean weight of the rabbits on the first day was 1.415±0.18 kg, whereas the mean weight of rabbits at the end of the fourth week was 1.475±0.145 kg, after one month of 0.9% saline injection in the control group, as shown in Table 4. There was no significant difference between groups (t=2.092, 95% CI=0.125–0.005, P=0.066).
In this study, the mean weight of the rabbits’ right quadriceps muscle was 8.28±1.06 g, while the mean weight of the rabbits’ left quadriceps muscle was 8.385±1.06 g. The left muscle was significantly heavier than the right (t=2.677, 95%CI=0.193–0.016, P=0.025), as shown in Table 5 and Figure 3.
For the pyridostigmine-only group, the rabbits’ weight during one month of the study is shown in Table 6 and Figure 4. There was a high statistically significant difference among rabbits weight during four weeks of the study in this group (P=0.002), which was more being in the third week (mean±SD=1.485±0.175 kg).
In the pyridostigmine-only group, the mean weight of rabbits on the first day was 1.45±0.227 kg, which was lower than the mean weight of the rabbits at the end of the fourth week, which was 1.475±0.178 kg after one month of oral pyridostigmine, as shown in Table 7. There was no significant difference between groups (t=1.103, 95%CI=0.076–0.026, P=0.299).
In the pyridostigmine-only group, the mean weight of the rabbits’ right quadriceps muscle was 8.73±1.23 g, which was higher than the mean weight of the rabbits’ left quadriceps muscle, which was 8.635±1.14 g, but the difference was not significant (t=1.57, 95% CI=-0.042– ‐0.232, P=0.151), as shown in Table 8 and Figure 5.
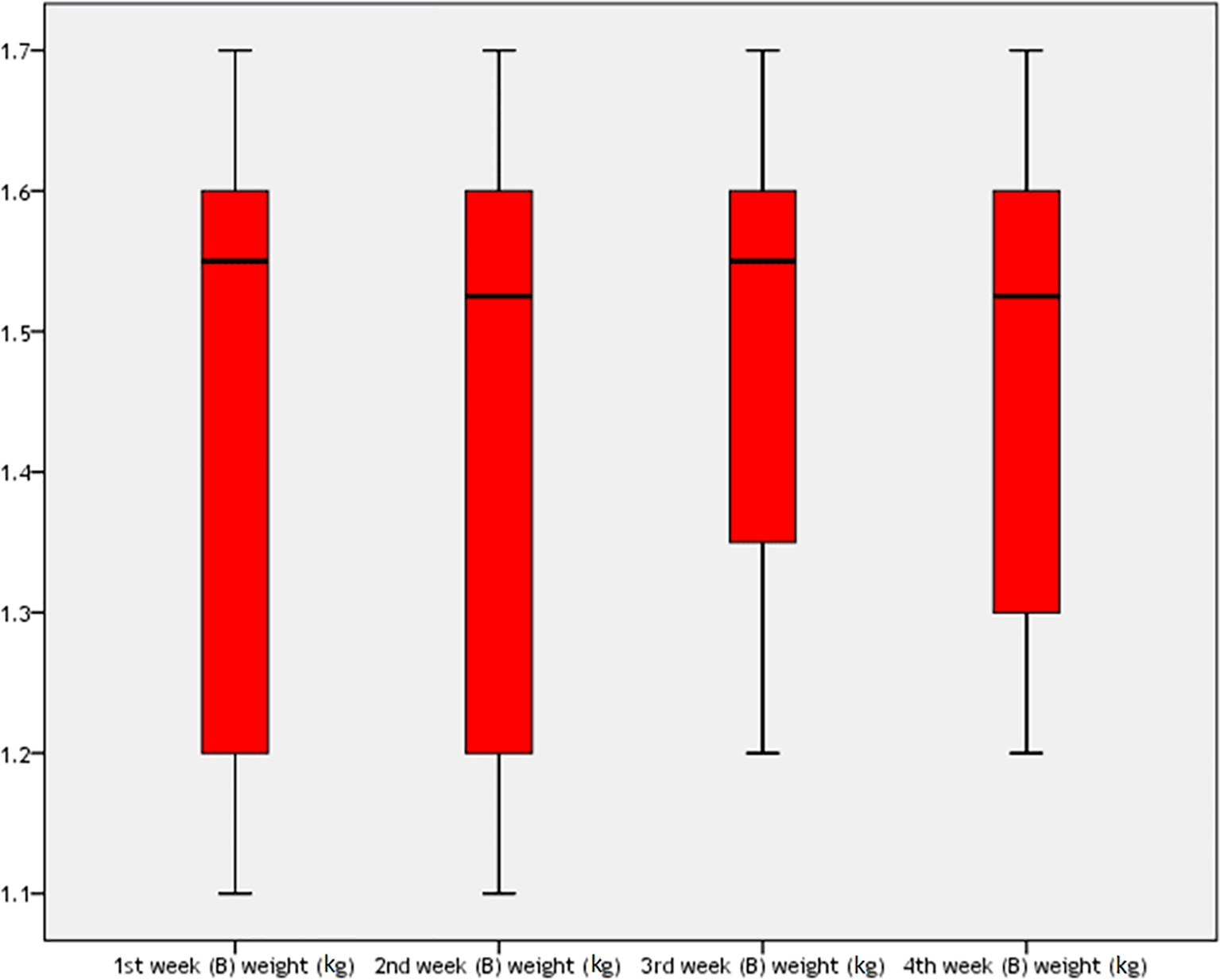
For the BoNT injection group, the rabbits’ weight during one month of the study is shown in Table 9 and Figure 6. There was a high statistically significant difference among rabbits weight during four weeks of the study in this group (P=0.033), which was more being in the first week (mean±SD=1.55±0.2 kg) but then dropped subsequently in the fourth week (mean±SD=1.345±0.13 kg).
The mean weight of the rabbits on the first day was 1.55±0.2 kg, which was higher than the mean weight of the rabbits at the end of the fourth week, which was 1.345±0.13 kg, after one month of BoNT injections, as shown in Table 10. There was a highly significant difference between groups (t=5.156, 95% CI=0.115–0.295, P=0.001).
In the Botox group, the mean weight of the rabbits’ right quadriceps muscle was 6.573±1.3 g, which was lower than the mean weight of the rabbits’ left quadriceps muscle, which was 8.09±1.2 g, a highly significant difference (t=21.795, 95% CI=1.674–1.36, P=0.0001), as shown in Table 11 and Figure 7.
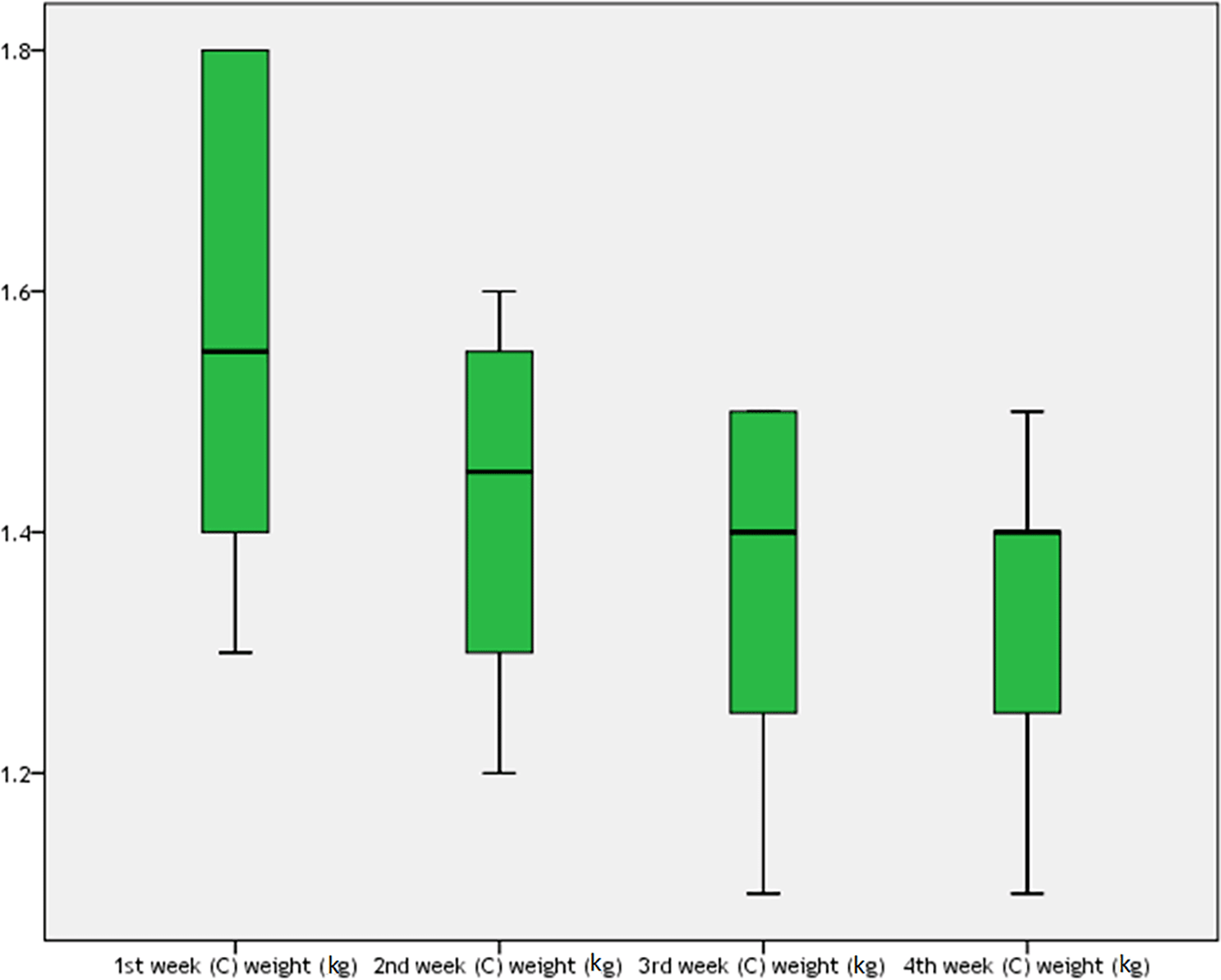
For the pyridostigmine and BoNT injection group, the rabbits’ weight during one month of the study is shown in Table 12 and Figure 8. There was a statistically significant difference among rabbits’ weight during four weeks of the study (P=0.027), which was more being in the first week (mean±SD=1.455±0.2 kg).
Thus, the mean weight of the rabbits on the first day was 1.455±0.2 kg, which was the same as the mean weight of rabbits at the end of the fourth week, which was 1.455±0.18 kg, after one month of BoNT injection, as shown in Table 13. There were no significant differences between the groups (t=0, 95% CI=N/A, P=1).
In this study, the mean weight of the rabbits’ right quadriceps muscle was 5.564±0.615 g, which was lower than the mean weight of the rabbits’ left quadriceps muscle, which was 8.36±0.55 g, a highly statistically significant difference (t=18.656, 95% CI=3.135–2.457, P=0.0001), as shown in Table 14 and Figure 9.
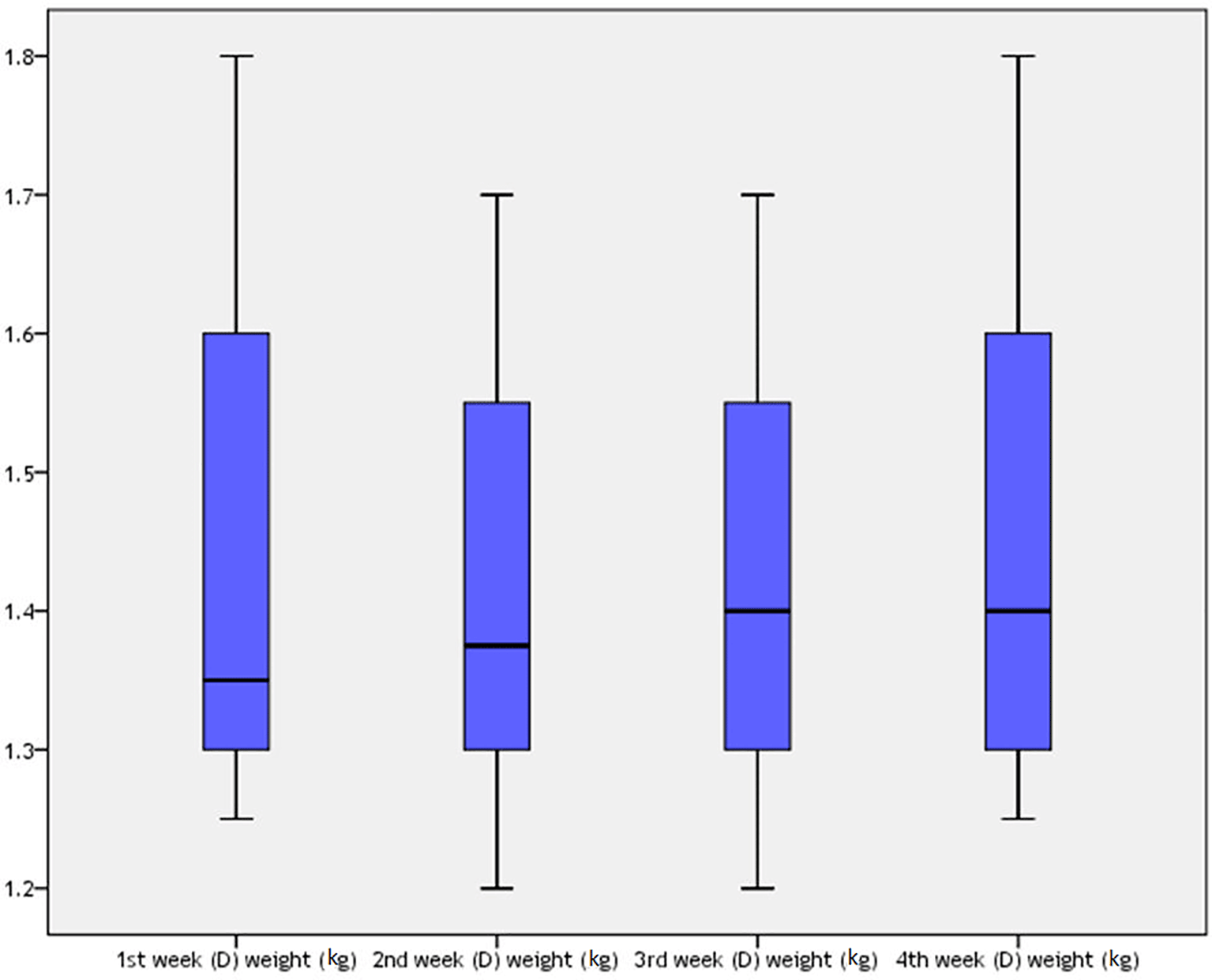
Table 15 and Figure 10 show the ANOVA analysis and linear correlation among different groups of the study in relation to the control group in the first week. There was a significant relationship between group A and B in the first week (P=0.024). There were no associations between group A and the other categories.
| Control group | 1st week weight (kg) | ||
|---|---|---|---|
| B | C | D | |
| ANOVA (F) | 15 | 0.574 | 0.88 |
| P value | 0.024 | 0.743 | 0.593 |
Table 16 and Figure 11 show the ANOVA analysis and linear correlation among different groups of the study in relation to the control group in the second week. There was a significant relationship between group A and D in the second week (P=0.047).
| Control group | 2nd week weight (kg) | ||
|---|---|---|---|
| B | C | D | |
| ANOVA (F) | 1.369 | 1.097 | 9.41 |
| P value | 0.43 | 0.511 | 0.047 |
Table 17 and Figure 12 show the ANOVA analysis and linear correlation among different groups of the study in relation to the control group in the third week. There was a significant relationship between group A and B in the third week (P=0.015).
| Control group | 3rd week weight (kg) | ||
|---|---|---|---|
| B | C | D | |
| ANOVA (F) | 21.52 | 0.44 | 1.001 |
| P value | 0.015 | 0.82 | 0.545 |
Table 18 and Figure 13 show the ANOVA analysis and linear correlation among different groups of the study in relation to the control group in the fourth week. There was no significant difference between group A and the other groups.
| Control group | 4th week weight (kg) | ||
|---|---|---|---|
| B | C | D | |
| ANOVA (F) | 12.8 | 0.258 | 2.341 |
| P value | 0.074 | 0.926 | 0.332 |
Figure 14 shows the correlation among different groups of the study in relation to the rabbits’ right quadriceps muscle weight. There was a highly statistically significant difference among groups according to the ANOVA analysis (F=18.515, P<0.0001).
Figure 15 shows the correlation among different groups of the study in relation to the rabbits’ left quadriceps muscle weight. There was no statistically significant difference among groups according to the ANOVA analysis (F=0.475, P=0.7).
Recently, a model for BoNT intoxication was developed in rabbits for the first time.21 This is in accordance with the animal rule of the US FDA.22 Rabbits are the third most commonly used species for experimental research in the western countries.23 Rabbits are larger than mice, so blood sampling and intravenous administration of drugs are easy.24 Phylogenetically, rabbits are closer to humans than rodents, and due to their anatomical, physiological, genetic, and biochemical similarities, they are used as animal models for human diseases in a variety of medical research fields.25
In this series, among the control group, the rabbits weight increased, reaching peak weight in the third week, with a statistically significant difference (P=0.03). The mean weight of rabbits on the first day was 1.415±0.18 kg, whereas at the end of the fourth week it was 1.475±0.145 kg, without significant difference between groups (P=0.066). Furthermore, the mean weight of the rabbits’ right quadriceps muscle was lower than the mean weight of the rabbits’ left quadriceps muscle, with a highly statistically significant difference (P=0.025). This is the same as found in Morsch et al.’s,26 experiment, except they used citric acid monohydrate, sodium citrate dehydrate, methyl paraben, propyl paraben, and NaCl in sterile water (pH 5.1). However, in the saline group of Richtsfeld et al.,27 there were no such changes, whereas an agreement was seen in saline injected group that showed gradual increased in the body mass of animals.28
In the pyridostigmine only group, the weight during the study peaked in the third week, with a highly statistically significant difference (P=0.002). The mean weight of rabbits on the first day was lower than the mean weight of rabbits at the end of the fourth week. The mean weight of the rabbits’ right quadriceps muscle was higher than the mean weight of the rabbits’ left quadriceps muscle, with no significant difference (P=0.151). This agrees with the findings of Morsch et al.26 They delivered pyridostigmine to animals systemically and continuously for seven to nine days via a minipump. Haigh et al. found that the effect of different doses of pyridostigmine on whole-blood acetylcholinesterase activity was recorded seven days after ingestion.29 Morsch et al.26 found rabbits treated with pyridostigmine lost weight and developed severe muscle weakness within two weeks. Hence, rather than preventing muscle weakness, pyridostigmine was capable of precipitating this weakness. This is in disagreement with what was reported by Richtsfeld et al., who found that animals in the pyridostigmine group lost 10% of their initial body weight during administration of pyridostigmine.27
Morsch et al. concluded that pyridostigmine remains the only approved symptomatic drug for the treatment of BoNT intoxication, and one week of ingestion was enough to exacerbate neuromuscular impairment.26
In the BoNT injection group, the rabbits’ weight was more in the first week (mean±SD=1.55±0.2 kg) but then subsequently decreased by the fourth week (mean±SD=1.345±0.13 kg); this was a highly statistically significant difference (P=0.033). Although, the mean weight on the first day (1.55±0.2 kg) was higher than the mean weight at the end of the fourth week (1.345±0.13 kg) of the study; this was a high significant difference (P=0.001). The mean weight of the rabbits’ right quadriceps muscle was 6.573±1.3 g, which was lower than the mean weight of the left quadriceps muscle (8.09±1.2 g), with a highly significant difference (P=0.0001). In the pyridostigmine and BoNT injection group, the rabbits’ weight gradually declined during subsequent injections. There was a statistically significant difference between the rabbits’ weights (P=0.027). Thus, the mean weight of rabbits on the first day was 1.455±0.2 kg, which had decreased at the end of the fourth week to 1.455±0.18 kg, with no significant between groups (t=0, 95% CI=N/A, P=1). In addition, the mean weight of the rabbits’ right quadriceps muscle was lower than the left quadriceps muscle, with a highly statistically significant difference (P=0.0001). Similarly, the animals lost weight and became weak after two weeks in Morsch et al.’s study.26 Also, our study’s results are consistent with previous experiments,30 and an agreement has been seen in Botox mice by Warner et al.28
Torgeman et al. used different symptomatic rabbits dosing group with increased in a dose-dependent manner: 50%, 75%, and 80% with toxin doses of 0.5 ng/kg, 0.65 ng/kg, and 0.75 ng/kg, respectively. They found that 100% of the rabbits became symptomatic only in the 0.85 ng/kg dose, despite its lethal exposure dose.21
In our ANOVA analysis of this study, we showed the following findings between the control group and the other groups, such as a significant relationship between group A and B in the first week (P=0.024). There was a significant relationship between group A and D in the second week (P=0.047). There was a significant relationship between group A and B in the third week (P=0.015). The correlation among different groups in relation to the rabbits’ right quadriceps muscle weights had a highly statistically significant difference among groups according to the ANOVA analysis (P<0.0001). However, there was no statistically significant difference in the correlation with the rabbits’ left quadriceps muscle weight (P=0.7). Similarly, there were significant differences in the weights of the tibialis muscle between groups in different study periods.27
In mice series, BoNT treatment significantly diminished right hindlimb muscle mass in both the quadriceps (102.9±31.2 vs. 195.3±13.9 mg, −47.3%, P<0.001) and calf (58.4±13.3 vs. 145.0±3.9 mg, −59.7%, P<0.001) compared to saline mice. Muscle mass in the contralateral hindlimb (left) of the BoNT-treated mice was also significantly diminished compared with that of the saline-treated mice, but to a lesser extent (quadriceps: 166.0±5.9 vs. 199.9±2.7 mg, minus 17.0%; calf: 120.8±4.2 vs. 145.0±1.6 mg, minus 16.7%, both P<0.001),28 which seem similar to the current study’s findings.
Increasing evidence indicates that prolonged exposure to pyridostigmine was an etiological factor for Gulf War syndrome, which includes skeletal muscle symptoms.31 This could be explained by the fact that certain muscles appear to be affected more than others, for reasons unknown, but possibly due to quantitative differences in etiology.30,32,33
To the best of our knowledge, this is the first study conducted in Iraq to investigate the antidote effect of pyridostigmine against BoNT injections. Pyridostigmine and saline caused increased weight in rabbits in comparison to BoNT-injected rabbits. No significant effect was seen on muscles after pyridostigmine administration, while the inverse was observed in the BoNT group. Pyridostigmine can act as a strong antidote against BoNT intoxication.
Alhasan Huda Salim, Jawad K. Hasan, & Sawsan S. Al-Haroon. (2022). The effect of Pyridostigmine As Antidote for Botulinum type A. https://doi.org/10.5281/zenodo.7250444. 34
The ARRIVE guidelines 2.0: author https://arriveguidelines.org/sites/arrive/files/documents/Author%20Checklist%20-%20Full.pdf
Huda Salim, Jawad Hasan, & Sawsan Alharoon. (2022). ARRIVE checklist of Pyridostigmine and BoNT. Zenodo. https://doi.org/10.5281/zenodo.7362881. 35
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Neuropharmacology, neuroscience, pain research, botulinum toxin investigation
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
References
1. Diamant E, Pass A, Rosen O, Ben David A, et al.: A Novel Rabbit Spirometry Model of Type E Botulism and Its Use for the Evaluation of Postsymptom Antitoxin Efficacy.Antimicrob Agents Chemother. 2018; 62 (4). PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Pharmacology, neurotoxins, botulinum, antitoxins, ex vivo, in vivo, in vitro models, stem cells.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 21 Dec 22 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)