Keywords
Sorafenib, Epigallo-3-Catechin Gallate, Vascular Endothelial Growth Factor, Microvascular Density, N-Nitrosodiethylamine
Sorafenib, Epigallo-3-Catechin Gallate, Vascular Endothelial Growth Factor, Microvascular Density, N-Nitrosodiethylamine
Hepatocellular carcinoma (HCC) is the most common primary type of liver cancer. In 2013, the prevalence of liver and bile duct cancer in a developed country like the United States was 30,640.1,2 Meanwhile, a high incidence of HCC was discovered in South and East Asia, Central and West Africa, Melanesia, and Micronesia/Polynesia. It has been estimated that there are more than 749,000 new cases of HCC in men and 226,000 in women every year.3,4 In 2020, liver cancer was considered the sixth most common cancer and the third leading to cancer-related deaths in the world.5
Vascular endothelial growth factors (VEGF) play an essential role in HCC tumor growth. Several carcinogens and tumor promoters initiate inappropriate activation of nuclear factor kappa B (NF-kB), which mediates the inflammation process and tumorigenesis. Meanwhile, overexpression of VEGF increases blood vessels permeability, leading to the differences between oxygen flow and delivery. A high level of VEGF is also typical in chronic liver disease that often triggers HCC.6,7 Micro-vessel density (MVD) is a tumor indicator of angiogenesis that needs to be examined in HCC since a higher level of MVD shows a poor prognosis. This high angiogenic activity can be inhibited through the administration of anti-angiogenic drugs.8
The most common management of HCC for operable cancers is liver resection, while chemotherapy and targeted therapy are also used. It was discovered that 80% of HCC patients are diagnosed with advanced-stage or inoperable cancer. Systemic therapy with Sorafenib is required to change the condition at the operable stage. Sorafenib has been proven to be the first systemic therapy that successfully improved HCC patients’ survival rate. It is an oral multi-kinase inhibitor that targets vascular endothelial growth factor receptor (VEGFR)-1, VEGFR-2, and VEGFR-3, thereby, reducing tumor angiogenesis. The disadvantages of Sorafenib treatment include high cost and approximately 30% of all patients responded to the treatment. Monotherapy of Sorafenib can cause several patient complaints, resistance, and high costs, therefore, when given in low-dose and combination with herbal medicines, the same effect is expected which is more affordable in price.9–12
Epigallocatechin-3-gallate (EGCG) is an active ingredient that was proven to prevent the growth of blood vessels in experimental animals. Its mechanism of action is by inhibiting urokinase and tyrosine kinase, which activates VEGF, epidermal growth factor (EGF), and fibroblast growth factor (FGF).13 In 2005, a previous study in Japanese stated that EGCG induces both in vitro and in vivo liver cell apoptosis to improve the prognosis of HCC. In vitro studies showed that the effective concentration of EGCG varies from 1 to 100 mol/L. According to pre-clinical studies that were carried out in rats, only less than 5% of oral catechin taken as a constituent of tea can reach systemic circulation, therefore, intraperitoneally administration is considered more effective. EGCG is the right choice to be combined with Sorafenib in advanced HCC, which uses the synergism of the two drugs. This combination can lead to similar effects as the Sorafenib standard dose.14,15
Therefore, this study aims to investigate the effectiveness of anti-angiogenic activity between Sorafenib standard dose and the combination of low dosage Sorafenib with EGCG. It was presented in adherence to the checklist of ARRIVE reporting guidelines.
This study used a randomized control trial post-test only design method (Figure 1). A total of 25 male Wistar rats (PT Biomedical Technology Indonesia), which were 7 weeks old with bodyweight 200–250 grams were placed in a cage with a controlled temperature of 22°C under 12 hours of light and dark cycle. The rats were given free access to food with AIN76 standard dietary formula for rodents, which was 67.7% carbohydrates, 11.5% lipids, and 20.8% protein from the Food Engineering Laboratory, IPB, Bogor, Indonesia, purchased from PT Surya Science and Beverages.
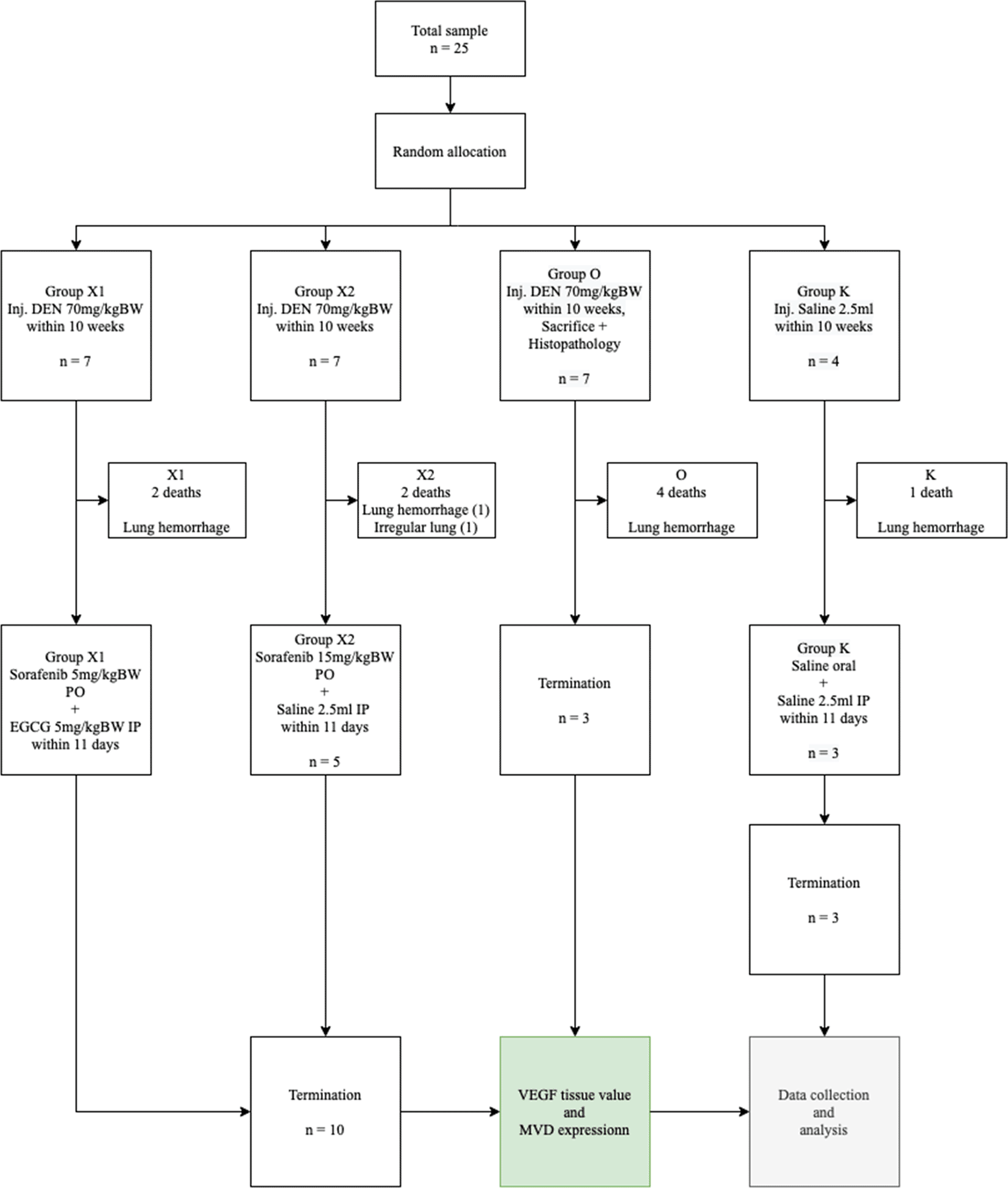
DEN: Diethyl-Nitrosamine, EGCG: Epigallocatechin-3-gallate, MVD: Micro-vessel density, VEGF: Vascular endothelial growth factor.
Diethyl-Nitrosamine (DEN) (N0756) with a molecular weight of 102.14 and Epigallocathecin-3-O-Gallate (Y0001936; primary pharmaceutical grade standard) with a molecular weight of 458.37 was purchased from Sigma-Aldrich. While, each tablet of Sorafenib contains 200 mg as Tosylate (Nexavar).
This study followed the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and was approved by Mochtar Riady Institute for Nanotechnology Ethics Committee (MRIN EC) with protocol number 2101001-AS06. The inclusion criteria were healthy and active 7-week-old male Wistar rats weighing 200–250 grams, while unhealthy male Wistar rats with anatomical anomalies are excluded. During the experiment, any infected or dead Wistar rats were also dropped out.
The sample size was calculated using the degree of freedom (Minimum and Maximum sample) formula. Rats were randomized and allocated into 4 groups, consisting of 7 rats in each group, except the control (K) group that contained 4 rats, with a minimal sample size of three. Subsequently, DEN was injected intraperitoneally in the abdominal area below the umbilicus on 21 rats for two treatment groups and a control group, with 70 mg/kg BW/week for 10 weeks.16,17 After 10 weeks, all rats were randomly divided into 4 groups, namely sham (K), Sorafenib 5 mg/kg BW + EGCG 5 mg/kg BW (X1), Sorafenib 15 mg/kg BW (X2), and without treatment (Group O). Group K was injected with saline for 10 weeks parallel with other groups. After the administration of DEN, group O was sacrificed, and a pathologist carried out a histopathological examination of liver tissue to show the formation of HCC. During the examination, anaplastic cells, oval nucleus, pleomorphic, coarse chromatin, and nucleolus invasive growth into stroma were observed, which confirmed HCC.
Sorafenib was dissolved in a maximum of 1.5 mL saline (maximum 10 mL/kg BW/day) and administered orally at a dose of 5 mg/kg BW and 15 mg/kg BW. Subsequently, EGCG 5 mg/kg BW/day was dissolved in approximately 1.5 mL saline (maximum 20 mL/kg BW/day) and administered by intraperitoneal injection once a day for 14 days. The sham group (K) was administered a saline solution orally and intraperitoneally, while the sorafenib-only group (X2) received intraperitoneal saline and oral Sorafenib. Meanwhile, the combination group of EGCG and Sorafenib (X1) received intraperitoneal EGCG and oral Sorafenib and the bodyweight of the rats was measured once a week. At the end of the experiment, the rats were sacrificed, exsanguination was done on deeply anesthetized animals with ketamine 80 mg/kg BW and xylazine 100 mg/kg BW intramuscularly to ameliorate any suffering, and the liver tissues were resected and examined microscopically. Moreover, a veterinarian carried out a necropsy when any rat died during the experiment to investigate the cause of death investigate the cause of death (Figure 2).
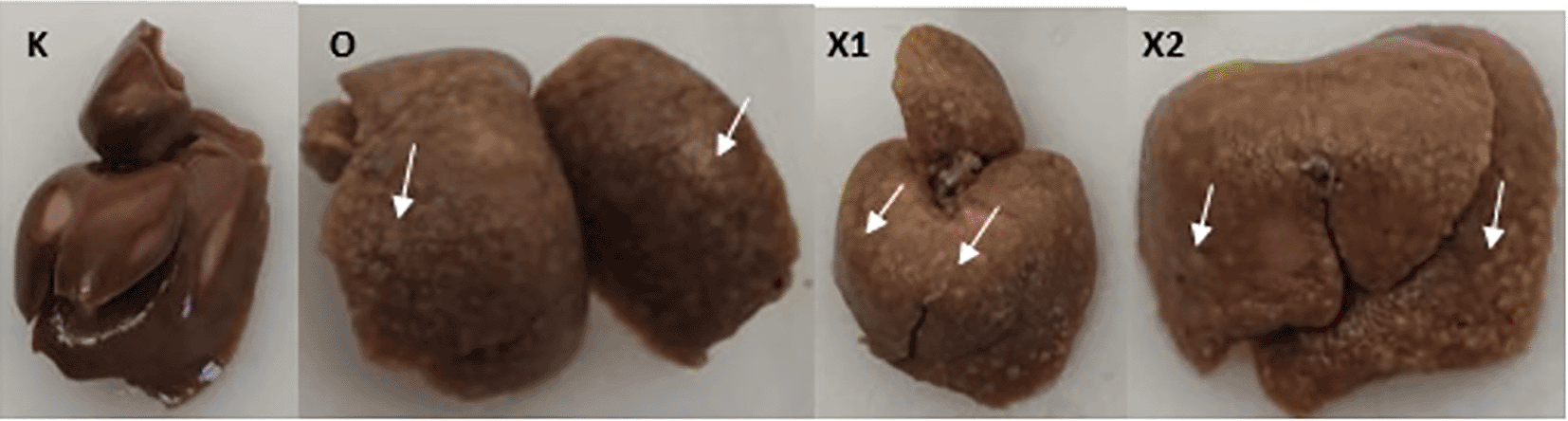
Group X1, X2, and O present multiple white nodules (White arrow); group (K) did not develop any nodules.
In liver tissue, VEGF was evaluated using an enzyme-linked immunosorbent assay (ELISA) quantitative methods, while MVD was calculated using Immunohistochemistry (IHC). The intensity and area of sinusoidal endothelial staining were measured quantitatively using a microscope at 100× magnification. Furthermore, the hot spots from the immunohistochemistry were selected using the “color selection” function and the “area/density (intensity)” function (ImageJ, RRID:SCR_003070) to calculate the values.
To prepare lysate from tissue, tissue of interest was dissected with clean tools. Dissected tissue was placed in microcentrifuge or Eppendorf tubes. Lysis buffer consisting of NP-40 buffer, sodium chloride, NP-40, Tris pH 8.0, and Triton X-100 or NP-40) was added to 5 mg of tissue and homogenized rapidly. Next, it was centrifuged at 4°C for 20 minutes. After carefully removing the tubes and placing them on ice, any supernatant was aspirated, and the pellet were discarded.18,19
A Bradford, a Lowry, or a bicinchoninic acid (BCA) assay was conducted to calculate protein concentration. Bovine Serum Albumin (BSA) is usually used as a standard protein. Each sample was frozen at -20°C for immunoprecipitation. 200μL of 1X Bradford reagent, 5 μL of BSA, and 30 μL of the unknown sample were added to each test tube. Absorbance was determined using sipper or individual cuvettes at 595 (VIS lamp).
All standards and samples were prepared twice as recommended and stored at room temperature. Each well containing standard and sample were incubated for 2.5 hours at room temperature. The washing process using 300 μL of Wash Buffer was repeated four times. All liquid was eliminated after each step to achieve the best result. After the last wash, the plate was inverted and blotted using a paper towel.
Approximately 100 μL of 1× Detection Antibody was titrated and incubated at room temperature for one hour. All liquid was removed, 100 μL of Streptavidin solution was added and incubated at room temperature for 45 minutes. Approximately 100 μL of TMB One-Step Substrate Reagent (Item H) was added and incubated in dark condition for 30 minutes. Lastly, 50 μL of Stop Solution (Item I) was added and absorbance was recorded at 450 nm.
Deparaffinization was done in incubator at 60°C for 45 minutes, followed by deparaffinization in xylene for 10 minutes. Next, 96% ethanol, 80% ethanol, and 70% ethanol were subsequently added to the formalin-fixed paraffin-embedded tissue for 5 minutes. Tissue was washed using distilled water. Antigen retrieval buffer (citrate buffer + tween) was placed into a jar and microwaved in full power for 20 minutes. The jar was removed and chilled on ice for 20 minutes.
Several drops of Hydrogen Peroxide Block were added to the section, incubated for 10 minutes, and rinsed two times in buffer. Protein Block was then added, incubated for 10 minutes, and rinsed once in buffer. Primary MVD polyclonal antibody (MBS 2520154) was added (1:100) in PBS-T, incubated in 4°C for 2 hours, and rinsed four times in buffer. A Biotinylated Goat Anti-Polyvalent was added, incubated for 10 minutes, and rinsed four times in buffer. Streptavidin Peroxidase was added, incubated for 10 minutes, and rinsed four times in buffer.
Approximately 30 μL of DAB Chromogen was applied into 1.5 mL of DAB Substrate. It was incubated for 3 seconds and rinsed four times in buffer. Next, Hematoxylin was used as a counterstain, incubated for 20 minutes, and rinsed in tap water. Tissues were dehydrated using 70% ethanol, 80% ethanol, and 96% ethanol, each for 1 minute. The samples were observed under 10×, 40×, and 100× magnification.20 For MVD, the Spearman’s correlation coefficient (rho) was 0.93 (p < 0.01), while intra-observer agreement (Kappa) were 0.88 for cut-off using mean.20,21
All data were expressed as mean ± standard deviation of the mean. The statistical analysis was carried out using SPSS 28 (IBM SPSS Statistics, RRID:SCR_019096). All data were normally distributed and the comparisons between groups were analyzed using ANOVA. Post hoc analysis using the least significant difference (LSD), where a p-value < 0.05 was considered statistically significant.
This study showed that the Sorafenib-only group effectively reduced VEGF tissue level better than without the treatment group. However, there was no significant difference in reducing MVD expression compared to the combination of low-dose Sorafenib and EGCG group, which indicated better overall results than the Sorafenib-only group. During the experiment, 9 rats died due to pulmonary hemorrhage and one rat died with irregular lung surface.
A total of 13 rats survived the 11 weeks of the experiment, although some looked unhealthy. One group reached the minimum sample size based on the calculation of the degree of freedom, the Institutional Animal Care and Use Committee (IACUC) Guidebook, and the World Health Organization (WHO). At the end of the experiment, the whole group of mice was terminated according to the euthanasia techniques based on IACUC and the American Veterinary Medical Association (AVMA) Guidelines.
The Shapiro-Wilk test results were used to calculate the mean and the distribution of the rats’ body weight data and a p > 0.05 was obtained for all groups. Homogeneity test results with Levene’s test obtained p = 0.978, which showed that the data obtained is homogeneous.
From the 16 sample slides examined, it was discovered that the tumor growth was solid with anaplastic cells having round, oval, pleomorphic nuclei, coarse chromatin, and prominent nucleolus that grows invasively into the stroma (Figure 3).
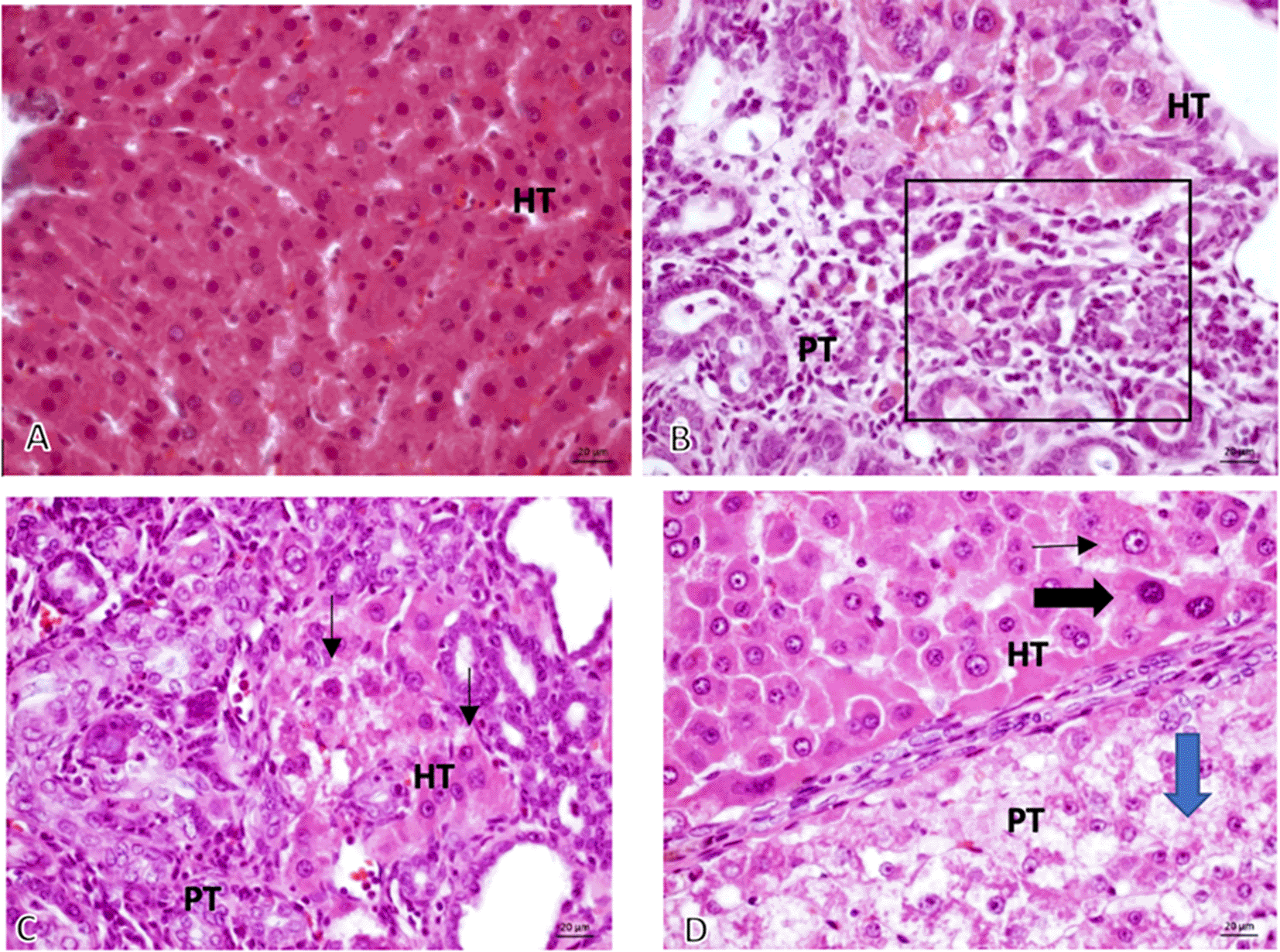
Microscopic 40× magnification on non-induced Diethyl-Nitrosamine (DEN) group: (A, Group K) normal hepatocyte; comparing graphic on DEN induced group: (B, Group O) Bile Duct hyperplasia (Black square area); (C, Group X1) Hyperchromatic cells (black-arrow) and (D, Group X2) Prominent cell (thin-arrow), Hyperchromatic cell (thick black – arrow), Ballooning degeneration (Blue-Arrow). HT, Hepatocyte; PT, Porta tract.
The mean VEGF values (Figure 4) between group Sorafenib 5 mg/kg BW + EGCG 5 mg/kg BW (X1) (106,682 ± 41,024) and group Sorafenib 15 mg/kg BW (X2) (214,5162 ± 67,717) had significant difference (p < 0.05), which showed that group X1 had the strongest effect in reducing VEGF values. Furthermore, the VEGF values between group X1 and the group without treatment (O) (318,101 ± 55,078) was significantly different (p < 0.05). A similar result was seen since VEGF values between group X2 and O was significantly different (p < 0.05).
MVD evaluation was carried out using 10× and 40× magnification to measure the intensity and area of sinusoidal endothelial staining (Figure 5). Subsequently, the hot spots from the immunohistochemistry were selected, and values were calculated. The mean MVD expression between the group Sorafenib 5 mg/kg BW + EGCG 5 mg/kg BW (X1) (36 ± 4.416) and group Sorafenib 15 mg/kg BW (X2) (26.2 ± 4.55) had no significant difference. Based on the results, MVD expression (Figure 6) between all groups and group O (176 ± 19) showed a significant difference (p < 0.05).
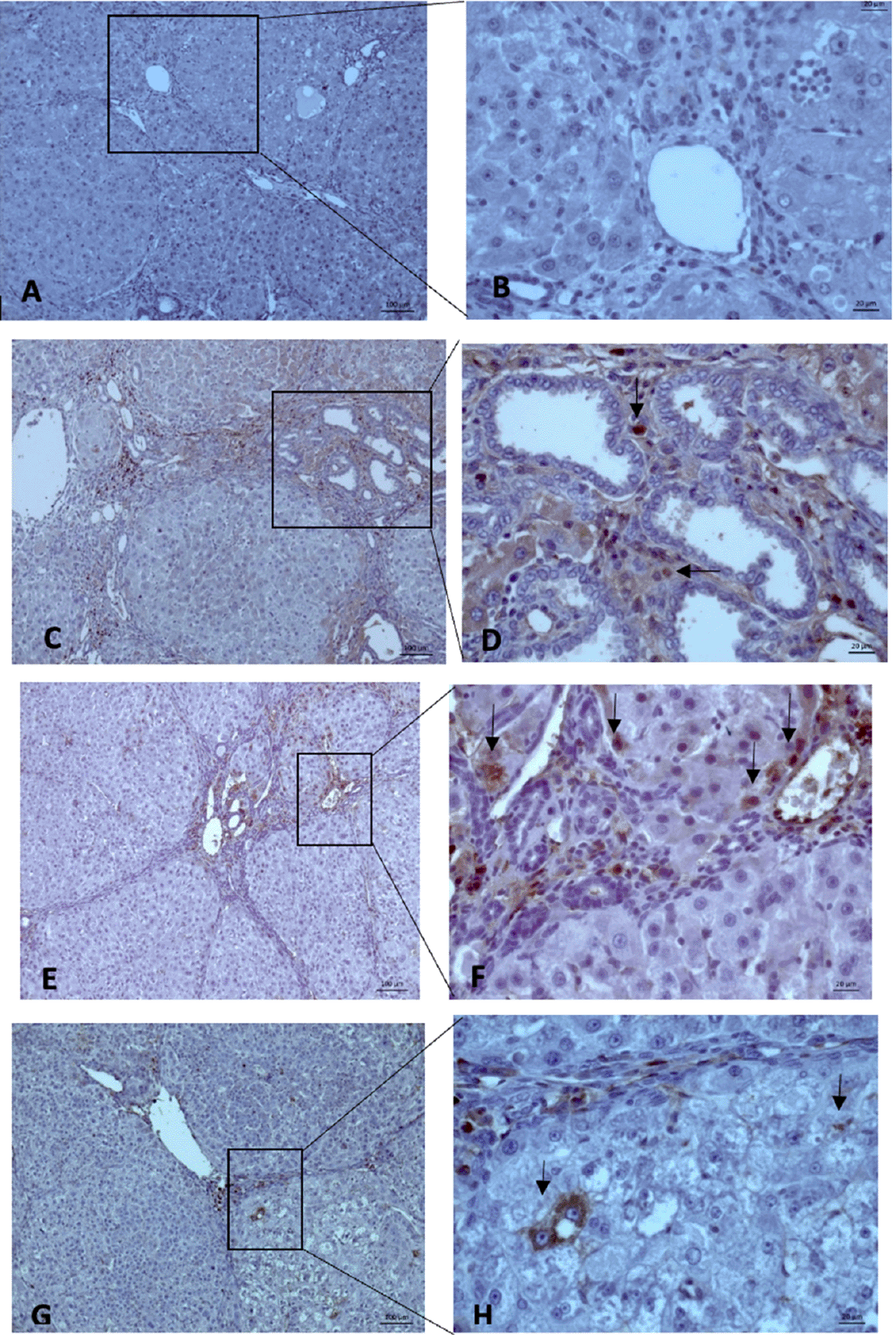
All three groups showed a “hot spot” area, which means there were positive results.
This study showed that the benefits of the combination of Sorafenib and EGCG are the same as an anti-neoplastic drug, which is as effective as anti-angiogenic. The results of VEGF values between 2 groups, namely the combination group Sorafenib 5 mg/kg BW and EGCG 5 mg/kg BW (X1) compared with Sorafenib 15 mg/kg BW alone (X2) showed that both treatments can reduce VEGF values. However, the X1 group was significantly more potent in decreasing VEGF value compared to group X2. This has exceeded the expectations, where the combination of low-dose Sorafenib and EGCG was more effective than only standard-dose Sorafenib. This indicated that Sorafenib and EGCG act synergistically with strengthening effect in anti-angiogenesis.
In vivo and in vitro studies have proved the effect of EGCG as a chemo-preventive, anti-angiogenic, anti-invasive, anti-proliferative, anti-inflammatory, and antioxidant substance. It was shown that EGCG blocks NF-kB activation by inhibiting IκBα degradation and the mitogen-activated protein kinase (MAPK) pathway. Meanwhile, downregulation of inducible nitric oxide synthase (iNOS) transcription and nitric oxide (NO) production from macrophages is dependent on NF-kB inhibition. It was reported that EGCG blocks NF-kB activation in human endothelial cells and inhibits monocyte chemotactic protein-1 (MCP-1) expression. Similarly, EGCG also prevents the apoptosis process by reducing mRNA expression of Bax and caspase 3 activity. It also inhibits cyclooxygenase-2 (COX-2) expression, proteasome-dependent degradation, MAPK pathways, and growth factor-dependent signaling, namely insulin-like growth factor-I (IGF-I), VEGF, and EGF.22
The results also suggested that several factors are responsible for the less effectiveness of the administration of Sorafenib as a single drug. Firstly, Sorafenib is accumulated in cancer cells, followed by an increase in the expression of enzymes to metabolize Sorafenib, which affects drug exposure. Thirdly, the presence or absence of tumor influences the concentration of Sorafenib and its primary metabolites based on assessing resistance to Sorafenib administration.23
MVD expression was significantly different between X1 and X2 groups compared to group O. This showed that the combination of low-dose Sorafenib and EGCG is also effective as standard-dose Sorafenib-only by decreasing MVD expression intratumorally. However, there is no significant difference in the discovery of MVD expression between the X1 and X2 groups. These are influenced by time length because the formation of MVD is affected by the growth of the capsule in the tumor. This is in line with the results by Kuczynski EA et al., who showed that therapy with Sorafenib significantly inhibits MVD (p < 0.001 vs. controls), while the Sorafenib-resistant group showed no evidence of continued angiogenesis.24 The evaluation of MVD expression is critical to determine the prognosis. According to Poon RTP et al., MVD-CD34 tumors were the only significant predictor of disease-free survival in patients with HCC or tumor size < 5 cm.25
Although the result was different from the hypothesis, a very satisfying conclusion was successfully obtained. The combination of low-dose Sorafenib with EGCG had better effectiveness than the standard-dose of Sorafenib in lowering VEGF values and was equally effective in reducing MVD expression in Wistar rats induced by DEN. Based on the results, it was concluded that EGCG adds a supplementary anti-angiogenic effect for HCC. Therefore, the use of low-dose Sorafenib combined with EGCG acts as a more cost-effective therapy is recommended to potentially increase drug compliance. Similarly, it also provides a satisfying therapeutic effect for advanced HCC.
After 10 weeks of administering DEN 70 mg/kg, macroscopic gross liver tissues showed irregular surfaces and pale colors, the histopathologists also confirmed HCC characteristics. The length and dosage of DEN induction were in line with Atmodjo et al., while the liver carcinogenesis or the beginning of HCC was recorded.26 There was a force majeure event in the experimental animal since 9 rats were found dead. Meanwhile, 8 rats died because of pulmonary hemorrhage, and one died with irregular lung surface due to lung injury. The hypothesis of this study stated that the suppression of the immune system increases the probability of lung disorders due to respiratory infections or fibrosis, or early malignancy in the lungs when rats were injected with DEN.27 This is because one rat that was not administered DEN died due to lung hemorrhage. This can be explained by Kun MW et al., who discovered the other cause of Wistar rat’s lung problem was lung infection due to A.Cantonensis.28 M. Pulmonis causes a different type of infection as explained by the study of Chawla et al., which showed gross and histopathological discoveries of severe congestion of lungs with suppurative and necrotizing pneumonia.29 Wang Y et al. also evaluated the induction effect of DEN in a rat model and discovered that the induced rat had liver dysfunction and damage, which is characterized by diffuse lesions with extensive interstitial inflammatory cell infiltration, alveolar edema, and bleeding. Meanwhile, minor injuries were discovered in the spleen, kidney, large intestine, heart, and other organs.30 Atmodjo et al. also noted the same discoveries for lung hemorrhage.26
There are several limitations to this study, firstly, the unhealthy condition of the samples after 10 weeks of administration of DEN can affect the number of samples. This led to the consideration of the decision of earlier termination. Secondly, the method of administering EGCG was unclear with the best effectiveness, oral EGCG which is associated with poor absorption (<5% absorption rate). Therefore, the intraperitoneal injection method was used to administer EGCG. This study recommends further investigation whether the administration of EGCG in form of nanoparticles orally or parenterally can increase the absorption and bioavailability of EGCG in the intestine and plasma.12,13
The combination of low-dose Sorafenib with EGCG has a more potential anti-angiogenic effect in liver cancer by reducing VEGF values compared to the single standard-dose Sorafenib. It also has similar effectiveness as single standard-dose Sorafenib in reducing MVD expression compared to the control group. Meanwhile, further study on the anti-angiogenic effect of low-dose sorafenib combined with EGCG is recommended.
Zenodo: Underlying data for ‘Anti-angiogenic effect of the combination low-dose sorafenib and EGCG in HCC-induced Wistar rats.’ https://doi.org/10.5281/zenodo.6044890.
This project contains the following underlying data:
Zenodo: ARRIVE checklist for ‘Anti-angiogenic effect of the combination low-dose sorafenib and EGCG in HCC-induced Wistar rats.’ https://doi.org/10.5281/zenodo.6044890.
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
Conception and design: Andry Irawan, Erik Prabowo, Ignatius Riwanto
Administrative support: Andry Irawan, Erik Prabowo, Wahyuni Lukita Atmodjo
Provision of study materials or patients: Wahyuni Lukita Atmodjo
Collection and assembly of data: Andry Irawan
Data analysis and interpretation: Ignatius Riwanto, Wahyuni Lukita Atmodjo
Manuscript writing: All authors
Final approval of manuscript: All authors
We thank Pelita Harapan University for facilitating this study and Dr. Ricarhdo Valentino Hanafi for organizing the manuscript.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: HCC, epidemiology, cancer, hematology
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 3 (revision) 21 Dec 22 |
read | read | |
|
Version 2 (revision) 21 Nov 22 |
read | ||
|
Version 1 09 Mar 22 |
read | read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)