Keywords
Inspiratory muscle training, Abdominal surgery, Systematic review, Postoperative complications, Functional capacity
This article is included in the Manipal Academy of Higher Education gateway.
Inspiratory muscle training, Abdominal surgery, Systematic review, Postoperative complications, Functional capacity
The early postoperative period following abdominal surgery is associated with fatigue and limited chest movements due to surgical pain, restricted diaphragmatic mobility, anesthetic effect, site, and length of the surgical incision, reduced physical activity, and positional dependence.1 These factors alter the thoracoabdominal mechanism and length-tension relationship, reducing chest mobility and various post-surgical impairments such as inadequate air entry and impaired cough mechanism leading to postoperative pulmonary complications (PPCs).2,3
Alteration in the breathing pattern due to the effect of general anesthesia and peri-operative drugs causes changes in neural drive, which further reduces functional residual capacity (FRC) post-operatively, leading to ventilation-perfusion mismatch and eventually leading to hypoxemia and increase in respiratory rate.1,4
Additional factors such as surgical incision around the diaphragm and abdominal muscles, and length of incision influence the development of PPCs. The site of an incision limits the respiratory movement due to reflex inhibition of the phrenic nerve and response to pain stimulus from nerve innervating abdominal muscles. Also, as the length of the incision increases, the peritoneal area near the abdominal viscera is severely affected. As a result, open abdominal surgery has a higher chance of developing PPCs than laparoscopic surgery.2,4,5
Postoperative pain, limited respiratory movements, and analgesics are believed to be important factors associated with cough impairment.6 Inadequate cough reflex post-surgery is commonly related to the pathophysiological basis of PPCs as it leads to excessive secretion accumulation, reduced vital capacity, and increases the risk of respiratory infection eventually causing PPCs.6,7
Similarly, preoperative patient-related risk factor causes PPC. Risk factors such as age, pre-existing respiratory disease, obesity, and smoking history alter normal respiratory physiology.8 Thus, identification of preoperative risk factors and modification of modifiable risk factors is essential to reduce the occurrence of PPCs.9,10
Diaphragmatic weakness pre-operatively and post-operatively are identified as potential modifiable contributors in developing PPCs.11,12 The incidence of postoperative lung atelectasis and pneumonia is the main pathophysiological mechanism behind the development of PPCs. Hypoventilation due to altered consciousness, respiratory muscle weakness, decrease FRC with dependency in supine position causes reversible alveolar collapse resulting from obstruction of airways due to impaired mucociliary function and inadequate cough reflex, resulting in the retention of mucus and altering ventilation-perfusion ratio.13
Pre-rehabilitation and preoperative chest physiotherapy include deep breathing techniques, splinted active coughing, incentive spirometry (IS), inspiratory muscle training (IMT), and education regarding early mobilization helps in reducing the occurrence of PPCs. Effective training improves respiratory function pre-operatively and benefits in improving lung expansion postoperatively than no intervention.8,14
However, recent literature suggests that the effectiveness of incentive spirometry post-abdominal surgery has no impact on reducing PPCs and that IMT with or without additional therapy, given as pre-rehabilitation has a beneficial effect of reducing PPCs and length of hospital stay.8,11
Clinical trials of preoperative IMT have revealed fewer declines in inspiratory muscle strength postoperatively by promoting deep breathing and reducing the occurrence of PPCs.8 IMT aims to increase strength and endurance by applying a resistive load to inspiratory muscle to achieve a training effect.3 It helps to restore lung function rapidly thus assisting in improving lung expansion which facilitates forceful expiratory maneuver for secretion clearance and earlier recovery in the postoperative period.12
Despite the beneficial effect of IMT, there are few limitations such as improper experimental design depending on participants pre-surgical status, type of surgery, control of training intensity, and patient selection as its benefits changes according to training dose given, i.e., starting load, load increment, duration of intervention, frequency, duration of the training, number of sessions and degree of supervision.11,15
Various outcome measures are used by researchers to observe the clinical course of IMT with its relationship with PPCs, most notable being such as length of hospital stay, respiratory muscle strength (RMS), lung volume, and capacities. It has been observed that RMS reduces following major abdominal surgery due to surgical pain, exertion while breathing, and maybe pre-cursor of PPCs affecting lung volume and capacities postoperatively.12
Thus, this review aims to synthesize findings from a systematic review that evaluates the effectiveness of IMT on abdominal surgery.
An evidence-based review, on IMT for participants undergoing abdominal surgery was undertaken. The review adheres to the PRISMA checklist for reporting the systematic review and the same has been deposited into the online repository. (OSF registry (DOI 10.17605/OSF.IO/K8NGV). This review was registered in PROSPERO (177876).
The following criteria describe the scope of review
A. Population: This review included adult participants of either gender undergoing elective abdominal surgery. Abdominal surgery such as bariatric surgery, abdominal oncological surgery, abdominal aortic aneurysm, urological, esophageal, gastric, and biliary surgery, affecting peritoneal area due to surgical incision. Reviews on non-abdominal surgery such as cardiac, pulmonary, or thoracic surgery were excluded. Systematic reviews on mixed populations, i.e., focusing on abdominal surgery and other types of surgery were only included if data for abdominal surgery were presented separately.
B. Intervention: The review focused on the intervention of IMT (pre-operatively or postoperatively) as prescribed by the therapist for participants undergoing abdominal surgery.
C. Comparator: Comparison between the intervention of IMT and no IMT, sham IMT, or usual care such as deep breathing exercises, splinted coughing, and incentive spirometry was studied in this review.
D. Outcomes: All outcome measures presented in primary Randomized controlled trials (RCTs) were eligible for the review.
I. A systematic review consisting of both RCTs and observational studies on the comparison between IMT and other rehabilitation care.
II. Systematic Reviews of RCTs on pre rehabilitation without IMT or intervention other than IMT were excluded.
III. Literature reviews that did not have a specific research question, search strategy, or process of selecting articles were excluded.
Search strategy: Electronic databases searched were Medline/PubMed (RRID:SCR_004846), Cochrane database of a systematic review (RRID:SCR_013000) and ClinicalKey. The search was limited to English-language publications. Search strategies were developed to use across databases, combining terms of keywords of “Inspiratory muscle training, abdominal surgery, and systematic review.” Search terms were combined using Boolean operators ‘AND’ & ‘OR.’ To identify further relevant reviews, a reference list of screened articles was assessed for eligibility.
Study selection: Searches were done on Medline/PubMed, Cochrane, and ClinicalKey that were downloaded on Mendeley (RRID:SCR_002750) and de-duplicated. Two researchers (SK) and (KS) independently screened titles and abstracts. Any paper identified as potentially eligible for review by either researcher was studied in full text and independently screened by both reviewers.
The full-text articles were excluded for the following reasons:
Data extraction: The data was extracted under the following titles:
I. Study characteristics, i.e., name of the author, year of publication
II. Intervention
III. Comparator
IV. Outcome measures
One researcher completed the data extraction, and the second researcher cross-checked. Discrepancies were cross-checked by both researchers at a second review and reached a consensus. Although all outcome measures were extracted and presented in tables, only those that were measured in two or more studies were synthesized for meta-analysis.
Quality of evidence assessment: Quality assessment of systematic review was done using the AMSTAR-2 tool, a validated quality assessment tool for the systematic review. Quality assessment was done by one researcher and checked by the second researcher. Discrepancies were resolved in the discussion.
Meta-analysis was completed using RevMan software (RevMan 5.3) (RRID: SCR_003581). This review included both dichotomous and continuous outcome measures. Risk ratio (RR) with 95% confidence interval (CI) was computed for the dichotomous outcome, whereas, for continuous outcomes, mean difference/standardized mean difference with 95% CI was computed.
All meta-analyses were presented in the forest-plot graph. The random-effect model was adopted for the meta-analysis. To identify heterogeneity, chi2 statistic (p < 0.1 was considered statistically significant) and evaluated heterogeneity with I2 statistic (>60% considered substantial heterogeneity).
From the electronic database search, 1249 articles were identified; 40 were eligible for full-text review, and four systematic reviews and meta-analyses were included in the final review (Figure 1). Reviews were excluded because of unsuitable title and/or study design, primary intervention other than IMT, and report written other than the English language. Data for 276 participants were analyzed, 137 in the intervention group, and 139 in the control group.
The description of characteristics of the systematic review and primary RCTs are documented in table format (Table 1). The most common outcome measures reported were respiratory muscle strength (Maximum Inspiratory Pressure/Maximum Expiratory Pressure), the incidence of PPCs, pulmonary function test, length of hospital stay, and functional capacity (Table 2).
| Author, year | Type of review | Number of studies related to abdominal surgery | Number of studies included in the review | Intervention | Comparator | Outcome measures |
|---|---|---|---|---|---|---|
| Mans Christina et al., 201412 | Systematic review and meta-analysis of randomized controlled trials or quasi-randomized controlled trial | 3 | 8 | IMT | no pre-operative training or sham inspiratory muscle training or usual pre-operative care: early mobilization, coughing, wound support, deep breathing exercises, walking |
|
| Katsura M et al., 20158 | A systematic review of RCTs | 7 | 12 | IMT | Non-exercise intervention or no intervention, usual care: deep breathing exercise, incentive spirometry, coughing, and early mobilization | |
| Filipa Kendall et al., 201711 | Systematic reviews and meta-analysis of RCTs | 7 (pre-operative studies:6, post-operative studies:1) | 17 | IMT (before and after surgery) | Sham IMT, breathing exercise, incentive spirometry | |
| Xiaoqing Ge et al., 201816 | Systematic reviews of RCTs | 4 | 13 | IMT | No preoperative training or sham inspiratory muscle training |
| Author, year | Treatment dosage | Outcome measure | ||||||
|---|---|---|---|---|---|---|---|---|
| Control group | IMT group | Primary outcome measure | Secondary outcome measure | |||||
| Initial load | Number of sessions per week | Duration of each session | Session per day | Number of weeks of training | ||||
| Dronkers et al., 20083 | Deep breathing exercises (DBE), incentive spirometry (IS), coughing, and forced expiratory technique (FET) | 20% of MIP | 6 sessions, 6 days per week | 15 minutes | 2 weeks before surgery | |||
| Dronkers et al., 201017 | DBE, IS, coughing, FET, and home-based exercise program (30 mins per day) | 10-60% of MIP (supervised) And 20% of MIP (home-based) | 2 times per week in OPD | 15 minutes | 2-4 weeks before surgery | |||
| Kulkarni et al., 201018 | Group A: no training Group B: DBE Group C: IS | 20-30% of MIP | 15 minutes | 2 | 2 weeks before surgery | |||
| Barlbalho-Moulim et al., 20117 | Usual care | 30% of MIP | 6 times per week under the supervision | 15 minutes | 1 | 2-4 weeks before surgery | ||
| Casali et al., 20112 | IMT sham training | 40% of MIP | daily | 30 minutes | 1 | Post-operative day 2 to postoperative day 30 | ||
| Soares et al., 20131 | Pre-operative: no treatment Post-operative till POD7: DBE, huffing and coughing, airway clearance, and active limb mobilization | 15% of MIP | daily | 15 minutes | 2-3 weeks before surgery | |||
| Llorens et al., 201414 | Usual care and incentive spirometry | 30% of MIP | daily | 20 minutes | 30 days before surgery | |||
| Heynen et al., 2012 (conference article)19 | Usual care | 60% of MIP | 7 times per week | 2 weeks before surgery | ||||
| Da Cunha et al., 2013 (conference article)20 | Breathing exercise associated with upper and lower limb exercises | 60% of MIP, 3 series of 12 repetitions | 5 times a week | 2 weeks before surgery | ||||
The RCTs included in this review varied in terms of age, lifestyle, and type of surgery. Elderly populations were focused on the study Dronkers et al. (2008)3 and Dronkers et al. (2010).17 The obese population was focused on studies of Casali et al.,2 Llorens et al.,14 and Barbalho-Moulim et al.7 variation in the type of abdominal surgery was also observed. Relation of age, lifestyle, and type of surgery is being discussed under the discussion section.
The four systematic reviews focused on 9 RCTs. Overlapping between systematic reviews was observed. The overlapping of review has been summarized in Table 3.
| Dronkers et al.3 (2008) | Dronkers et al.17 (2010) | Kulkarni et al.18 (2010) | Barbalho-Moulim et al.7 (2011) | Casali et al.2 (2011) | Heynan et al.19 (2012) | Soares et al.1 (2013) | Da Cunha et al.20 (2013) | Llorens et al.14 (2014) | |
|---|---|---|---|---|---|---|---|---|---|
| Christina Mans et al.12 (2014) | + | + | + | ||||||
| Katsura M. et al.8 (2015) | + | + | + | + | + | + | + | ||
| Filipa Kendall et al.11 (2017) | + | + | + | + | + | + | + | ||
| Xiaoqing Ge et al.16 (2018) | + | + | + | + |
Quality assessment done by AMSTAR-2 has been summarized in Tables 4 and 5. Out of 4 systematic reviews, 3 systematic reviews were reported as high-quality reviews.
| PICO | Deviation from protocol | Explanation from study design | Comprehensive search strategy | Study selection in duplicate | Data extraction in duplicate | Justification from exclusion | Adequacy of detail | RoB assessment | funding | Meta-analysis: measures for statistics | Impact of RoB | Impact of RoB on results | Explanation for heterogeneity | Test for publication bias | Conflict of interest | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Christina Mans et al.12 (2014) | Y | Y | Y | PY | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y |
| Katsura M. et al.8 (2015) | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Filipa Kendall et al.11 (2017) | Y | PY | Y | PY | Y | Y | PY | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Xiaoqing Ge et al.16 (2018) | Y | N | Y | Y | Y | Y | PY | Y | Y | Y | Y | Y | N | N | Y | Y |
| Sr no. | Author name | Level of evidence (AMSTAR 2) |
|---|---|---|
| 1 | Mans et al.12 | High-quality review |
| 2 | Katsura M. et al.8 | High-quality review |
| 3 | Filipa Kendall et al.11 | High-quality review |
| 4 | Xiaoqing Ge et al.16 | Low-quality review |
This review involved meta-analyses of the following outcome measures:
1. Respiratory Muscle Strength
i. Maximum inspiratory pressure (MIP): Six studies were analyzed for MIP,1–3,7,14,17 observing 93 participants in the intervention group and 87 participants in the control group. Heterogeneity [I2] was 53% (Pheterogeneity = 0.06). The mean difference was 4.97 (95% CI -5.07 to 15.01) for the intervention group versus the control group (Figure 2).
ii. Maximum expiratory pressure (MEP): Two studies were analyzed for MEP,7,14 observing 38 participants in the intervention group and 38 participants in the control group. Heterogeneity [I2] was 0% (Pheterogeneity = 0.49). The mean difference was 3.32(95% CI -9.10 to 15.74) for the intervention group versus the control group (Figure 3).
2. Postoperative pulmonary complications:
i. Atelectasis: Three studies were analyzed for the occurrence of an adverse event of PPC (atelectasis),1,3,19 observing 35 participants in the intervention group and 37 participants in the control group. Heterogeneity [I2] was 0% (Pheterogeneity = 0.93). The risk ratio was 0.40 (95% CI 0.19 to 0.88) for the intervention group versus the control group. The test for overall effect (P = 0.02) was statistically significant, favoring intervention (Figure 4).
ii. Pneumonia: Five studies were analyzed for the occurrence of the adverse effect of PPC (pneumonia)1,17–20 observing 74 participants in the intervention group and 76 participants in the control group. Heterogeneity [I2] was 0% (Pheterogeneity = 0.57). The risk ratio was 0.41 (95% CI 0.14 to 1.19) for the intervention group versus the control group. Out of the five studies analyzed, four studies favor intervention (Figure 5).
3. Pulmonary function test
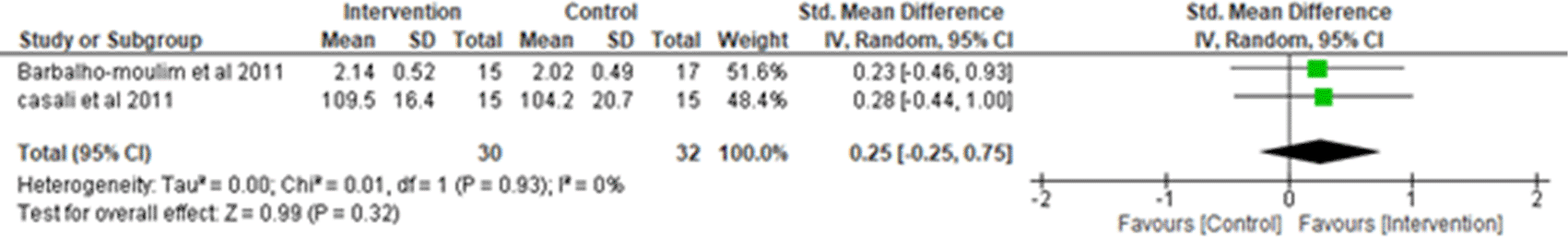
i. Forced vital capacity: Two studies were analyzed for forced vital capacity (FVC),2,7 observing 30 participants in the intervention group and 32 participants in the control group. Heterogeneity [I2] was 0% (Pheterogeneity = 0.93). The mean difference was 0.25 (95% CI -0.25 to 0.75) for the intervention group versus the control group (Figure 6).
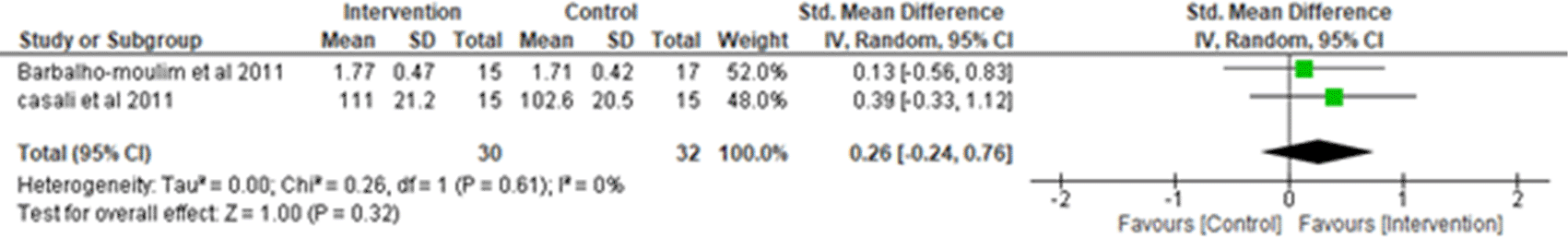
ii. Forced Expiratory Volume (FEV1) in the first second: Two studies were analyzed for FEV12,7 observing 30 participants in the intervention group and 32 participants in the control group. Heterogeneity [I2] was 0% (Pheterogeneity = 0.61). The mean difference was 0.26 (95% CI -0.24 to 0.76) for the intervention group versus the control group (Figure 7).
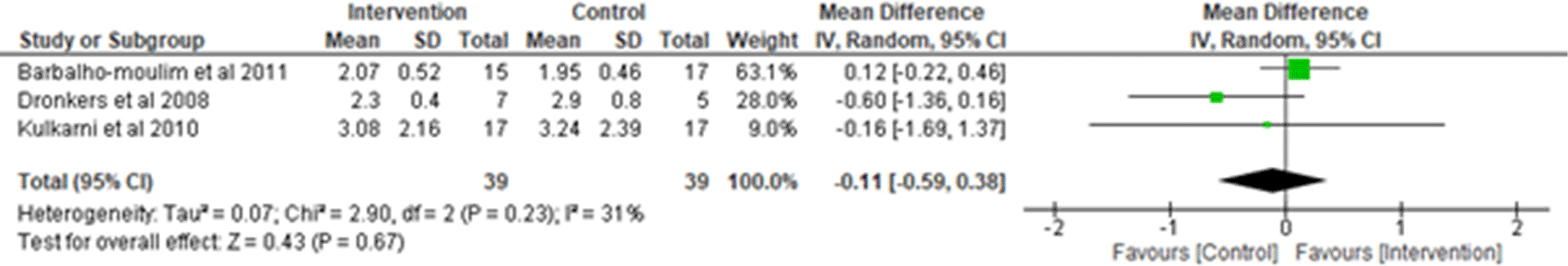
iii. Inspiratory vital capacity (IVC)/Vital Capacity (VC): Three studies were analyzed for IVC/VC3,7,18 observing 39 participants in the intervention group and 39 participants in the control group. Heterogeneity [I2] was 31% (Pheterogeneity = 0.23). The mean difference was -0.11 (95% CI -0.59 to 0.38) for the intervention group versus the control group (Figure 8).
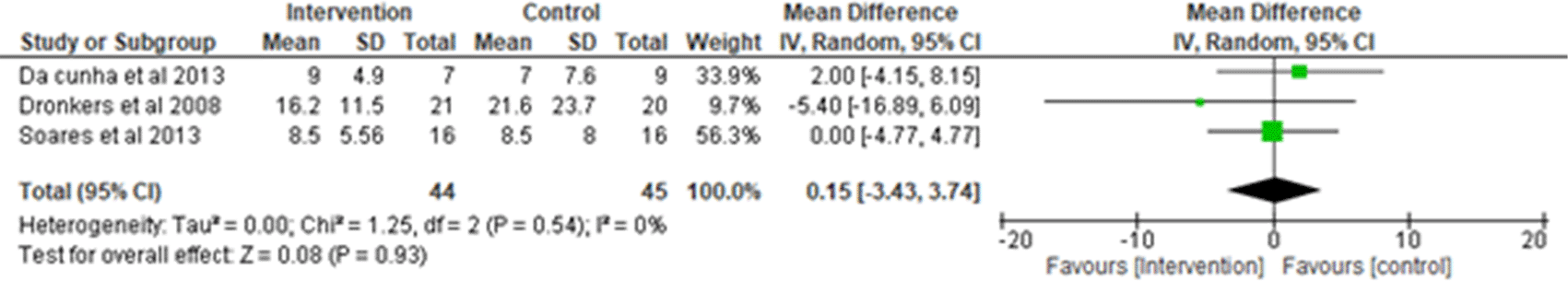
4. Length of hospital stay: Three studies were analyzed for the length of hospital stay1,17,20 observing 44 participants in the intervention group and 45 participants in the control group. Heterogeneity [I2] was 0% (Pheterogeneity = 0.54). The mean difference was 0.15 (95% CI -3.43 to 3.74) for the intervention group versus the control group. The test for overall effect (P = 0.93), concludes data to be statistically insignificant (Figure 9).
5. Functional capacity: One study reported functional capacity using a six-minute walk test. The preoperative and postoperative six-minute walk distance value (median and range) in the intervention group was higher as compared to the control group (Table 6).
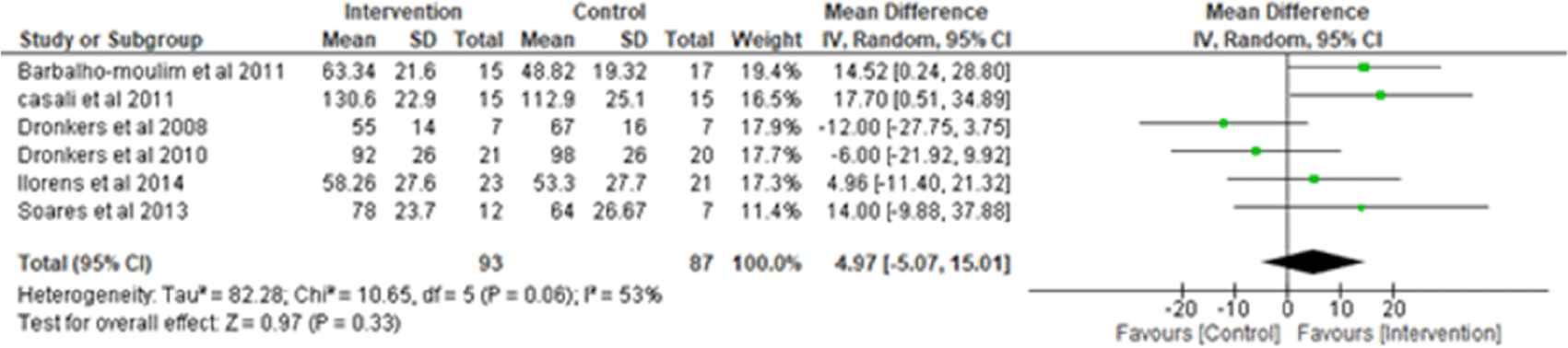
I2 = heterogeneity/test for heterogeneity, CI = confidence interval.
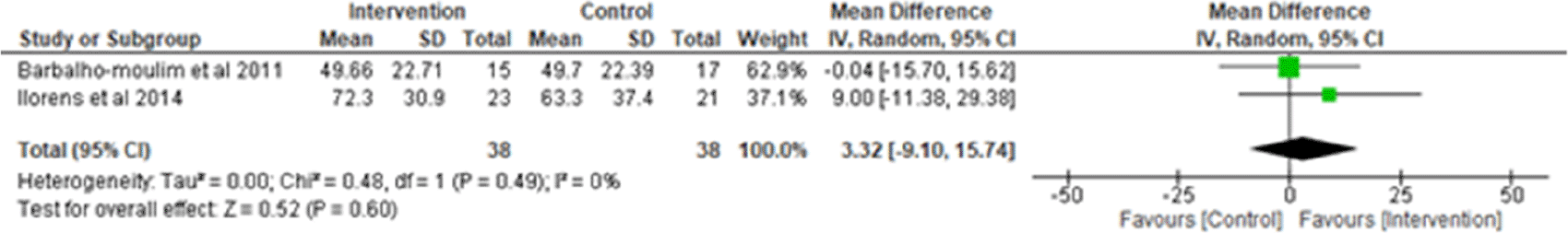
I2 = heterogeneity/test for heterogeneity, CI = confidence interval.
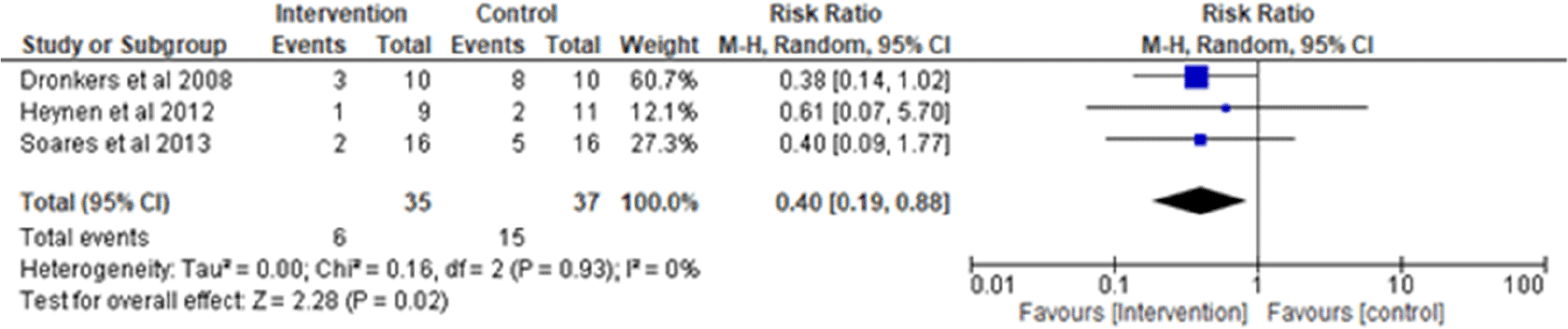
I2 = heterogeneity/test for heterogeneity, CI = confidence interval.
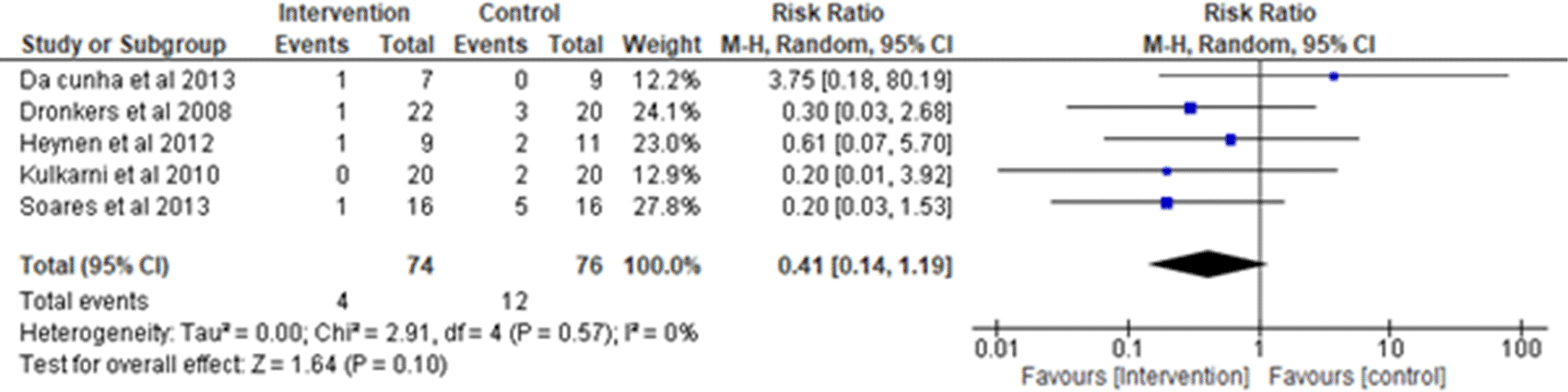
I2 = heterogeneity/test for heterogeneity, CI = confidence interval.
I2 = heterogeneity/test for heterogeneity, CI = confidence interval.
I2 = heterogeneity/test for heterogeneity, CI = confidence interval.
I2 = heterogeneity/test for heterogeneity, CI = confidence interval.
I2 = heterogeneity/test for heterogeneity, CI = confidence interval.
| 6MWD in meters: Soares et al. 20131 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention group | Control group | ||||||||||
| Pre-operative median | Pre-operative range | Pre-operative (N) | Post-operative median | Post-operative range | Post-operative (N) | Pre-operative median | Pre-operative range | Pre-operative (N) | Post-operative median | Post-operative range | Post-operative (N) |
| 514.4 | 460.8-557.5 | 12 | 486 | 392.3-562.3 | 12 | 441.5 | 412.3-505.9 | 8 | 447.3 | 373.7-465.8 | 8 |
This review was summarized using 4 systematic reviews and meta-analyses. The included systematic reviews highlighted the effect of IMT not only on abdominal surgery but also included cardiac and thoracic surgeries. However, this review only focused on abdominal surgery. Abdominal, cardiac, or thoracic surgeries lead to PPCs but the incision close to the diaphragm or respiratory muscle may have a higher incidence of PPCs compared to others. The reason can be viable tissue damage during surgery, pain, and diaphragm dysfunction.21
In cardiac surgeries, the most common procedure sternotomy has reported less respiratory muscle dysfunction as compared to abdominal surgery and thoracic surgery where the respiratory muscles are directly affected.22 The inspiratory muscle diaphragm accounts for 60–70% change in lung volume and capacities and, also, the inspiratory tone in the diaphragm prevents abdominal viscera from compressing lungs.23
Following abdominal or thoracic surgery, due to respiratory muscle dysfunction, a restrictive pattern develops in the immediate post-operative period. As per Laplace’s law, “curvature of the diaphragm is an important determinant of its ability to produce pressure.” Thus, in abdominal surgery, it is common to find a decrease in maximal inspiratory, transdiaphragmatic, and expiratory muscle pressure.22
In addition, surgery-associated conditions such as peritonitis, abdominal trauma, and fluid shifts can cause respiratory failure by increasing intra-abdominal pressure. This increase in intra-abdominal pressure can decrease chest wall compliance and diaphragmatic excursion.23
The meta-analysis was performed to compare various outcome measures for the participants undergoing abdominal surgery. The different outcome measures for quantitative analysis were respiratory muscle strength (MIP/MEP), PPCs (atelectasis and pneumonia), pulmonary functional capacity, and length of hospital stay, and qualitative analysis on functional capacity Six Minute Walk Distance (6MWD) The result suggests that IMT as preoperative or postoperative intervention plays a significant role in improving inspiratory capacity and then eventually reduces the incidence of PPC and health-related cost of care.
In a meta-analysis of MIP, the studies Barbalho-Moulim et al., Llorens et al., Soares et al. found a significant difference in pre-intervention and Casali et al. in post-intervention MIP level in the intervention group vs. control group, whereas Dronkers et al. (2008) and Dronkers et al. (2010) could not find any significant difference. Meta-analysis showed moderate heterogeneity between studies I2 = 53% and found a combined positive effect of IMT on MIP.1–3,7,14,17
Studies have shown that better MIP values among intervention groups are acknowledged due to the recruitment of motor units of respiratory muscle that promote increased muscle strength. Respiratory muscle, like any skeletal muscle, responds to the increasing load imposed by IMT and follows the physiological principle of muscle training. Threshold IMT offers a flow-independent one-way valve to ensure constant resistance. Thus, increment in frequency, duration, and intensity of IMT provides more load/resistance, improving muscle function and observing morphological changes in the diaphragm.24,25
Post-surgery, participants show restrictive ventilator defects due to reducing chest movement and modified breathing patterns. Threshold IMT is characterized by active recruitment of diaphragm and abdominal muscles by providing constant specific pressure for strength and endurance training of inspiratory muscles. Post IMT training, it has been observed that by increasing the inspiratory muscle function, there is an improvement in lung volume and capacities and improving physical capacity.
The result of the incidence of PPC atelectasis was found clinically and statistically significant. PPC pneumonia was found clinically significant, favoring the intervention group. IMT helps to achieve higher MIP pre-operatively and helps to retain higher MIP, as compared to control, postoperatively as well, by restoring pulmonary function rapidly. Filipa Kendall et al. suggest that IMT provides a better result as it helps to maintain strength and endurance postoperatively and prevents the decline of MIP in the early postoperative period and may reduce the occurrence of PPC.11
Postoperative respiratory muscle dysfunction reduces vital capacity, tidal volume, and total lung capacity, eventually leading to a reduction in FRC and causing atelectasis. According to Kulkarni et al., post IMT training participants’ ability to overcome elastic load to inhalation improved and helped in maintaining baseline level vital capacity. The author suggests that the maintenance of vital capacity improves cough efficacy and reduces changes to PPC. Similarly, Casali et al. reported that IMT was associated with early and faster recovery of FEV1, Peak Expiratory Force (PEF), and forced expiratory flow 25-75 (FEF25-75). Improved respiratory muscle strength permits larger lung volumes and provides an improvement in expiratory flow volumes.18
The length of hospital stay was neither clinically nor statistically significant. The incidence of PPC may increase the length of hospital stay. However, there are other reasons than PPCs such as pain, systemic complications, or wound dehiscence, which further affects the course of postoperative management effectively.
Functional capacity was measured using a 6MWD, which showed results favoring the intervention group, pre-operatively as well as postoperatively. A recent study done by Keeratichananont W. et al. reported that pre-operative 6MWD less than 325 m had a sensitivity of 77% and specificity of 100% to predict a high risk of PPC. However, only one study reported the use of 6MWD as an assessment of functional capacity. Similarly, a study done by Cargnin et al. showed that post-IMT there is a positive linear association between lung function and functional capacity due to increase inspiratory capacity which eventually leads to higher tolerance to fatigability and improve functional capacity.26,27 This concludes that 6MWD can be used as an alternative predictor of PPCs.
Most of the systematic review and primary RCTs focused on preoperative IMT training, only one study (Casali et al.) Focused on postoperative IMT training from post-operative day (POD)-2 to POD-30. Studies on preoperative IMT training show a positive effect on the reduction of PPC and prevent significant changes in MIP postoperatively. However, preoperative IMT training suggests elective abdominal surgery. Thus, more studies are required, which concentrate on postoperative IMT to provide better information regarding its effect on emergency surgeries. The primary RCTs included in the systematic review had a smaller sample size, thus providing a larger confidence interval among studies affecting pooled data analysis.
The population among studies varied according to their age, gender, and body mass index (BMI). Studies on elderly participants,3,17 obese participants,2,14 and obese female participants7 provide a broader spectrum to focus, and its effect on pooled data. Llorens et al. and Barbalho-Moulim et al. suggested that obese participants develop an increased risk of hypoxemia postoperatively because of altered body mechanics such as fat deposition over the abdominal region, abdominal viscera affecting descent of the diaphragm, and compression over the chest wall. This compression results in excessive strain to the diaphragm and causes mechanical disadvantage to the muscle and affects effective training.7,14 Dronkers et al. (2008) had a study population with age group younger in the control group (CG) as compared to the intervention group (IG) (mean (SD) of CG VS IG 59 (6) vs. 70 (6), respectively). The physiological changes that occur with age and the effect of surgery, cumulatively, might affect data analysis.3
Abdominal surgery involves a large variety of surgical procedures. Different types of surgery, based on the type of incision, i.e., open abdominal or laparoscopic surgery, area of the incision, length of incision, and the number of organs affected, helps to acknowledge the level of severity on the diaphragm and intercostal muscles and eventually to affect the biomechanics of respiration. The review had a heterogeneous group of upper and lower abdominal surgical procedures producing different levels of impairment on muscles of respiration. Kulkarni et al. focused on colorectal, gastrointestinal, vascular, and urological surgery, Llorens et al., on laparoscopic bariatric surgery, Barbalho-Moulim et al. and Casali et al. on bariatric surgery, Dronkers et al. (2008) on abdominal aortic aneurysm surgery, Dronkers et al. (2010) on elective abdominal oncological colon surgery and Soares et al. on surgery of esophagus, stomach, and biliary tract.1–3,7,14,17,18 Thus, variation in the type of surgery may have a varied effect on lung function and capacity.
The review involved a different type of surgical procedure, varied type of population, and lack of proper guidelines on IMT for upper and lower abdominal surgery. The starting load, maximal load, and dosage were different among studies. However, the maximum number of studies had starting load in the range of 20-30% of their MIP, pre-operatively,1,3,7,14,18 and Dronkers et al. (2010) had 10-60% of MIP as their starting load. The increment of the load depends upon participants' tolerance subjectively on Borg rating of perceived exertion (RPE). In most of the preoperative IMT studies, the duration of training is 15 minutes per day,1,3,7,17 Kulkarni et al.18 had two sessions of 15 minutes per day, and Llorens et al.14 had two sessions of 10 minutes.
In the only study on postoperative IMT, Casali et al. had two sessions of 20 minutes and 40% of MIP as their starting load from discharge to POD-30 for 30 minutes a day.2 Although the studies provided information regarding the starting load and duration of the training, authors of primary RCT failed to report on the number of breathing cycles, respiratory flow and pattern, and rest interval between or within each set. Subjective increment based on RPE was adopted by most of the therapists. However, the chances of participants achieving a maximal level of load according to their capacity are debatable. Fear of eliciting pain, fatigue, and low quality of adherence are some of the factors affecting proper increment.
The ideal respiratory maneuver should include alveolar inflating pressure, time for alveolar inflation, and inflating volume.28 Thus, the lack of knowledge on parameters such as inspiratory breathing pattern, volume near vital capacity or tidal volume, number of breaths per set, required flow, perioperative use, and progression of the procedure i.e., increment in frequency or dosage or both hinder various ideas about the methodological aspects of training and its effect on results and identify best effective IMT protocol.29 Also, supervision played a significant role. Studies with increment strictly imposed by the therapist and supervised had a longer duration of the training, higher progressive loading, and adhered to the given protocol, which indicates that such groups had a higher final maximal load and greater chance to produce better results.
The present review consists of only a systematic review of RCT. This review includes quantitative analyses of all objective outcome measures covered in RCTs and covered all types of abdominal surgery (bariatric, oncological, urological, oesophageal, gastric, and biliary). Thus, provides a wide range of understanding of the effect of IMT on abdominal surgery. There are fewer RCTs on abdominal surgery. Therefore, meta-analysis includes a smaller number of studies and a smaller population in few objective outcome measures. This study failed to focus on the effect of confounding factors (elderly population and obesity) on meta-analyses. Less number of RCTs that observe the effect of IMT in the post-operative period. Also, most of the studies were elective abdominal surgery; leading to the requirement of a study that focuses on IMT in the postoperative period, for emergency surgeries.
Inspiratory muscle training proves to be a beneficial intervention in improving MIP in patients undergoing abdominal surgery. Improvement in MIP has a positive association in reducing PPCs and improving lung function and capacity. Most of the systematic review included in this review agreed on the intervention of 2 weeks before surgery for 15 minutes can bring effective and positive outcomes in a wide range of the high-risk population. However, factors concerning breathing cycles, respiratory flow, and rest interval should be observed for better management post-operatively.
Conceptualization: Sampath Kumar Amaravadi, Khyati Shah, Stephen Samuel.
Data curation: Sampath Kumar Amaravadi, Khyati Shah.
Methodology: Sampath Kumar Amaravadi, Khyati Shah, Stephen Samuel, Ravi Shankar.
Resources: Sampath Kumar Amaravadi, Khyati Shah.
Writing – original draft: Sampath Kumar Amaravadi, Khyati Shah, Stephen Samuel, Ravi Shankar.
Writing – review & editing: Sampath Kumar Amaravadi, Khyati Shah, Stephen Samuel.
Formal analysis: Ravi Shankar
All data underlying the results are available as part of the article and no additional source of data is required.
PRISMA checklist for ‘Effect of Inspiratory Muscle Training on Respiratory Muscle Strength, Post-operative Pulmonary Complications and Pulmonary Function in abdominal surgery- Evidence from Systematic reviews’. doi: 10.17605/OSF.IO/K8NGV.30
Data are available under the terms of Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Partly
Is the statistical analysis and its interpretation appropriate?
Partly
Are the conclusions drawn adequately supported by the results presented in the review?
Partly
If this is a Living Systematic Review, is the ‘living’ method appropriate and is the search schedule clearly defined and justified? (‘Living Systematic Review’ or a variation of this term should be included in the title.)
Not applicable
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Exercise oncology, prehabilitation, cancer survivorship, exercise capacity, trial methodology, systematic reviews
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Partly
Is the statistical analysis and its interpretation appropriate?
Partly
Are the conclusions drawn adequately supported by the results presented in the review?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Pneumology and evidence-based medicine.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 03 Mar 22 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)