Keywords
practice guideline; reporting guideline; RIGHT statement; protocol; EQUATOR
This article is included in the Research on Research, Policy & Culture gateway.
practice guideline; reporting guideline; RIGHT statement; protocol; EQUATOR
AGREE: The Appraisal of Guidelines for Research and Evaluation
E&E: Explanation and Elaboration
EQUATOR: Enhancing the QUAlity and Transparency Of health Research
GIN: Guideline International Network
GRADE: The Grading of Recommendations, Assessment, Development and Evaluation
PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses
PROSPERO: International prospective register of systematic reviews
RIGHT: Reporting Items for practice Guidelines in HealThcare
RIGHT-P: RIGHT for practice guideline protocols
SR: Systematic Review
SPIRIT: Standard Protocol Items: Recommendations for Interventional Trials
WHO: World Health Organization
Trustworthy practice guidelines can inform clinical practice and decision-making and improve clinical outcomes.1 However, high-quality guidelines are currently scarce,2–5 despite many methodological advancements in the field,6–8 and the fact that most guideline developers have produced manuals and handbooks to support their processes.9–11 One potential explanation for this could be the lack of a standardized processes to develop and report the guideline protocols in a transparent format.
A research protocol is intended to help researchers to improve the quality of that research by defining a priori the methods to be followed and contributing to its transparent reporting. It is essential to have a standardized way to report these protocols. For example, the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) extension for protocols (PRISMA-P) was developed in 2015 to improve the reporting quality of systematic reviews and meta-analysis protocols.12 One study13 showed that the reporting quality of protocols of clinical trials has improved by 8.8% since the publication of the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) 2013 statement.14
To our knowledge, there are no widely agreed on guidelines or standards for how guideline protocols should be reported. The World Health Organization (WHO) guideline development handbook11 proposes that guideline protocols should address 17 topics. A 2019 study found that the contents of existing guideline protocols are diverse and vary.15 In 2017, a multi-international group of experts developed Reporting Items for practice Guidelines in HealThcare (RIGHT) statement reporting guideline for practice guidelines.6 Given the importance of guideline protocols, we will form an international group of stakeholders to develop the reporting guideline for guideline protocols. We intend to develop the RIGHT-Protocol (RIGHT-P), as an extension of the RIGHT statement for practice guideline protocols. The purpose of this paper is to describe the methods to be used for the development of the RIGHT-P.
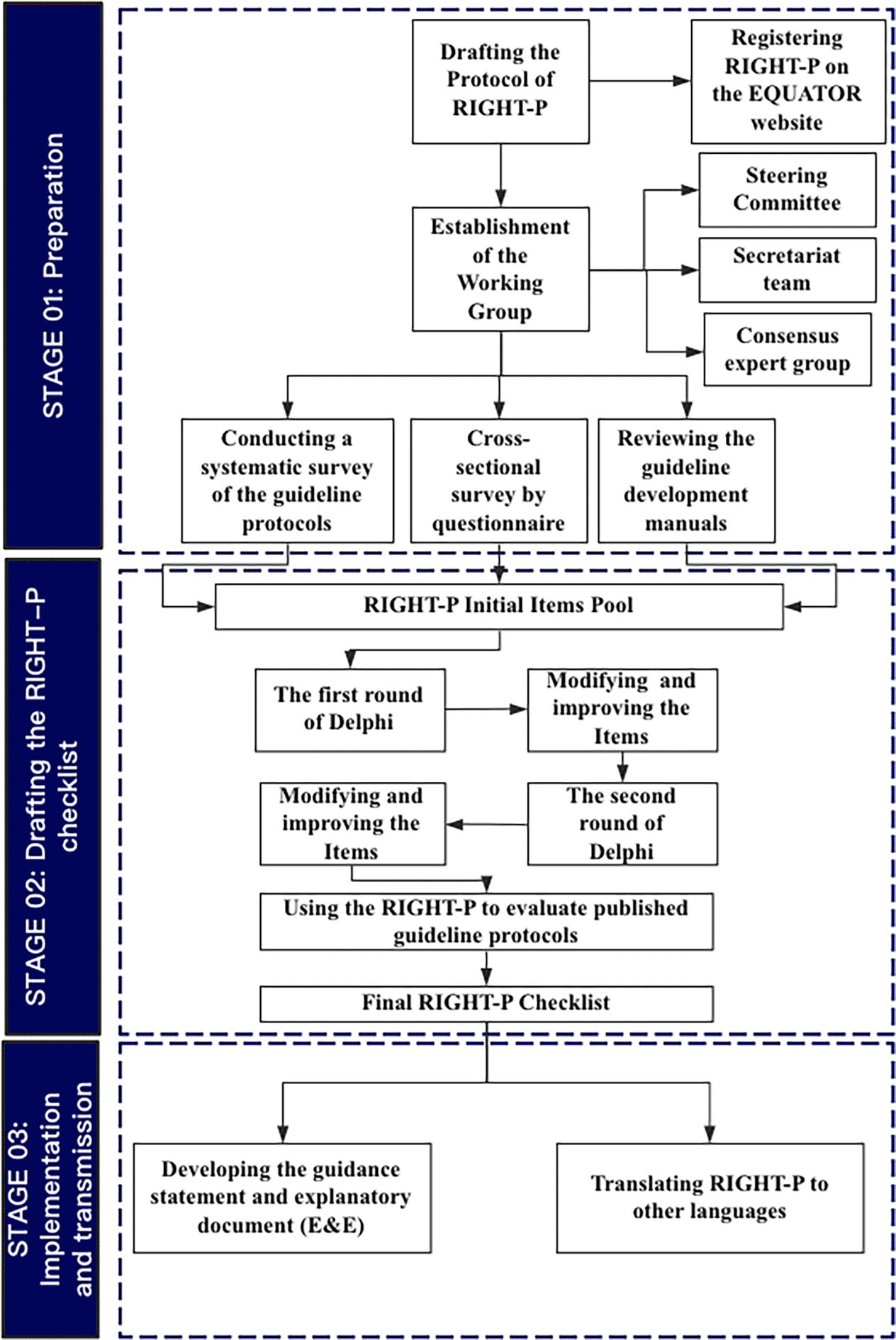
In this study, we define a guideline protocol as a document detailing the processes and methods to be followed for developing, adapting or updating guidelines. We will follow the toolkit on how to develop a reporting guideline recommended by Enhancing the QUAlity and Transparency Of health Research (EQUATOR) Network to develop the RIGHT extensions for guideline protocols.16 We will build RIGHT-P as an extension to the RIGHT checklist6 while building on the WHO guideline development handbook,11 as well as other guideline manuals collected through a systematic search. The target users of RIGHT-P are guideline developers/adapters, guideline evaluators, and guideline stakeholders. We expect to complete the development of RIGHT-P within two years, from August 2021 to December 2022. The process will consist of three stages: preparation, drafting the RIGHT-P checklist, and testing and dissemination. The detailed development process is illustrated in Figure 1, and the timetable is showed in Table 1.
Draft the protocol
To enhance the transparency and quality of the RIGHT-P development, we registered it on the EQUATOR network. We have also developed the protocol we are reporting in this paper, and the development of RIGHT-P will be initiated after the publication of this protocol.
Establish RIGHT-P working groups
We will establish three expert groups, a steering committee, a consensus expert group and a secretariat team. The steering committee will consist of 3-5 experts with rich experience in guideline development and reporting standards. The steering committee will form the consensus expert group, manage conflicts of interest of all participants in the project, provide consultation on the RIGHT-P development process, assure the quality of the development process, and approve the final version of the RIGHT-P.
The secretariat team includes three members from the RIGHT Working Group, of which Xufei Luo is the team’s leader and contact person for the RIGHT-P project. The team's responsibilities are to coordinate the consensus expert group and the steering committee, conduct the background research, such as, a systematic review on published guideline protocols, draft the initial RIGHT-P checklist, organize Delphi meetings, manage email communications, and draft the final RIGHT-P article.
The consensus expert group will consist of 20-30 multidisciplinary and international experts whose role is to participate in the Delphi survey and approve the final version of the RIGHT-P checklist. We will invite experts from the Guideline International Network (GIN), the Appraisal of Guidelines for Research and Evaluation (AGREE) Collaboration, the RIGHT Working Group, and the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) Working Group. We will also invite representatives of guideline developers/adapters and guideline users to participate in this expert group.
Conduct systematic survey of the guideline protocols
We are conducting a systematic review of published guideline protocols; that review has been registered on the PROSPERO (International prospective register of systematic reviews) website (CRD42021135732). We intend to extract the information reported for the protocols with published guidelines and then try to generate an initial pool of items for consideration in the RIGHT-P.
Cross-sectional survey and review the guideline manuals
We will design questionnaire to investigate the knowledge, attitudes, and behaviors of guideline developers/adapters, guideline users, and other stakeholders regarding the guideline protocols. The questionnaire consists of three parts: (1) the basic information about participants (e.g., gender, age, working time, title, units of employment, profession); (2) the knowledge, attitudes and behaviors with regards to developing and publishing a guideline protocol, and the degree of support for the development of the RIGHT-P; (3) request to propose 3-5 items that the RIGHT-P should address. We plan to use a convenience sample of 20-30 stakeholders outside the consensus expert group. We will use SurveyMonkey for data collection.
In addition, we will review the guideline development manuals and handbooks to collect the potential items. We will systematically search and analyze the guideline development manual, extract the relevant contents about the guideline protocols, and then classify and compile the relevant contents into the initial pool of RIGHT-P items.
Generate the initial pool of items for consideration
Based on the findings of the systematic review of the guideline protocols, the results of the cross-sectional survey and the review of handbooks, we will formulate a pool of initial items for consideration in the RIGHT-P. In addition, we will categorize the initial items into basic information, background, development methods, evidence, recommendations, and other information. We will use Microsoft excel 2016 software in the collection and management of all data and items.
Delphi consensus meeting
We will use a modified Delphi method to reach consensus on the RIGHT-P items. We will use a 7-point Likert scale for determining whether an item should be included. Participants will score each initial item on a scale of 1-7 for inclusion, with a score of 1 indicating strong disagreement with the inclusion of the item and a score of 7 indicating strong agreement with the inclusion of the item.17 We plan to have two rounds of the Delphi survey to collect the opinions, comments, and suggestions of the participants via the SurveyMonkey. If necessary, we will hold online meetings to help reach consensus through the Zoom Cloud Meeting App.
We will send the initial list of items to the consensus expert group members via the SurveyMonkey, and the first round of Delphi survey will allow the experts to score each item of the initial RIGHT-P checklist and give suggestions and modifications. At the same time the participants can add the items they consider important and not included in the initial item pool.
After collating the results of the first round of Delphi survey (median score for each item), we will generate the second round of Delphi questionnaire. If the median score of an item ≥ 6, we will include the item; if the median score of an item is between 3 (exclusive) and 6 (exclusive), we will enter it in the second round of Delphi survey; if the median score of an item is ≤ 3, we will exclude it.17 We will collate suggestions and comments, and consider them when drafting the list of the second round of Delphi survey. Suggestions and comments from experts will be recorded and responded to one by one.
We will conduct the second round of Delphi survey two months after the first one, using the same scoring scheme as in the first round. We will exclude items with a median score ≤ 3 and include items with a median score ≥ 6. For items with a median between 3 and 6, the steering committee will make the final decision regarding inclusion/exclusion after considering expert suggestions and comments from the second round.
All ideas and comments will be recorded and documented by the secretariat team, and after the second round of the Delphi survey, the steering committee will generate a RIGHT-P checklist draft version 1 and revise for final approval via email.
Use the RIGHT-P to evaluate published guideline protocols
To assess the feasibility and applicability of the RIGHT-P checklist draft version 1, we plan to use it to evaluate published guideline protocols that included in prior systematic survey. We will use the results of that step to copy with any further edits to the draft RIGHT-P checklist version 1.
Quality assurance and final approval
Upon revision of the RIGHT-P checklist based on the results of the evaluation for guideline protocols, we will generate a draft version 2 of the RIGHT-P checklist. We will invite the guideline developers/adapters, guideline users, and other stakeholders to provide feedback and perform external review regarding the draft version 2 checklist. Finally, we will send the revised draft checklist version 2 to the steering committee and the consensus expert group for final approval. To ensure that the RIGHT-P project runs smoothly, we plan to record each step of the process in detail and post it on the RIGHT statement website after the RIGHT-P checklist is published.
Develop the Explanation and Elaboration (E&E) document
Once the final RIGHT-P checklist is finalized, we intend to develop detailed explanations and elaborations document to explain and provide example(s) for each item to facilitate the understanding and adoption of the reporting checklist by the target users.
Translate RIGHT-P to other languages
To facilitate the understanding and dissemination of the RIGHT-P, we plan to contact those interested to translate the RIGHT-P checklist into non-English languages such as Chinese, French, Japanese, Korean, and Spanish. We will post the different langue version on the RIGHT statement website as soon as they are finalized. In addition, the readers who are interested can contact the RIGHT-P developers to collaborate on its translation into the language of their choice.
Patient and public involvement
Patient and public will not be involved in this study.
Study status
Currently, we are working on reviewing the guideline manuals and generating the initial pool of RIGHT-P items.
Despite the fact that few guideline protocols have been published, their importance to guideline development cannot be overstated. This is analogous to the importance of protocols to clinical trials,14 and systematic reviews.12 This project will develop an extension of the RIGHT statement for protocols, following the standard methods of international reporting guidelines development.16 Also, it will engage relevant stakeholders from WHO, AGREE, GIN and other international guideline development organizations.
Since the publication of the RIGHT statement in 2017,6 guideline developers have increasingly emphasized reporting on the guidelines and their different procedures, types, fields, and sections. Researchers have developed extensions for patient version guideline (RIGHT-PVG),18 Traditional Chinese Medicine (RIGHT-TCM).19 Extensions for acupuncture (RIGHT-Acu)20 and guideline adaptation (RIGHT-Ad@pt)21 are currently in development. We aim to enhance and ensure the quality of guideline development by promoting and standardizing the writing and reporting of guideline protocols. The RIGHT Working Group aims to inform guideline developers of the importance of registration22 and protocol drafting, and promote and advocate for protocol drafting in the guideline development process.
We hope that the RIGHT-P will be helpful to guideline developers/adapters in the planning stage of guideline development/adaptation in terms of clarifying the rigorous schedule steps and improving the quality of reporting in the protocol.
The RIGHT-P project is funded by “the Fundamental Research Funds for the Central Universities (lzujbky-2021-ey13)”. The funders will have no role in the study design, data collection and analysis, writing of the article or the decision to submit it for publication.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the rationale for, and objectives of, the study clearly described?
Partly
Is the study design appropriate for the research question?
Yes
Are sufficient details of the methods provided to allow replication by others?
Partly
Are the datasets clearly presented in a useable and accessible format?
Not applicable
Competing Interests: I am employed by the UK National Institute of Health ad Care Excellence (NICE), which produces health and social care guidelines.
Reviewer Expertise: Guideline development methods, systematic reviewing, evidence-based healthcare, qualitative evidence synthesis.
Is the rationale for, and objectives of, the study clearly described?
Partly
Is the study design appropriate for the research question?
Yes
Are sufficient details of the methods provided to allow replication by others?
No
Are the datasets clearly presented in a useable and accessible format?
Not applicable
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: epidemiology, public health, reporting guidelines, mental health, meta research, science communication
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 04 Mar 22 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)