Keywords
eggplant; gas exchange; lipid peroxidation; physiological parameters; proline; salinity; tolerance
This article is included in the Agriculture, Food and Nutrition gateway.
This article is included in the Plant Science gateway.
eggplant; gas exchange; lipid peroxidation; physiological parameters; proline; salinity; tolerance
AR: analytical reagent
CRD: completely randomized design
DAI: days after induction
DAS: days after sowing
EC: electrical conductivity
Fv/Fm: maximum photochemical efficiency of PS II;
H2O2: hydrogen peroxide
HSD: honestly significant difference
kg: Kilograms
L: Liter
MDA: malondialdehyde
NaCl: sodium chloride
NPK: nitrogen, phosphorus, and potassium
POD: peroxidase
PPFD: photosynthetic photon flux density
RWC: relative water content
SPAD: soil plant analysis development
SIR: salt induction response
TCA: tri-chloroacetic acid
TIR: temperature induction response
Agriculture is the primary source of food, and it also provides raw materials for many industrial purposes. Agriculture plays a vital role in stable economic development of developing countries.1 The challenges are the global water demand, which has increased by 600% in the past few decades, salinization of irrigated areas and how to produce 70% more yield for satisfying the food demand of a rapidly increasing human population as well as livestock.2–4 In addition to this challenge, factors like biotic and abiotic stresses can devastate agricultural production in multiple ways. Abiotic stresses are some of the most significant factors that affect plant growth that can lead to decreased crop productivity.5 Salinity is a major abiotic stress that affects crops on 20% of the land worldwide, i.e., nearly 950 million hectares, and it is increasing day by day. Saline soils may produce ionic toxicity and osmotic stress, which results in inhibition of growth in crop plants.6 Such growth reduction can lead to senescence of the plants. Salinity stress disturbs plant physiological and metabolic processes. The accumulation of Na+ and Cl− in plant tissues causes harmful effects.7 Salinity stress may inhibit seedling growth and cause closure of stomata through an induced synthesis of abscisic acid, which reduces photosynthesis.8
Eggplant (Solanum melongena) is a common vegetable crop across the globe,9 and it is sensitive to salinity.10 Salinity stress negatively affects eggplant germination rate,11 vegetative growth12 and fruit yield.13 To address this problem, different strategies have been used, such as breeding tolerant eggplant varieties, using optimal irrigation regimes, providing appropriate fertilization, and applying various stress ameliorative measures.14 The salt induction response (SIR) is an innovative technique which was inspired from the temperature induction response (TIR) concept.15 The SIR principle is based on exposing seeds to sub-lethal salinity and subsequent exposure to challenging salinity, and then further evaluating-growth and recovery. An earlier study reported results of using SIR in mungbean for screening germplasm. Even though there were efforts to examine the plant responses towards salinity stress, there are no reports available on tolerance potential and mechanism of SIR in plants as well as the effect of SIR at the whole-plant level.16 We hypothesized that salinity induction induces a response, which leads to an increase in inherent salt tolerance in eggplant seedlings and mature plants. The objectives of the present studies were to identify the challenging salinity (NaCl) concentration, standardize the SIR cycle for eggplant seed germination, to quantify the effect of salinity on eggplant growth, physiology, and yield, and to elucidate the mechanism(s) of tolerance to salinity stress.
A salinity induction response (SIR) experiment was performed in the crop physiology and genomics lab and shade nethouse located at the School of Agricultural Innovations and Advanced Learning, Vellore Institute of Technology, Vellore, Tamil Nadu, India [12.92°N 79.13°E, 220 m above the mean sea level]. Eggplant (Solanum melongena L. cv. CO2) seeds were chosen for this study. This particular CO2 eggplant variety is agronomically important that it is being widely cultivated by the farmers of Tamil Nadu moreover, it is moderately sensitive to salinity and it is a pure line selection from Varikkathiri developed by Tamil Nadu Agricultural University, Coimbatore, Tamil Nadu India.
Eggplant seeds were surface-sterilized using 70% alcohol solution for one minute before sowing, followed by 5% sodium hypochlorite for three minutes and further seeds were washed with sterile distilled water once accordingly. The eggplant seeds (10 seeds/Petri dish) were sown on three layers of Whatman filter paper (grade 1) in glass Petri dishes (90×25 mm). NaCl (extrapure analytical reagent (AR) grade, were acquired from Sisco Research Laboratories (SRL), Maharashtra, India) and solutions were prepared at 60, 80, 100, 125, 200, 300 and 400 mM concentrations respectively. The pH of the prepared solutions was measured using pHep pH tester, (Hanna Instruments, Woonsocket, RI, USA) and electrical conductivity was measured with a Field Scout electrical conductivity meter, (Spectrum Technologies, Inc. Plainfield, IL, USA). The corresponding data is given in Supplementary Table S1. A control treatment (0 mM NaCl) was also used, and seeds were supplied with solutions prepared with distilled water throughout the experiment. The Petri dishes were organized in a completely randomized design (CRD) with six replications. Each Petri dish received 1 mL of the prepared NaCl solution (0, 60, 80, 100, 125, 200, 300 and 400 mM NaCl) respectively. Petri plates were kept at room temperature (27°C) and normal laboratory light conditions (200–400 μmol m-2·s-1) photosynthetic photon flux density. After 2 weeks, the percentage of seed germination was calculated to identify the optimum challenging salinity concentration, a 90% reduction to only 10% germination. Seed germination percentage was expressed as a percentage and was calculated by the following formula; Germination percentage (%) = [number of seeds germinated/total seeds]×100. The optimum salinity concentration was 125 mM NaCl (Figure 1) for the Petri plate experiment. This optimum challenging salinity exhibited less than 7.5% germination recorded at 125 mM NaCl (Figure 1) and a sub-lethal dose of 100 mM NaCl considered as challenging salinity to the further pot experimental study. Based on the identification of optimum challenging salinity and earlier SIR report in mung bean as supportive data 60, 80, 100 and 125 mM NaCl concentrations were chosen for the SIR cycle.16
Eggplant seeds were exposed to an induction cycle as above at 60, 80, 100 and 125 mM NaCl for 0, 30, 60, 90, 120 min. (Extended data, Figure S2). After each time interval, the treated seeds were transferred to Petri dishes, and they were supplied with distilled water until the seeds germinated. Seed germination percentage was expressed as a percentage and was calculated by the following formula: Germination percentage (%) = [number of seeds germinated/total seeds]×100. After analyzing the results, the 120-min interval was determined to be an optimum time considering high germination rate at 120-min interval for the salt induction treatments, when compared with other time intervals from 0, 30, 60, 90 min appropriately.
Surface sterilized eggplant seeds were exposed to the following three treatments: induced (NaCl treatment), non-induced (NaCl treatment) and absolute control (distilled water) by the method in Figure 2. Seeds were treated for a single induction cycle of 120 min with 60, 80, 100 or 125 mM NaCl. In the non-induced treatment, the seeds were exposed to a lethal level salinity (125 mM) for 120 min. All the seeds of induced and non-induced treatments were transferred to Petri dishes and irrigated with distilled water for three days. Treated seeds were maintained at room temperature (27°C) for three days for recovery. The recovery period was determined based upon the previous reports on the temperature [13] and salt induction [14] responses. The absolute control was treated with distilled water only. After three days of recovery, 125 mM NaCl solution (challenging salinity) was applied to all Petri plates (Induced, Non-induced and Absolute control), and the responses were observed. After 11 days of the 125 mM NaCl challenge treatment, the germination percentage, root length, vigour index, seedling biomass and relative water content were determined. The biomass of single seedlings was measured using a balance. After measuring the fresh seedling mass (g), seedlings were subjected to dry biomass analysis. Seedlings were put into a paper packet and placed in a hot air oven (80°C) for 48 h. Seedling dry masses (g) were measured with a balance for calculating the vigour index by the following formula: [Vigour index = germination percentage × seedling dry mass].17 Tissue water content (%) was calculated using the fresh and dry masses of leaves [Tissue water content = (fresh mass − dry mass)/dry mass × 100].
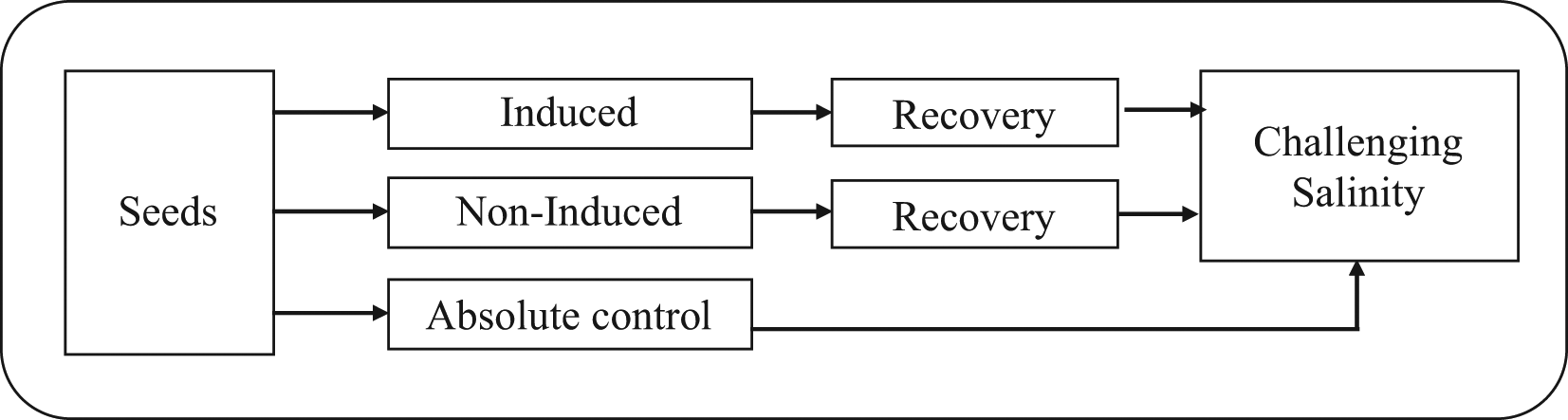
Induced: Seeds were subjected to an induction treatment (induction cycle of 120 min using 60, 80, 100 or 125 mM NaCl), recovery in distilled water for 3 days, then treatment with a challenging salinity of 125 mM). Non-induced: Seeds were subjected to a lethal level salinity (125 mM NaCl) for 120 min, a 3 day recovery interval, and then exposure to the challenging salinity. Absolute control: Seeds were directly exposed to a challenging salinity. A sub-lethal level challenging salinity (100 mM) or lethal level challenging salinity (125 mM NaCl) was given to all the seeds and maintained throughout the experiment. the challenging salinity was defined as the salinity level (100 or 125 mM NaCl) at which there was no or ≤10% germination.
Pot studies was carried out in a shaded nethouse. Seeds from the induced (60, 80, 100 or 125 mM NaCl), non-induced (125 mM NaCl) and absolute control treatments were transferred to protrays and irrigated with distilled water for 15 days and raised as seedlings in the greenhouse. Seedlings were transplanted to pots 15 days after induction (DAI) treatments ended. The pots [i.e., (25×24 cm) 10 L capacity] contained a soil mixture (15 kg/pot) consisting of red soil (Laterite soil): sand: compost (2:1:1). All the pots were arranged in a completely randomized design with six replications. Pots were irrigated with distilled water (0 mM NaCl) for seven days to avoid transplanting shock. A sub-lethal dose of saline water (100 mM NaCl) was prepared by mixing NaCl with fertilizer (NPK 19-19-19 at a concentration of 1.0 g L−1). Irrigated with 1 g/L (only fertilizer) or 100 mM NaCl was applied to pots manually throughout the experiment. Irrigations were scheduled at three days interval and each pot received 2 L of water to maintain soil continuously moistened in the pots. The growth conditions inside the shade nethouse were maintained by NMC-Junior, Crop Management Technologies, Netafim Irrigation and as follows: max/min temperature 34/26°C day/night, respectively, relative humidity 80% and average photosynthetic photon flux density (PPFD) 900 μmol m−2 s−1.
The leaf physiological and antioxidant measurements were recorded at 30 days after saline water irrigation. Relative water content (RWC) was calculated using the Weatherly methodology18 by the formula: RWC (%) = [(fresh weight-dry weight)/(turgid weight-dry weight)] × 100. Leaf chlorophyll content was measured with a soil plant analysis development (SPAD) meter 502 plus. The Fv/Fm ratio was calculated using the Mini PAM II fluorometer (MINI PAM II- Walz, Effeltrich, Germany). Leaf photosynthetic rate and stomatal conductance were measured with a portable photosynthesis system (LI-6800; LI-COR, Lincoln, NE, USA). Briefly, green leaves of 30 days after saline water irrigated plant and third fully expanded leaf from top to downward were enclosed in the portable photosynthesis system under a light intensity of 800 μmol m−2 s−1 PPFD and 400 μmol mol−1 CO2 at 25°C leaf temperature and relative humidity between 40 and 55%. Leaf osmotic potential of eggplant leaves was measured with a Wescor, VAPRO 5500 vapour pressure osmometer (Wescor, Logan, UT, USA) as described by Ball and Oosterhuis.19 The leaf proline content was determined, according to Bates et al.20 The fifth fully grown leaf sample (0.5 g) was taken and homogenized in 5 mL of sulphosalicylic acid (3%) using pestle and mortar. Around 2 mL ninhydrin reagent and 2 mL of glacial acetic acid were added to it. The whole reaction mixture was kept in 100°C water bath for 30 min. Once the reaction mixture cools down, 6 mL of toluene was added to the reaction mixture and later the mixture was transferred to a separating channel followed by thorough blending. The chromophore comprising toluene was isolated and absorbance was taken at 520 nm in a spectrophotometer against toluene blank. Proline concentration was evaluated by comparing it to a standard curve of proline and calculated as μg g−1 fresh weight. Hydrogen peroxide (H2O2) content was estimated using the Loreto and Velikova method,21 0.5 g of leaf sample was taken and homogenized in 3 mL of 1% (w/v) of tri-chloroacetic acid (TCA). The homogenized sample was centrifuged for 10 min at 10,000 rpm at 4°C. Consequently, 0.75 mL from supernatant has been further added to 0.75mL of 10 mM K-phosphate buffer of pH 7.0 and 1.5 mL of 1 M of potassium iodide. The H2O2 concentration from the supernatant was estimated by comparing its optical density towards a standard calibration curve at 390 nm using a spectrophotometer, and the values expressed as μmol g−1 fresh weight. The level of malondialdehyde (MDA) content in the sample was estimated by using the method performed by Weisany et al.22 About 0.2 g of leaf sample were homogenized with 5 mL of 0.1% (w/v) trichloroacetic acid (TCA) and centrifuged at 12,000 rpm for 5 min at 4°C. To 1 mL of supernatant 4 mL of 0.5% (w/v) thiobarbituric acid in 20% trichloroacetic acid. Further, the samples were kept in water bath boiling at 90°C and then the sample was cooled to stop the reaction. Once again, the reaction mixture was centrifuged at 10,000 rpm for 5 min. The absorbance was evaluated at A532 nm and A600 nm wavelength respectively. The malondialdehyde content was determined by using this formula MDA (nmol g−1 FM) = [(A532−A600) × V × 1000/ε] × W, and the specific extinction coefficient (ε =155 mM cm−1), where V is the quantity of grinding medium, W is the fresh weight of the leaf taken. The peroxidase (POD) activity was determined by using procedure described by Hemenda and Klein,23 and the reaction mixture contained 25 mmol L−1 phosphate buffer saline (pH 7.0), 0.05% guaiacol, 10 mmol L−1 H2O2 and enzyme. The peroxidase activity was estimated by the increase in absorbance at 470 nm due to guaiacol oxidation. Harvesting of fruits was done on 130 days after saline water irrigation, and fruit yield (g/plant) was recorded. After fruit harvest, leaf area for the whole sampling unit was measured by using a Leaf Area Meter (Model LI-3100 of LI-COR Inc.) and data given in Supplementary Table S2. After harvesting the whole plant was dried in a hot air oven (80°C) for 48 h, and total dry mass (g/plant) was calculated. The treatments were organized in a completely randomized design. The data for each parameter (growth, physiological, biochemical, antioxidants, yield) was statistically analyzed by analysis of variance (ANOVA) with p<0.05 as the measure of significance for each parameter using JMP 2007 (SAS Institute, Cary, NC, USA). Post-hoc comparisons were evaluated using the Tukey Honestly Significant Difference (HSD) test at p<0.05 for the treatment effects.
Growth parameters
The Petri plate experiment results indicated that the seed survival of the eggplant treated with gradually increasing concentrations of NaCl (induced treatment) was 77%, whereas with a level of 125 mM concentration of NaCl (non-induced seedlings) has 57% when observed after 14 days (Figure 3a). Similarly, the eggplant seedlings treated with a gradual increase of 60 to 125 mM concentration of NaCl had increased root length compared to non-induced and control treatments (Figure 3b). Induced seedlings had a greater vigour index than the control treatment (Figure 3c). Thus, the SIR indicated better quality seedlings than the non-induced and control seedlings. Likewise, the tissue water content and fresh and dry biomass of control seedlings were less than induced seedlings (Figure 3d–f). Therefore, salt induction of eggplant seedlings allowed an improved response to the challenge of 125 mM NaCl.
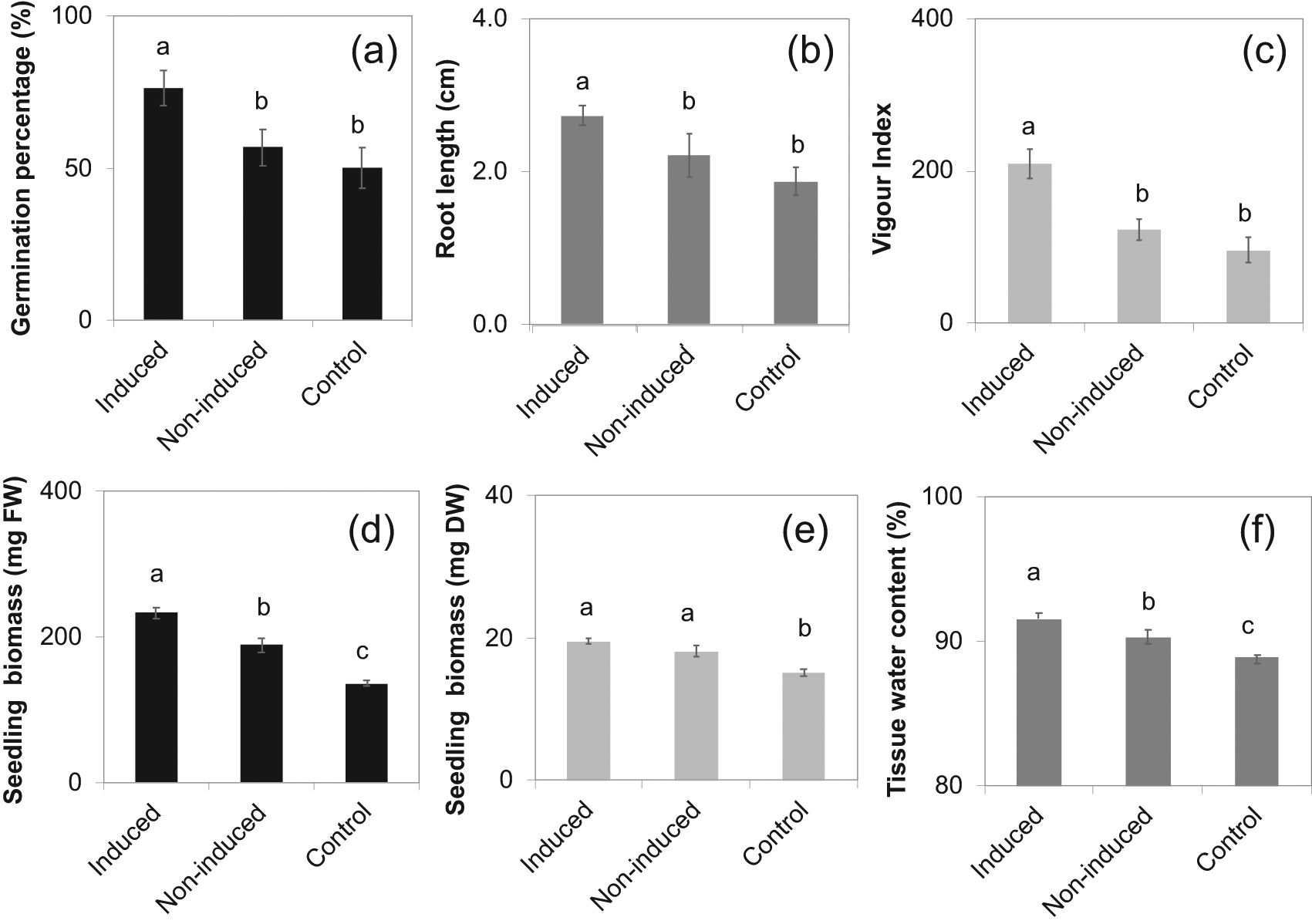
The means ± standard error are from six replications. Means with different letters are significantly different by Tukey Honestly Significant Difference (HSD) test at P≤0.05.
Physiological parameters
Induced seedlings growing in pots irrigated with 100 mM NaCl solution (sub-lethal level challenging salinity) had significantly greater relative water content over the control and non-induced treatments (15% and 9%, respectively) (Figure 4a). The leaf osmotic potential of the induced plants (−1.08 MPa) was less negative than the non-induced (−1.45 MPa) and control (−1.60 MPa) treatments under 100 mM NaCl concentration (Figure 4b). There were no differences in relative water content and leaf osmotic potential among the treatments with 0 mM NaCl irrigation (Figures 4 a,b). With a few exceptions, leaf photosynthetic rate, stomatal conductance, SPAD chlorophyll content, and the Fv/Fm ratio was significantly higher for induced plants, intermediate for non-induced plants, and lowest for control plants with both 0 and 100 mM NaCl irrigation, and lower for all treatments at 100 mM NaCl than at 0 mM NaCl (Figure 5).
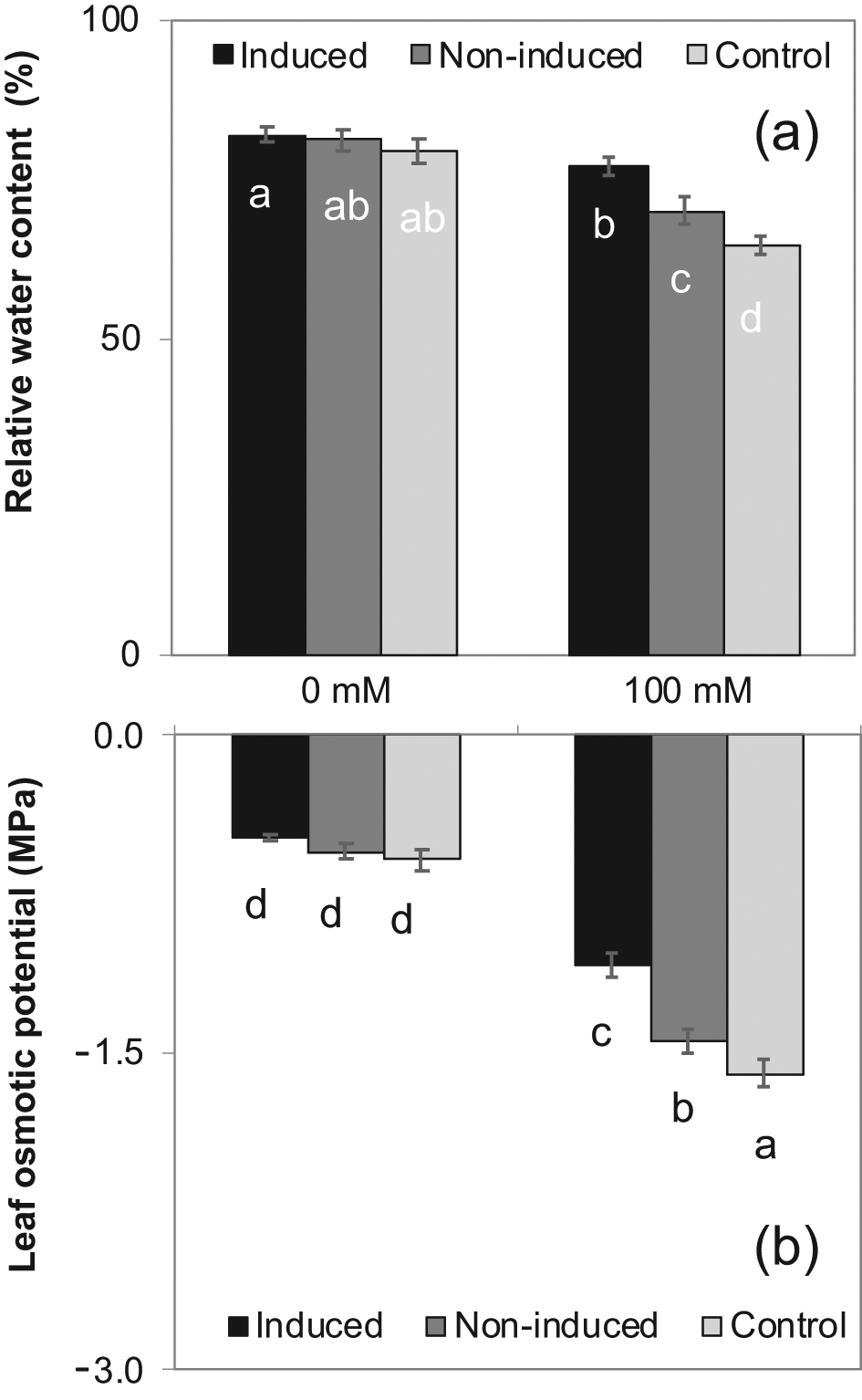
The means ± SE standard error are from six replications. Means with different letters are significantly different by Tukey Honestly Significant Difference (HSD) test at P≤0.05.
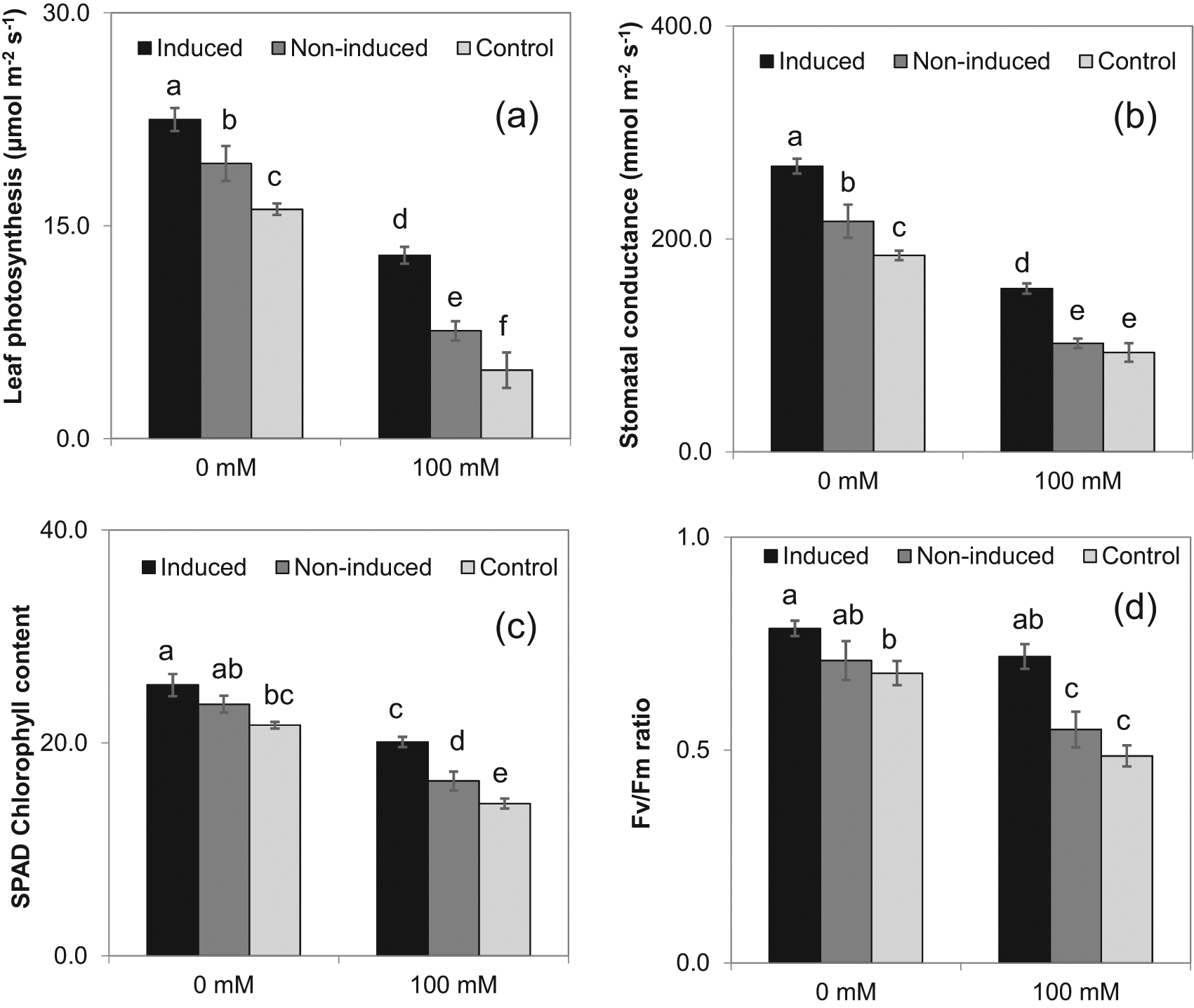
The means ± SE standard error are from six replications. Means with different letters are significantly different by Tukey Honestly Significant Difference (HSD) test at P≤0.05.
Antioxidant parameters
The antioxidant parameters indicated that the induced plants synthesized significantly more proline than the non-induced and control plants under 100 mM NaCl irrigation but showed no difference at 0 mM NaCl (Figure 6a). Lipid peroxidation was less for induced plants at both NaCl irrigation treatments than the control and non-induced plants (Figure 6b). Hydrogen peroxide content of induced plants was significantly less than the control with both irrigation treatments (Figure 6c). Leaf peroxidase (POD) activity of induced plants was significantly greater than non-induced and control plants (100 mM NaCl irrigation but all plant types were the same with no NaCl in the irrigation treatment (Figure 6d).
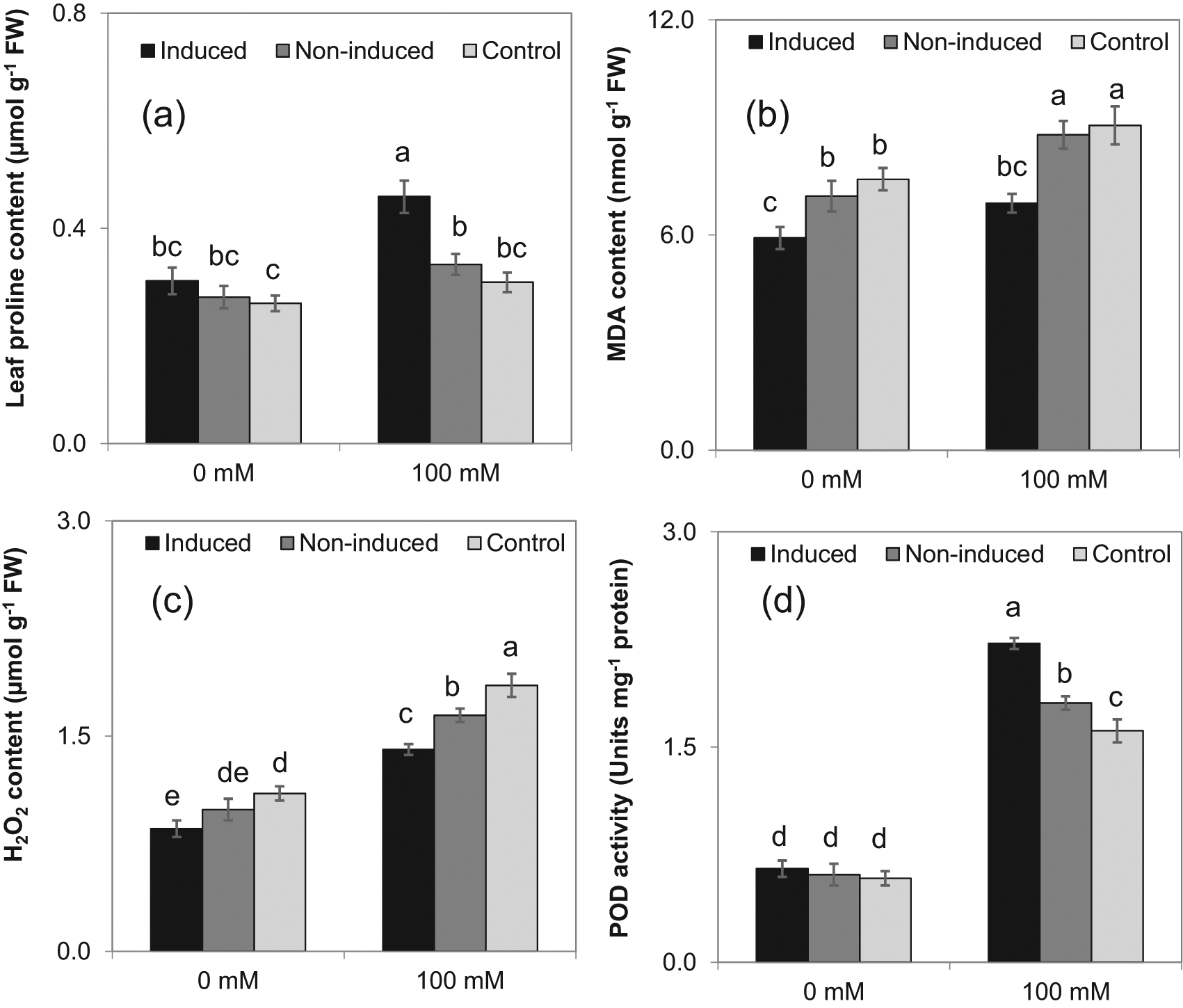
The means ± SE standard error are from six replications. Means with different letters are significantly different by Tukey Honestly Significant Difference (HSD) test at P≤0.05.
Growth and yield parameters
The fruit yield and dry mass of the plants were significantly reduced by 100 mM NaCl irrigation irrespective of pre-treatments (Figure 7). However, fruit yield and total dry biomass of induced plants were significantly greater than of non-induced plants which were greater than control plants in both irrigation treatments. The fruit yield of induced plants was significantly higher (66%) followed by the non-induced plants (24%) compared to control plants under saline irrigation. The total dry mass of induced plant was 94.9% and 88.6% higher than the control plant supplied with 0 and 100 mM NaCl, respectively.
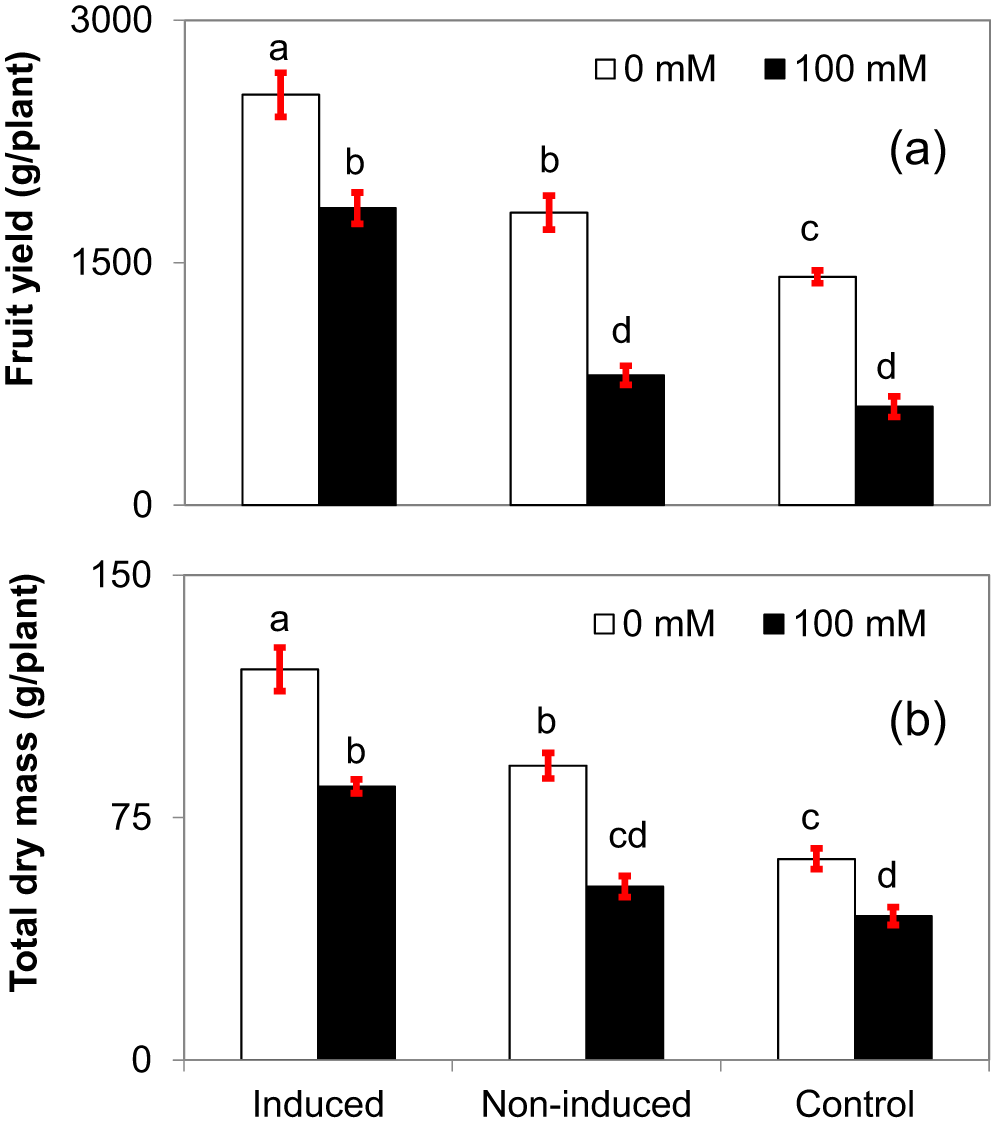
The means ± SE standard error are from six replications. Means with different letters are significantly different by Tukey Honestly Significant Difference (HSD) test at P≤0.05.
Salinity stress affects plants at all growth stages but it is more critical during seed germination as it can lead to seedling mortality.24 In the current study, the results showed that induced seedlings had significantly increased growth parameters (root length, vigour index, tissue water content). Salt induction initiated the salt acclimation process in seedlings, as well as vigorous radicle growth, which could be a major reason for better germination and initial growth. A salt induction treatment had an assimilative impact on the adaptive process in rice and soybean.25,26 Mung bean seeds treated with NaCl solution exhibited a positive root growth response compared to untreated seeds,27 similar to root growth and biomass of induced eggplant seedlings at challenging salinity in the current study. The predominant effects of salinity include inhibition of root initiation and reduction in growth.27 These effects observed with the eggplant plants from control and non-induced pre-treatments in the present study. An increased salt concentration causes a low water balance in tissue water,28,29 and reason for this reduction in fresh biomass of eggplants under non-induced and control treatments.
The pot experiment results indicated that the induced plants had higher relative water content and less negative leaf osmotic potential under the 100 mM NaCl irrigation. The change in leaf osmotic potential could be due to salt induction reducing dehydration by maintaining a better osmotic balance. Present findings followed the results of Al Hassan and co-workers on salinity induced changes in beans.20 Induced plants exhibited a higher leaf photosynthetic rate and stomatal conductance than the other treatments under both irrigation treatments. Salt stress can cause growth inhibition, and these responses were greater in control and non-induced plants than induced plants irrigated with 100 mM NaCl solution. It also maintained higher leaf photosynthesis rate by induced plants in the presence of 100 mM NaCl was likely due to maintenance of stomatal conductance.30 Higher photosynthesis rate also relies on a well-developed root system, which allows higher water uptake, and this was reflected in the higher stomatal conductance and unaffected photosystem machinery (PSII efficiency).31 Salinity stress significantly affects photosynthetic parameters in the all plants, but not same degree in induced plants, and the responses were similar to the wild-type eggplant S. insanum.32 The high PSII efficiency (Fv/Fm ratio) of induced eggplants could be due to the stability of the pigment system along with the antioxidant mechanisms for coping with the salinity stress.33 Similar antioxidant defense mechanisms in chloroplasts and mitochondria were observed in salt-tolerant pea (Pisum sativum) cultivars.34 Ascorbate plays a central role in protecting the chloroplast ultrastructure under salt stress conditions, and acts as a reactive oxygen species scavenger and is a cofactor in dissipating the excess light energy.35
Proline is an effective amino acid, osmolyte, an osmoprotectant, and high leaf proline content has been used to characterize as salt-resistant crops.17 Similarly, the induced plants had significantly higher proline content with 100 mM NaCl irrigation (Figure 6a). In addition, the proline content results emphasized that the SIR did not affect the plants under control irrigation (0 mM NaCl). The present findings are in contrast to the response of proline content in wheat (Triticum aestivum L.) plants and the temperature induction response.36 These observations signify that the salt induced eggplants irrigated with saline water mainly depend on desired change in osmotic potential as well as osmoregulation, to maintain the antioxidant activity near average values than the control plants (Figure 6d). The hydrogen peroxide and MDA content were elevated in maize (Zea mays L.) plants when exposed to a saline concentration.37,38
The lipid peroxidation and the hydrogen peroxide was less in induced plants. Malondialdehyde is the end product of lipid peroxidation process, and acts as a signal of cell membrane damage in plants.39 The increased level of MDA content in noninduced and control plants was due to salinity stress. The induced plants appeared to cope with salinity stress better than the other treatments. The non-induced and control plants exhibited responses similar to maize under salinity stress.18 Enhanced activity of peroxidase detoxifies the H2O2 content of the cytosol and chloroplast under stressful conditions.40 The leaf POD activity was also greater in induced than non-induced and control plants with saline irrigation, results corroborated by Meloni and coworkers41 in cotton (Gossypium spp.).
The increase in proline content and POD activity along with lower lipid peroxidation and H2O2 content of induced plants under 100 mM NaCl irrigation affirms that the salt-induced plants had better physiological acclimation during salinity stress. Upregulation of antioxidant enzymes of induced plants at the subcellular level alleviates salt stress effects, and these results are corroborated by wild tomato (Lycopersicon pennellii) under saline irrigation.18
The fruit yield and dry mass of the plants were significantly reduced by saline irrigation water irrespective of induction treatments. A similar reduction in eggplant was reported previously,42 and it might be due to cumulative build-up of soil salinity. However, such reductions were less in the induced plants when compared to non-induced and control plants. The SIR maintained photosynthesis as well as the antioxidant response with saline irrigation and contributed to greater fruit yield and growth (dry mass) of induced plants. Salt induction had a positive effect on leaf relative water content, which may be a result of accumulating excess salt in cell vacuoles, which in turn reduces the osmotic potential leading to pulling more water into the leaves. These responses agree with the leaf water status and photosynthetic rates of plants in the present study. Therefore, saline water irrigation had less effect on plant growth and fruit yield of induced plants, in contrast to a report of eggplant under saline irrigation by Hegazi and co-workers.43 Future improvements in the SIR should be focused on molecular changes such as a proteomic interaction or gene expression patterns related to the salt induction process.
The improvement of salt-tolerance in vegetable crops is a desirable technique to maintain production during salinity stress. The present SIR results elucidated an optimized protocol for eggplant as well as unravelled likely tolerance mechanism(s) protecting against a challenging salinity concentration during irrigation. Increased seed germination, vigour index, and seedling growth of induced seedlings showed the capability of quality seedling production with salinity tolerance. The responses to salt induction confirmed desirable leaf physiological parameters of eggplant such as relative water content, photosynthetic rate, and proline content. The fruit yield and dry mass were greater in induced plants than non-induced and control plants. The salt induction cycle applied to eggplant seeds improved tolerance of seedlings as well as to mature plants when irrigated with a sub-lethal level of NaCl (100 mM NaCl) and could make it suitable for growing eggplant using saline water. The present results of the SIR will be extended to studies of other crops and will be further studied at molecular and proteomic levels.
Open Science Framework: Inducing inherent salinity tolerance of eggplants by salt induction response DATA, https://doi.org/10.17605/OSF.IO/NQS95.44
Underlying data SIR.xlsx
Open Science Framework: Inducing inherent salinity tolerance of eggplants by salt induction response DATA, https://doi.org/10.17605/OSF.IO/NQS95.44
Extended Data SIR.pdf (Supplementary figure 1. Seedling germination of eggplants at 7 days after sowing (DAS) under different NaCl treatments. Supplementary figure 2. Standardization of interval time for the salt induction response. Supplementary figure 3. Seed treatment of the induction cycle in different NaCl concentrations at 120 minutes time interval. Supplementary figure 4. Seedling morphology of eggplants at 25 days after induction (DAI) under induced, non-induced and control treatments. Supplementary figure 5. Schematic representation of salinity induction response mechanism. Supplementary table 1. The pH and electrical conductivity (EC) of different NaCl solutions. Supplementary table 2. Leaf area (cm2) of eggplants grown in pots at 130 days after saline water irrigation under induced, non-induced and control treatments. Supplementary table 3 Soil electrical conductivity (EC) analysis of eggplants grown in pots after harvest (130 days after saline water irrigation) under induced, non-induced and control treatments.)
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
Authors are grateful to Fast Track Research Initiative (FTRI) 2019 and VIT Seed Grant, Vellore Institute of Technology, Vellore.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Abiotic stress physiology; plant nutrition and hormonal physiology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 28 Feb 22 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)