Keywords
Medicinal plants, free radicals, antioxidants
This article is included in the Plant Science gateway.
Medicinal plants, free radicals, antioxidants
ALT: Alanine aminotransferase
AST: Aspartate aminotransferase
CT: Control
CT-B: Control+Baccharis
DB: Diabetes
DB-B: Diabetes+Baccharis
DM: Diabetes mellitus
HBA1c: Glycated hemoglobin
HPLC: High-Performance Liquid Chromatography
OGTT: Oral glucose tolerance test
ROS: Reactive Oxygen Species
TBARS: Thiobarbituric acid reactive substances
Diabetes mellitus (DM) is a public health problem, and according to the International Federation of Diabetes’ data, the number of diabetic patients is increasing. In total, 463 million cases were registered in 2019, and by 2045, 700 million cases are estimated (IDF 2019). The mechanisms involved in diabetes mellitus and hyperglycemia are complex; however, in the past few years, this pathology has been related to oxidative stress and high levels of reactive oxygen species (ROS) (Retnakaran et al. 2017). DM, in the long-term, may damage human health, cause failure or dysfunction in several organs including the eyes, nerves, heart, kidney, and blood vessels (Retnakaran et al. 2017; Elbatreek et al. 2019; Sani et al. 2019).
Diabetes without the correct diagnostic and treatment may lead to death (Guo et al. 2020). Therefore, researchers are seeking new strategies for the treatment of DM, and those which involve natural products have been receiving special attention (IDF 2019; Sani et al. 2019; ADA 2020). An example of a natural product is Baccharis dracunculifolia, a plant with potent antioxidant activity, which may prevent mitochondrial oxidative damage.
B. dracunculifolia, known as the rosemary-of-the-field, belongs to the Asteraceae family, and it is a shrubby, colonizer, and invasive plant. B. dracunculifolia is a medicinal plant popularly utilized because of its anti-inflammatory, anti-ulcers properties and also because of its effects against hepatic diseases. From this plant, green propolis is obtained, which is a highly efficient product against microorganisms, and much valorized in the international market (Dallaqua and Damasceno 2011; Guimarães et al. 2012; Bonin et al. 2020). B. dracunculifolia antioxidant activities were studied by Hocayen (2016), who performed extractions with different solvents (ethanol, methanol, and acetone). The results showed that all extracts have antioxidant effects, especially the one extracted with methanol solvent (Yan 2014; Halim and Halim 2019; Kaludercic and Di Lisa 2020).
Studies have been relating the production of ROS with diabetes mellitus observing the fact that lipid peroxidation biomarkers increase, while endogenous antioxidant syntheses decrease (Fatani et al. 2016; Egini et al. 2017; Ghazizadeh et al. 2019; Halim e Halim 2019). Thus, a way of avoiding the harmful effects triggered by ROS is the supplementation with compounds that possess antioxidant activities. Once the antioxidants react rapidly in the organism, the damages caused by the free radicals may be delayed or even prevented (Incalza et al. 2018; ADA 2020; Wang et al. 2020). Therefore, the present study analyzed the effects of the methanolic extract of B. dracunculifolia on glucose, creatinine, urea, plasmatic transaminases, glycated hemoglobin (HbA1c) and thiobarbituric acid reactive substances (TBARs) levels, as well as histological parameters in diabetic rats.
All the experimental procedures in this study were approved by the Institutional Ethics Committee on Animal Use (protocol no. 029/2012) of Midwest State University of Paraná, Guarapuava, Paraná, Brazil. All efforts were made to ameliorate any suffering of animals by performing the experimentation under the regulations of the Brazilian National Council for the Control of Animal Experimentation. This study is reported in line with the Animal Research: Reporting of in vivo Experiments (ARRIVE) guidelines (Aparecido Pereira 2021).
This study included 24 male adult albino Wistar rats, housed in a temperature-controlled environment (22 °C ± 2 °C), with an inverted light-dark cycle (12/12 hours) and acclimated for two weeks before the study (Animal Facility of the Midwest State University of Paraná, Guarapuava, Paraná, Brazil). Potable water and a standard diet were available ad libitum throughout the experiment. The animals were allocated into four groups of 6 animals each: Control (CT), nondiabetic animals that received water, 0.75 mL/100 g as treatment; Control+Baccharis (CT-B), nondiabetic animals that received B. dracunculifolia extract at 50 mg.kg−1; Diabetes (DB), diabetic animals that received water, 0.75 mL/100 g as treatment and Diabetes+Baccharis (DB-B), diabetic animals that received B. dracunculifolia extract at 50 mg.kg−1. All treatments were daily, uninterrupted, and performed orally by gavage. For sample size calculation was used software G Power (Faul et al. 2007), based on Cohen's principles (Charan and Kantharia 2013). The groups were randomly formed using a table of random numbers. The animals were placed in identified boxes and with markings on their tails to avoid confusion (Festing 2006).
After acclimatization, some animals were induced to an experimental model of diabetes by streptozotocin (Sigma, St. Louis, USA) was dissolved in cold citrate buffer at 0.1 M and pH 4.5. The intraperitoneal injection (IP) was performed after 12 h of fasting at a concentration of 50 mg.kg−1 (body weight) (Radenković et al. 2016). After seven days, this procedure was repeated. Fourteen days after the first application, fasting glycemic levels were estimated. Rats with fasting blood glucose (BG) values between 150 and 250 mg.dL−1 were considered diabetic and included in the study. Animals of non-diabetic groups underwent the same manipulations, being intraperitoneally injected with 0.9% saline solution, at the end of this initial phase, non-induced animals that presented glucose lower than 100 mg/dL were included in the study.
Samples of the sheets of Baccharis dracunculifolia (800g) were collected at Midwest State University, CEDETEG campus in Guarapuava, Paraná, Brazil. Harvesting geographic coordinates were: 25° 23′36″ south latitude and 51° 27′19″ west longitude. This region has an altitude of approximately 1100 m, an average annual rainfall of 1711 mm, and an average annual temperature of 16.7 °C. An exsiccate of the plant was deposited at the Museu Botânico Municipal (MBM) herbarium, and registered with the number 385123.
After harvesting, the plants were naturally dried for ten days on a clothesline in order to remove existing humidity and avoid microbiological contaminations. The methanolic extract of B. dracunculifolia was performed according to a standardized method (Hocayen et al. 2012). Firstly, the residual humidity test was performed according to the Brazilian Pharmacopoeia (2019). After, an agitation extraction method was applied. A proportion of 50g of B. dracunculifolia to 220 mL of methanol was utilized. This solution was maintained under constant stirring for one week, using the FANEM® Mod-255 kline shaker (Guarulhos, São Paulo, Brazil) at 180 revolutions per minute. Next, vacuum filtration with Kitasato was performed, and then, the filtered sample was put into a rotary evaporator RV 10 Basic® (IKA, Königswinter, Germany) to remove all methanol. The yield of the metanolic extract, cold dried (Re), was 12%. The obtained extract was diluted in Tween 80 solution and distilled water in a 1:8 proportion, aiming to improve extract solubilization and facilitate the administration, resulting in a solution of 125 mg.mL−1 concentration.
The chromatographic profile of the methanolic extract of Baccharis dracunculifolia was analyzed by high-performance liquid chromatography (HPLC). First, the samples were injected in a Varian equipment model 920-LC. A mixture of standards, described below, was subsequently injected into a C-18 Microsorb MV 100 reverse phase column (250 × 4.6 mm, 5 μm particle size). The standard flow was maintained at 1 mL min−1. The reverse-phase analysis consisted of solvent A (acidified water, 0.1% H3PO4, pH: 2.16) and solvent B (methanol). The gradient pumping system was 30% (B) at the start, 64% (B) at 15 min, 75% (B) in 26 min, 95% (B) in 28 min, returning to 30% (B) in 32 min, and remaining stable up to 40 min. The column was maintained at a constant temperature of 30 °C. The injection volume of each sample was 10 μL. The compounds were identified according to their elution order, and by comparing their retention time with those of their standard. The evaluated standards were: coumaric acid, ferulic acid, rutin, cinnamic acid, kaempferol, apigenin, galangine, pinocembrine, and quercitin (authentic analytical standards). All samples and standards were previously filtered with nylon membranes (0.45 μm pore diameter - Millipore).
At the start of the experiment, the animals were deprived of food for 12 hours to determine fasting glucose. The analysis was performed on days 0, 7, 14, and 28 of the experiment with glucose test strips and a portable glucometer (ACCU-CHEK® Active), utilizing tail vein blood.
After 28 days of treatment with B. dracunculifolia extract, animals of all experimental groups were fasted for 12 h and were anesthetized with IP of ketamine/xylazine (80/10 mg.kg−1). After complete anesthesia, all rats were euthanized by exsanguination (cardiac puncture), an appropriate method for tissue dissection and preservation (Pierozan et al. 2017). Blood samples were collected by cardiac puncture (without and with EDTA anticoagulant) for subsequent analyses such as glycated hemoglobin (HbA1C), cholesterol, triglycerides, creatinine, urea, aspartate aminotransferase (AST), alanine aminotransferase (ALT) and glucose levels. These biochemical parameters were analyzed utilizing specific kits by Labtest Diagnostic SA® (HbA1C Turbiquest REF. 385, Cholesterol Liquiform REF. 76, Triglycerides Liquiform REF. 87, Creatinine REF. 35, Urea UV Liquiform REF. 104, AST/GOT Liquiform REF. 109, ALT/GPT Liquiform REF. 108) and a semi-automatic spectrophotometer (Diaglobe CA-2006), and insulin levels were determined by radioimmunoassay (RIA).
The lipid peroxidation level of hepatic and renal tissues was analyzed utilizing Janero (1990) protocol, described briefly below. The degree of lipid peroxidation was measured by Thiobarbituric acid reactive substances (TBARS) assay. The organs (liver, kidneys and pancreas) tissue homogenates were added to a 10% trichloroacetic acid solution and a 0.67% thiobarbituric acid solution. After adding n-butanol, the colored supernatant was read in a spectrophotometer at 535 nm (UV Vis Spectrophotometer METROLAB 1700). A concentration curve was performed, with malondialdehyde as standard, at concentrations of 1.5, 3.0, 4.5, 6.0 and 10.0 mMol/mL. The TBARS results were expressed in mMol of TBARS/g of tissue.
Oral glucose tolerance test (OGTT) was performed on the 14th day of treatment. In order to perform the test, the animals were fasted for 14 hours. After fasting, a blood sample was collected from the tail vein, corresponding to time (0). Next, a glucose dosage was administrated (1 mg/kg – body weight) through gavage. Blood samples were collected at times 15, 30, and 60 min after glucose administration. Glycemia was determined by utilizing a portable glucometer (ACCU-CHEK® Active).
In order to evaluate the presence or absence of histological alterations, vital organs were collected, such as: pancreas, kidney, and liver. The samples were weighed and evaluated macroscopically concerning their shape, consistency, and color. After, the samples were placed in 10% buffered formalin solution to a subsequent preparation of histological slides. Tissues were included in paraffin blocks, cut in a microtome in slides of 3-4 micrometers, and colored by Hematoxylin-eosin technique (Cardiff et al. 2014). The mounted specimens were observed and were scored under light microscopy and the images were captured by a camera connected to an optical microscope (Olympus BX-40; Olympus, Tokyo, Japan), for a qualitative comparison of the structural changes.
All results are presented as mean ± S.D. Statistical analyses were performed in SPSS software, Windows 23.0 version (Chicago, IL, EUA). Analyses of normality were performed utilizing the Shapiro-Wilk test, followed by one-way ANOVA. Differences were considered statistically significant at p < 0.05. Post-hoc Student-Newman-Keuls was used to identify significant differences between the groups.
The results of HPLC analysis for the identification of the compounds present in the methanolic extract of B. dracunculifolia are shown in Table 1 (Aparecido Pereira 2021). Chrysin, pinocembrine, and coumaric acid were identified in the tested sample.
No clinical manifestation that could characterize the presence of another underlying disease in the animals was observed. Weekly results of capillary blood glucose, oral glucose tolerance test (OGTT), and plasmatic insulin levels may be observed in Figure 1. Capillary blood glucose levels of DB-B group animals were decreased when compared to the DB group from the 2nd week of treatment (80%, p < 0.05) (Figure 1A). After the start of treatments, no animals were excluded, all tests were performed with n = 6.
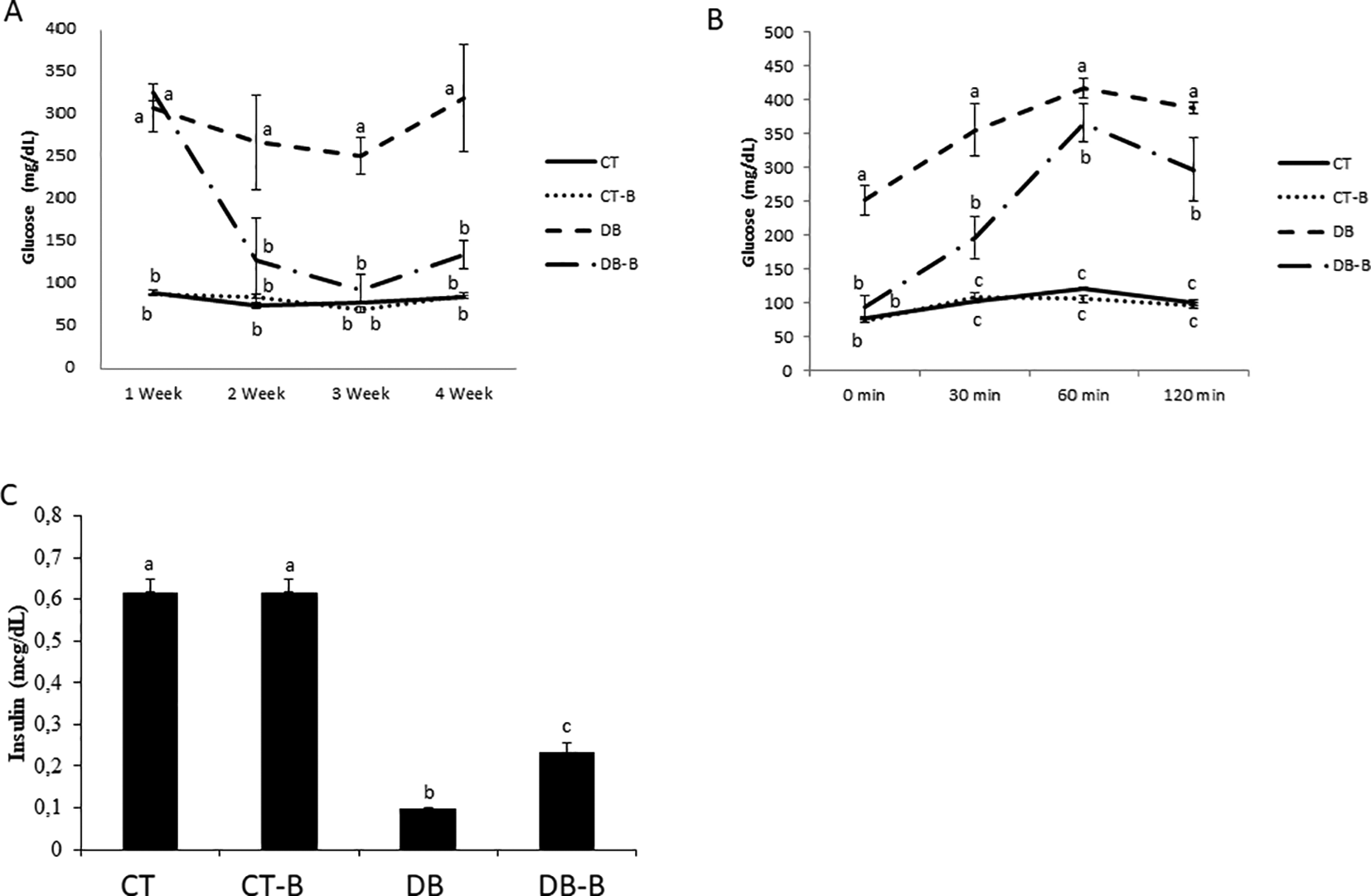
Data present mean ± ME (n = 24). (a,b,c) different letters are statistically different among groups (p < 0.05; Student-Newman-Keuls afer ANOVA oneway). The measurements were performed after 12 h of food fasting. CT – control; CT-B – control+Baccharis; DB – diabetes; DB-B – diabetes+Baccharis.
In the OGTT test, DB-B group animals presented lower levels of glucose at all times tested when compared to the DB group (32%, p < 0.05). In fasting measurements, the animals of the DB-B group did not present any significant difference compared to the CT group, but glucose levels were elevated in the 30 min (100%, p < 0.05), 60 min (200%, p < 0.05) and 150 min tests (150%, p < 0.05).
The DB-B group presented an increase in plasmatic insulin levels when compared to the DB group (52.17%, p < 0.01) (Figure 1C). Lower levels of plasmatic insulin were noticed in DB groups when compared to the CT group (200%, p < 0.001).
Biochemical values may be observed in Table 2. The DB group, when compared to the DB-B group, presented higher values of creatinine (26.42%, p < 0.05), urea (31.42%, p < 0.01), and triglycerides (60.80%, p < 0.01). DB-B group creatinine’s and triglycerides’ values (0.39 ±0.01 e 75.0 ±8.4) were similar to the CT group (0.32 ± 0.01 e 71.7 ± 5.4) and CT-B (0.39 ± 0.01 e 58.8 ± 4.5).
| Biochemical parameters | Groups | |||
|---|---|---|---|---|
| CT | CT-B | DB | DB-B | |
| HbA1C (Hb %) | 2.25 ± 0.17 | 1.80 ± 0.08 | 8.75 ± 0.40 | 8.58 ± 0.43 |
| Cholesterol (mg/dL) | 58.5 ± 4.62 | 64.7 ± 0.56 | 72.2 ± 2.85 | 62.5 ± 4.00 |
| Triglycerides (mg/dL) | 71.7 ± 5.4 | 58.8 ± 4.5 | 191.3 ± 15.5 | 75.0 ± 8.4* |
| Creatinine (mg/dL) | 0.32 ± 0.01 | 0.39 ± 0.01 | 0.53 ± 0.03 | 0.39 ± 0.01* |
| Urea (mg/dL) | 17.3 ± 0.02 | 39.5 ± 3.40 | 67.8 ± 8.78 | 46.5 ± 3.78* |
| AST (UI/L) | 259.0 ± 19.1 | 239.8 ± 11.9 | 396.0 ± 26.5 | 365.5 ± 46.6 |
| ALT (UI/L) | 92.0 ± 3.1 | 81.0 ± 4.9 | 257.0 ± 27.0 | 232.5 ± 45.2 |
The effects of B. dracunculifolia treatment over liver and kidney lipid peroxidation are presented in Figure 2. In the DB group, an increase of lipid peroxidation was noticed when compared to the CT group (80%; p < 0.05). The DB-B group presented a significant reduction in TBARs levels when compared to the DB group (liver 33.33% p < 0.05 and kidney 38.77%, p < 0.05), and similar levels when compared to CT group.
During the experiment, diabetic animals presented characteristics corresponding to the induced disease, considering the aggressiveness of the injuries caused by the model. The effects of streptozotocin in glucose homeostasis reflect the abnormalities induced by cellular toxicity due to biochemical alterations.
The histological samples, as well as the organs’ weight, are presented in Figure 3 (liver), 4 (pancreas), and 5 (kidney), with representative images of each experimental group of the present study. In histological evaluation, the CT group presented a typical histological structure. DT group demonstrated contraction in size, characterizing a fibroplasia. The deposited conjunctive tissue promotes structural contraction, altering the organ size, which is also stimulated by the decreased number of healthy cells, hypoplasia, and cellular death characterized by cellular necrosis. The preservation of organs’ structure was observed in histological samples of the rats treated with B. dracunculifolia, being the parenchyma and stroma preserved.

Micrographs of liver by the end of 28 days’ treatment. Micrographs of (A) CT; (B) CT-B; (C) DB and (D) DB-B. Optical microscope, magnification range of 400×. No use of image filters or brightness changes have been made to the images. It was observed: (A) Moderate fibroplasia with perivascular mononuclear cell inflammatory reaction associated with reactive hepatocytes; (B) Diffuse centrilobular necrosis with cytoplasmic vacuolization in hypertrophic hepatocytes, mild fibroplasia and stromal hyperemia; (C) Endothelial cells regularly distributed in vascular wall, acinar formation, hepatocytes regularly distributed and activated Kupffer cells in necrotic areas; (D) Perivascular tissue necrosis with parenchymal discrete swelling. Scale bar 25 μm. CT – control; CT-B – control+Baccharis; DB – diabetes; DB-B – diabetes+Baccharis.
Regarding organs’ weight, during the macroscope analyses, the DB group presented higher weight and volume to kidney and liver, characterizing hypertrophy. These issues also present brighter colors on the surface, paler than the CT group. No macroscope alterations were observed in the pancreas.
Differences regarding macro-structural morphology were observed in the kidney, liver, and pancreas of DB and DB-B animals, considering the size, shape, consistency and color of the organs. As a result of the injury caused by the diabetes model, DB group organs presented microscope alterations compatible with the disease. In the liver, the development of hepatocellular intracytoplasmic vacuolation with parenchymal alterations was observed, due to lipid accumulation (Figure 3).
The DB group presented pancreatic hypotropia, islets of Langerhans, in addition to leukocyte infiltration in the acinar region and fibrosis in the remaining islets (Figure 4). In nondiabetic rats, histological structures were typical.
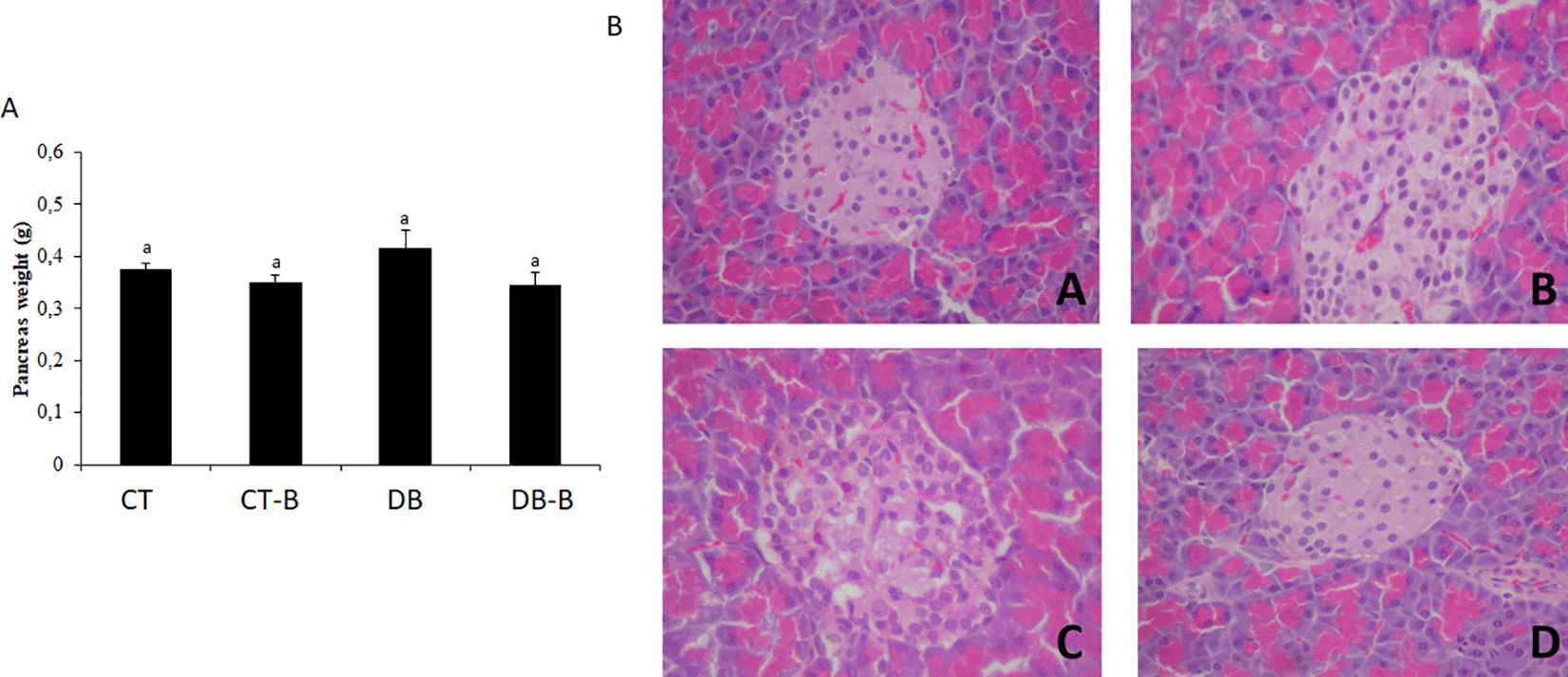
Micrographs of pancreas by the end of 28 days’ treatment. Micrographs of (A) CT; (B) CT-B; (C) DB; and (D) DB-B. Optical microscope, magnification range of 400×. No use of image filters or brightness changes have been made to the images. It was observed: (A) Hypocellularity of Langerhans islets secreting cells with mild necrosis and hemorrhage between acinar cells; (B) Hypocellularity of acinar cells with discreet hemorrhage and Langerhans islets swelling; (C) Hypotropia of Langerhans islets with hyperplasia of acinar cells and formation of discreet inter-acinar fibroplasia; (D) Swelling and vacuolization of Langerhans islets with necrosis of secreting cells and diffuse inter-acinar necrosis associated with vacuolization. Scale bar 25 μm. CT – control; CT-B – control+Baccharis; DB – diabetes; DB-B – diabetes+Baccharis.
In diabetic animals, the kidneys presented alterations such as swelling, peripheral displacement of the nucleus, due to intracytoplasmic water accumulation and membrane instability, increased nucleus/cytoplasm ratio, cellular swelling, and ribosome sedimentation forming eosinophilic granules (Figure 5). The Glomerulus showed little impairment, presenting tubular and interstitial injuries (Fakhruddin et al. 2017). In the CT and CT-B groups, no alterations were found.
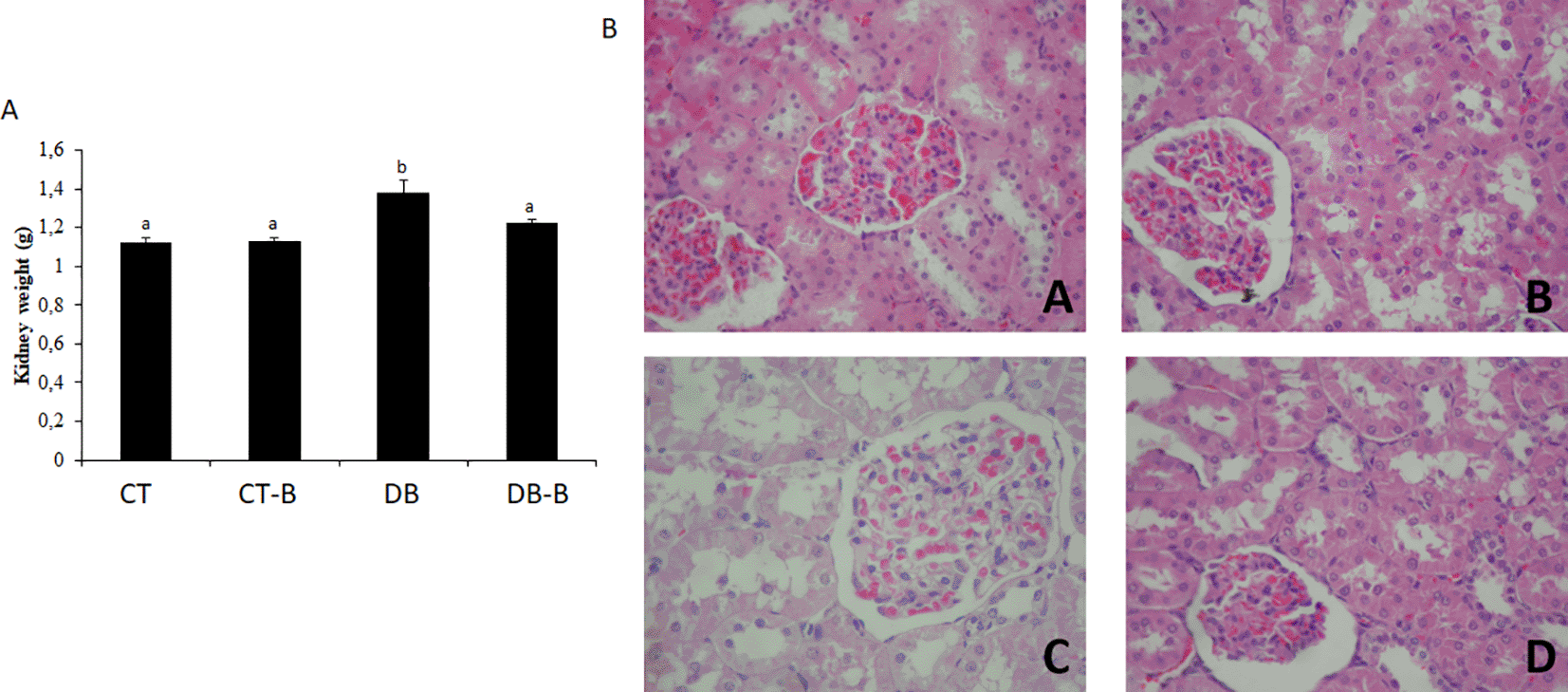
Micrographs of kidney at the end of 28 days’ treatment. Micrographs of (A) CT; (B) CT-B; (C) DB; and (D) DB-B. Optical microscope, magnification range of 400×. No use of image filters or brightness changes have been made to the images. It was observed: (A) Necrosis of proximal contorted tubules with hydropic degeneration, and discreet glomerular cell swelling with focal hemorrhage; (B) Necrosis of proximal contorted tubules associated with glomerular sclerosis and intense hydropic degeneration in tubular epithelium; (C) Proximal contorted tubules with epithelial cell hypotrophy associated with moderate fibroplasia and glomerular hypotrophy, but with Bowman’s space; (D) Necrosis of proximal contorted tubules, swelling and glomerular hemorrhage. Scale bar 25 μm. CT – control; CT-B – control+Baccharis; DB – diabetes; DB-B – diabetes+Baccharis.
After receiving the B. dracunculifolia treatment, animals presented decreased levels of creatinine and urea. These animals also presented a qualitative improvement in kidney morphological structure when compared to the DB group. Glomerular and proximal contorted tubules structures were conserved, in which glomerular swelling was evident as well as diffuse tubular necrosis, which may be associated with a minimization of renal damage after the plant extract ingestion.
In other studies (Kim and Kim 2012; Ahangarpour et al. 2017; Momin and Yeligar 2019), which also employed medicinal plants as treatments, the authors observed a reduction in triglycerides levels. These studies suggest that the antioxidant activity of the extracts may be responsible for the results found. The reduction in triglyceride levels may be explained by the control of certain lipoprotein hydrolysis (Achi et al. 2017; Prnova et al. 2020). The administration of methanolic B. dracunculifolia extract did not promote statistical differences between groups concerning hepatic enzyme levels (AST and ALT). However, a qualitative improvement in hepatic damage was observed in the diabetic animals treated with the plant extract.
Although the treatment with B. dracunculifolia was able to decrease fasting glucose levels and improve diabetic animals’ response to OGTT, no reduction in HbA1C levels was observed. A possible explanation of this fact is associated with the experimental model aggressiveness, which destroys part of β-pancreatic cells and reduces the sensitization of peripheric insulin receptors (Mirza and Panchal 2019). Due to this fact, after food intake, glucose levels take a longer time to return to basal levels, promoting higher glycosylation of hemoglobin in diabetic rats. Another explanation is associated with the average life of rats’ red blood cells, 120 days. Once the treatment was administrated for 28 days, there was no adequate time to cause a reduction in HbA1C levels.
After administrating B. dracunculifolia treatment in the DB-B group, higher concentrations of plasmatic insulin were observed when compared to the DB group and according to histological analyses of liver, kidney, and pancreas samples, the animals of the DB-B group presented fewer alterations when compared to the DB group. Studies employing different types of plants demonstrated an increase of insulin excretion in experimental models of diabetes (El-Sayed et al. 2009; Achi et al. 2017; Noor et al. 2017; Effiom et al. 2019).
The DB group also showed a significant increase in lipid peroxidation when compared to the CT group. This fact may be attributed to the activation of the oxidative system triggered by the pathology, which favors oxidative stress (Sharma et al. 2019). The DB-B group presented a significant reduction in lipid peroxidation (both in the liver and kidney tissue) compared to the DB group. The animals treated with B. dracunculifolia extract also presented similar TBARs levels to nondiabetic rats (Figure 2). These results suggest that the plant has antioxidant effects in the studied organs.
In HPLC analyses performed with the methanolic extract of B. dracunculifolia used in the present work, Iurckevicz et al. (2019) identified the same compounds presented in this study results (chrysin, pinocembrine, and coumaric acid), with an addition of two compounds, caffeic acid, and ferulic acids. Studies suggest these compounds may present antioxidant and anti-inflammatory properties (Delgadillo-puga et al. 2019; Falcão et al. 2019). Salazar and collaborators (2018) also performed a chromatographic study with B. dracunculifolia and found similar results.
Pinocembrin, a widely studied flavonoid, is found in different products of natural origin, especially in bee propolis and in plants of the Piperaceae family (Hu et al. 2019). Scientific studies have shown that pinocembrin presents several biological properties such as anti-inflammatory, antioxidant, anticancer, and neuroprotective (Şahin and Karkar 2019; Zhang et al. 2019). Chrysin is a natural compound found in flowers, such as passion fruit and trumpet flower, and foods such as mushrooms, honey, and propolis. Studies report numerous potent activities related to this compound, such as anti-inflammatory, antioxidant, anti-allergic, anti-hypertensive, and anxiolytic (Del Fabbro et al. 2019; Veerappan, Malarvili 2019). Therefore, the presence of these compounds may contribute to the hypoglycemic effects demonstrated in the present study. Many compounds may be responsible for the antioxidant and anti-inflammatory activities of Baccharis gender, such as polyphenolics like phenolic acids, coumarins, and flavonoids (Figueiredo-Rinhel et al. 2017; Bonin et al. 2020). The results of this study suggest that the anti-inflammatory activity of B. dracunculifolia methanolic extract may be responsible for its hypoglycemic effect, which may be histologically observed by the absence of cellular necrosis and inter acinar fibroplasia, characterizing a healing process of tissue that favors the integrity of the organs.
The results of the present study show that the treatment with methanolic extract of B. dracunculifolia decreases lipid peroxidation levels, increases insulin rate, and improves the response in oral glucose tolerance test. It is suggested that the antioxidant activity of the plant’s extract is responsible for the improvement of diabetes mellitus. Histologically, little vascular alterations were observed, characterizing a reduction in the conditions’ typical inflammatory process. Other improvements were observed, such as vascular permeability maintenance, regulation of fibroplasia (which maintains vascular integrity), and mainly, the reduction of hydropic degeneration rate. Therefore, cellular membrane permeability and homeostasis were maintained. The study indicates that the extract of B. dracunculifolia has relevant protective properties in hepatic, renal and pancreatic tissues in vivo.
Harvard Dataverse: Sub-chronic effects of Baccharis dracunculifolia treatment on biochemical, oxidative, and histopathological parameters in diabetic rats. https://doi.org/10.7910/DVN/EXDCGC (Aparecido Pereira 2021).
This project contains the following underlying data:
Harvard Dataverse: ARRIVE checklist for ‘Sub-chronic effects of Baccharis dracunculifolia treatment on biochemical, oxidative, and histopathological parameters in diabetic rats’ https://doi.org/10.7910/DVN/EXDCGC (Aparecido Pereira 2021).
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
The present study was gently supported by the Federal Institute of Paraná, the Midwest State University of Paraná, and the Central Analysis of Federal Technological University of Paraná (Pato Branco campus).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
No source data required
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Pharmacology
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Antidiabetic Mechanisms, Molecular Toxicology, Medicinal Plants, Bioinformatics
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Endocrinology, Animal Models, In-vivo experimentation, Medicinal chemistry.
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Phytotherapeutics, Clinical Biochemistry, Medical Genetics
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
|
Version 1 12 Jan 22 |
read | read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)