Keywords
Cefotaxime, LRTI, Community acquired pneumonia
Cefotaxime, LRTI, Community acquired pneumonia
The updated review article has critical and balanced view of this drug cefotaxime and has been compared to its biggest competitor – ceftriaxone on the basis of reported cases and clinical trials published till date. Both drugs have an identical antimicrobial spectrum, similar indications, and clinical use.
The drug have been listed as an essential medicine in various guidelines for antibacterial and antiviral use.
Cefotaxime is better, safe and cost effective; and also can be given in multi drugs resistant infections.
The above study shows a comparison with ceftriaxone for more than 5 years.
The following changes were added to the article:
The burden of LRTI across the globe was added and antimicrobial resistance and Microbial spectrum were excluded from the Abstract.
An update in data from the Global burden of disease from 2016 to 2019, The global action Plan for pneumonia (UNICEF) and Information about nosocomial pneumonia was added in the Introduction section.
Empirical treatment with antibacterial agents to reduce mortality and Information about cephalosporin was added.
Adverse effects of ceftriaxone derived from various studies have been mentioned in Most prescribed antibiotics for LRTIs.
Recent studies on the comparable spectrum of anti-microbial activity of Cefotaxime and Ceftriaxone were included in the Establishment of Cefotaxime over Ceftriaxone section.
The efficacy of Cefotaxime in preventing surgical infections, Coagulopathies and psuedocholelithiasis in conditions of infections and pediatric patients have been added to Cefotaxime use in various conditions.
Cefotaxime Toxicity has been well-established under the Contraindications of Cefotaxime section.
The conclusion has been improved with inclusions about Cefotaxime’s efficacy, fewer adverse effects and its affordability in the market.
Tables 2, 3 and 4 were replaced by guidelines on Cefotaxime Dosage, general dosing guidelines and its listing in the essential medicine.
Figure 2 was removed.
Classification of Cephalosporin was removed.
To read any peer review reports and author responses for this article, follow the "read" links in the Open Peer Review table.
Lower respiratory tract infections (LRTIs) rank fourth as a cause of mortality around the globe. As per the Global Burden of Disease (GBD) study (2016), the maximum number of LRTI episodes among children younger than five years across the world were reported in south Asia (18.76 million)1 whereas almost half a billion people, i.e. 257 million males and 232 million females suffered from LRTI globally, according to the GBD 2019.2 Nearly 489 million incident and 11.0 million prevalent cases of LRTI contributed to 2.49 million deaths and 97.2 million disability-adjusted life years (DALYs). Overall, 1.23 million deaths were seen for people 70 and older.3 LRTI includes clinically diagnosed and self-reported cases of pneumonia, respiratory syncytial virus (RSV), bronchiolitis, and influenza-like illness. The discovery and clinical application of antibiotics in the treatment of respiratory tract infections have changed the management of diseases like acute bronchitis, pneumonia, and acute exacerbation of chronic lung diseases (such as COPD or bronchiectasis).4 Respiratory tract infections have always posed a challenge in terms of morbidity and mortality.5 LRTIs constitute a major portion of critically ill patients are associated with prolonged hospitalization, higher costs, and possibly, an increase in mortality.6 Nosocomial pneumonia is associated with a significant prolongation of hospital stay and expenses.7 Pneumonia is the single largest cause of mortality among children worldwide and incidences of 24.8 per 10,000 adults in a year have been reported.8,9 Lack of (pneumococcal) vaccination coverage and the inability to hospitalize critical pneumonia patients on time are two major causes of increasing mortality. Apart from extremes of age, other risk factors which predispose to LRTIs include poor sanitization, severe malnutrition, lack of breastfeeding for infants, HIV infection, lack of immunization, chronic illness, family history of LRTI and exposure to tobacco smoke/air pollutants.10,11
The Global Action Plan for Pneumonia and Diarrhoea (GAPPD) was launched by the World Health Organization (WHO) and the United Nations Children’s Fund (UNICEF) in 2013 to reduce mortality from pneumonia in children under five years. The Millennium Development Goal (MDG) specific for 2025 are to (i) to reduce mortality from pneumonia in children less than five years of age to fewer than three per 1000 live births; and (ii) to reduce the incidence of severe pneumonia by 75% in children less than five years of age compared to 2010 levels.
In 2019, a few patients with pneumonia of an unknown cause were identified in Wuhan, China and the causative organism was recognized as a new coronavirus, later named the 2019 novel coronavirus (2019-nCoV)12; it has affected humanity worldwide and was declared a global pandemic by the WHO. Among patients with COVID-19-related pneumonia, co-infection with bacteria majorly by Streptococcus pneumoniae, Haemophilus influenzae, Chlamydia pneumoniae, and Staphylococcus aureus is one of the chief concerns.
The latest CAP guidelines released by the American Thoracic Society and Infectious Diseases Society of America recommend cefotaxime in combination with macrolides or doxycycline as one of the empiric antibiotics for the management of such infections.13,14 Resistance to newly developed antibiotics has been documented globally. In the aforementioned context, the rational use of older, effective antimicrobial agents mentioned in several guidelines with no multidrug-resistance (MDR) could serve as a helpful alternative for the treatment of MDR bacterial pathogens.3 From this background, the present narrative review aims to revisit the basic science of old, commonly prescribed cephalosporins for respiratory infections like ceftriaxone/cefotaxime and their clinical applicability in some of the most common bacterial infections, LRTIs. This article highlights the need for looking back at well-established, cost-effective and non-resistant antibiotics for LRTI patients. Apart from S. pneumoniae, other bacteria responsible for LRTIs have been tabulated in Table 1.15,16
| Gram-positive bacteria | Gram-negative bacteria | Virus |
|---|---|---|
| Streptococcus pneumonia | Pseudomonas aeruginosa | Human rhinovirus |
| Staphylococcus aureus | H. influnzae | Influenza virus |
| Klebsiella pneumonia | Human parainfluenza virus | |
| E. coli | Human coronavirus | |
| Moraxella catarrhalis14 | Human respiratory syncytial virus | |
| Legionella spp.17 | Human metapneumovirus | |
| Chlamydia pneumoniae17 |
Third-generation cephalosporins are generally prescribed for cases of bacterial infection, pneumonia and upper and lower respiratory tract infections. These cephalosporins are semisynthetic analogues with different chemical substitutions on the C7 acylamido chain and include ceftriaxone, cefdinir, cefixime, cefditoren, cefpodoxime, ceftazidime, cefoperazone, ceftizoxime, ceftibuten, cefotaxime and others. They are more active against Gram-negative bacteria and organisms resistant to first- and second-generation cephalosporins. Third-generation cephalosporins are used for empiric therapy in (i) acute LRTI due to community-acquired pneumonia (CAP) in adults and children; (ii) CAP patients requiring intensive care unit (ICU) admission; (c) suspected infection in Human Immunodeficiency Virus (HIV) patients (outpatients)18; and (d) patients with healthcare-associated and community-acquired SBP without hepatocellular carcinoma.18,19 Some third-generation compounds, like ceftriaxone, ceftazidime, and cefotaxime are poorly absorbed in the gastrointestinal tract and are administered only intramuscularly (IM) or intravenously (IV).
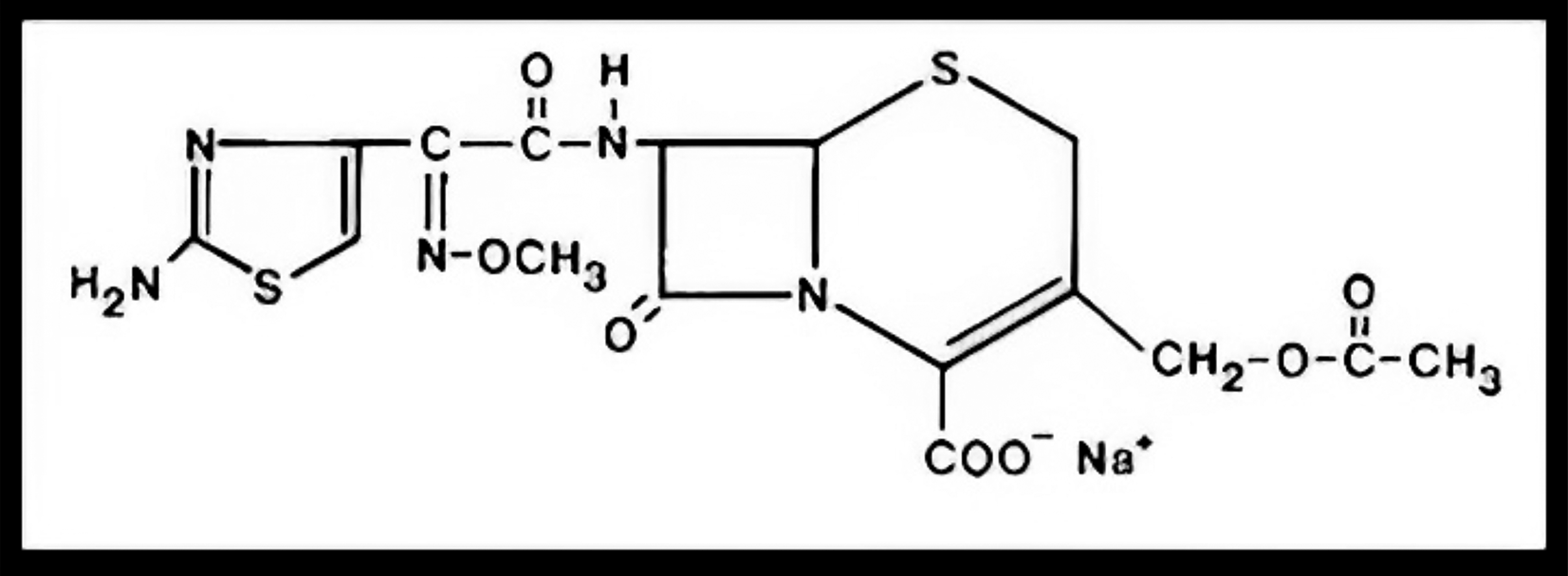
The most useful third-generation cephalosporins, ceftriaxone and cefotaxime have been used as listed in an essential medicine list, also recommended by various guidelines along with dosages; a few are listed in Tables 2, 3 and 4. Cefotaxime is the most commonly used drug in neonatal early-onset sepsis due to its wider therapeutic index, superior cerebrospinal fluid penetration, and lower incidence of nephrotoxicity. Its molecular structure is presented in Figure 1.18
| Name of guideline and year | Disease | Cefotaxime dose | Ceftriaxone dose | Reference |
|---|---|---|---|---|
| ICMR guidelines for antimicrobial use in common syndromes, 2019 | Antimicrobial therapy for CAP Adults dose Paediatric dose Acute bacterial meningitis | 2 gm thrice daily IV 30-35 mg/kg thrice daily IV 2g IV q 4-6 h | 2 gm once daily IV 50 mg/kg twice daily 2g IV q 12 h | 21 |
| National Treatment Guidelines for Antimicrobial Use in Infectious Diseases, 2016 | - Obstetric sepsis during pregnancy - Pediatric infections | -200 mg/kg/d, 3-4 divided doses | -2 gm IV OD+ Metronidazole 500mg IV TDS +/-gentamicin 7 mg/kg/day OD -100 mg/kg/day-2 divided dosage (for 10-14 days) | 22 |
| Lower respiratory tract infection (Above two months of age) | 50 mg/kg/dose 6h | 75-100 mg/kg/day in two divided doses, IV | ||
| Community acquired Pneumonia (severe case) | Amoxycillin clavulanate 1.2 g IV TDS Or Ceftriaxone 2 g IV OD Duration 5-8 days | |||
| Acute Bacterial meningitis | 2 g IV 4-6 hourly (10-14 days) | 2 g IV 12 hourly (10-14 days) |
| Organisations | Dose | References |
|---|---|---|
| Central Drugs Standard Control Organisation (CDSCO) | - Sterile Cefotaxime sodium USP eq. to Cefotaxime 500 mg/1000 mg+Sterile Sulbactam 250 mg/500 mg per vial injection for the treatment of lower respiratory tract injection and genito-urinary tract infection approved by FDC on 26.12.2003. - Cefotaxime sodium + Sulbactam sodium for injection (Additional Indication) for the treatment of lower respiratory tract infection in children was approved by FDC on 24.06.2008. | 23 |
| European Medicines Agency (EMA) | - List of nationally authorised medicinal products has cefotaxime. As mentioned in EMA/403947/2019 Human Medicines Evaluation Division on 11 July 2019. | 24 |
| Ministry of Health and family welfare | Cefotaxime is used as Anti-infective Medicine in Beta-lactam medicines (6.2.1.9) and also as anti-infective medicines-antiviral medicines (6.7.5.2) mentioned in the National list of essential medicine (2011), India. For essentiality of the requirement of medicines, it has been categorized as follows: - S and T denote essentiality at the Secondary and Tertiary levels. | 25 |
| World Health Organization (WHO) | * FIRST CHOICE for adults − Acute bacterial meningitis − CAP (severe) − Complicated intraabdominal − Complicated intrabdominal infections (severe) − Endophthalmitis − Enteric fever − Gonorrhoea − Hospital-acquired pneumonia − Necrotizing fasciitis − Pyelonephritis or prostatitis (severe) * FIRST CHOICE for children − Acute bacterial meningitis − Community acquired pneumonia (severe) − Complicated intraabdominal infections (mild, moderate and severe) − Hospital-acquired pneumonia − Pyelonephritis (severe) | 26 |
Ceftriaxone is an antibiotic agent frequently used in paediatric hospital practice for the treatment of severe bacterial infections. Ceftriaxone is the most common cause of cholelithiasis in pediatric practice,28 as well as nephrolithiasis due to the drug being excreted via biliary and renal routes. This medication is contraindicated in preterm infants up to 41 weeks of age and in full-term infants less than 28 days of age. Patients with hypersensitivity to cephalosporins may result in an anaphylactic reaction and ceftriaxone-induced encephalopathy symptoms feature limb weakness or numbness, and memory impairment; behavioural problems have been reported in a few cases and are more common in patients with end-stage renal disease (ESRD). Clinically, the symptoms manifest within one to seven days of antibiotic therapy and usually resolve within two to seven days after discontinuation of the medication.
Futagi et al., 2022 reported a case of an 84-year-old male patient with a single right kidney when started on IV ceftriaxone (CRO) (2 g, every 24 hours) for bacterial infection who had about one-tenth of total body clearance and one-third of the volume of distribution compared with healthy adults, and the elimination half-life was about three times longer. He developed tonic-clonic seizures of the left face and left upper extremity on the eighth day. The encephalopathy appeared to have been caused by the administration of CRO to a patient with a single kidney.29
CRO-associated encephalopathy (CAE) develops at a dose of 1 g/day and may increase blood and cerebrospinal fluid ceftriaxone levels.30 Neurotoxic symptoms such as psychosis, seizure, myoclonus, and choreoathetosis show no abnormalities in laboratory investigations, CT, or MRI. However, electro encephalogram (EEG) shows slow and generalized periodic discharge with triphasic morphology.31
Ceftriaxone-associated neurotoxicity occurred in a patient after pancreas-kidney transplantation, as reported by Hagiya et al. in 2017. Thus, a higher serum concentration of the drug can trigger neurotoxicity. Antibiotic-associated encephalopathy (AAE) is easily treatable by stopping the administration of the responsible drugs.32
In another case, a 49-year-old female patient with symptoms of upper respiratory tract infection and fever for five days, for which she received ceftriaxone for two days, was admitted to the emergency department with pruritic skin rash in both upper and lower limbs and swollen lips for one day. The patient had a history of penicillin allergy and was also allergic to some foods. Many studies have reported antibiotic-induced leukocytoclastic vasculitis caused by ceftriaxone, which healed with withdrawal of antibiotic use for 2, 5, 14 and 30 days; however, there was only one study on cefotaxime by Cure et al., 2007 in which patients recovered after five days of antibiotic.33,34 Resistance to ceftriaxone has been steadily increasing from 2015 to 2020. Therefore, the appropriate antibiotics should be selected based on susceptibility data.35
Ceftriaxone causes more severe clinical courses and more fatal outcomes than other drugs responsible for DIIHA (Drug-induced immune hemolytic anaemia).36 In a case where ceftriaxone was given for bilateral pneumonia to an 82-year-old male patient admitted to ICU, skin lesions formed after three days and led to the patient’s death due to acute and fatal cephalosporin-induced autoimmune haemolytic anaemia (CIIHA), due to strong agglutinating drug antibody effect. CIIHA is a rare and serious cause of livedo that should be suspected in patients exhibiting livedo and acute haemolytic anaemia within hours/days following cephalosporin administration.37 The temporal relationship between changing platelet counts and starting and discontinuing ceftriaxone suggests the diagnosis of drug-induced thrombocytopenia.38
The risk of third-generation cephalosporin (ceftriaxone) resistance is particularly high in nosocomially-acquired episodes of spontaneous bacterial peritonitis but also occurred in healthcare system-acquired cases.39 Ceftriaxone was responsible for the highest number of deaths in one study (49 cases). Of 20,877 (5.8%) reports, 1205 were related to ceftriaxone; 357 reports (30%) were categorized as serious including cardiac arrest, anaphylactic and anaphylactoid reactions. The most frequently reported serious events with ceftriaxone were cardiac arrest, and anaphylactic and anaphylactoid reactions.40
CIIHA is a potentially fatal complication.33 The reported fatality rate of CIIHA is as high as 40%. Acute renal failure and cardiovascular decompensation are the most lethal complications in pediatric patients with CIIHA. A seven-year old child underwent cochlear implantation of the left ear under general anaesthesia, and was given 1 g of IV ceftriaxone intra-operatively and subsequently daily post-operation. The child developed CIIHA after seven days of daily ceftriaxone administration and presented with sudden abdominal pain, shivering, sweating, and dark tea-coloured urine. Ceftriaxone therapy was immediately discontinued whereas intravenous immunoglobulin was used after the episode of hemolysis; hemoglobinuria then ceased by itself.41
A pilot study was performed with intravenous ceftriaxone (1 g/24 h) or cefotaxime (1 g/8 h) for at least three days to assess the emergence of third-generation cephalosporin-resistant Enterobacteriaceae, carbapenem-resistant Enterobacteriaceae, Pseudomonas aeruginosa, toxigenic Clostridium difficile, and vancomycin-resistant Enterococci. No significant difference in the emergence of resistance was observed.42
Also, cefotaxime and ceftriaxone have a comparable spectrum of antimicrobial activity, but differ in terms of pharmacokinetics. In an antimicrobial stewardship (AMS) program, ceftriaxone was replaced (64% reduction in application density from 2013 to 2019) with cefotaxime.43
Like other cephalosporins, cefotaxime inhibits bacterial cell wall synthesis. Its beta-lactam rings bind to penicillin-binding proteins (PBPs) and inhibit transpeptidation in peptidoglycan cell wall synthesis of susceptible bacteria. This leads to the autolysis of the bacteria. The effect of cefotaxime on several organisms has been tabulated in Table 5.44
Once administered, cefotaxime is metabolized in the liver and most of the molecule including its metabolites are renally excreted. Within the liver, cefotaxime converts to desacetylcefotaxime, which is further converted to desacetylcefotaxime lactone and subsequently to metabolites.45 More than 80% of cefotaxime is recovered in the urine, with one third being in the form of desacetylcefotaxime (des-CTX). Although des-CTX is the primary metabolite of cefotaxime, its activity is eight-fold lower than cefotaxime itself. 46,47 It is generally well-tolerated and hypersensitivity reactions such as rash, fever, and pruritus are uncommon.27 Cefotaxime has not been associated with hypoprothrombinemia/coagulopathies, or disulfiram-like reactions, as with other cephalosporins and pseudocholelithiasis.27,44
In an Indian study by Merchant et al., the incidence of nosocomial infection was documented at 1.8% in patients of general wards versus 16.7% in ICU patients. It reported cefotaxime (34%) as a commonly utilized antibiotic, followed by amikacin and gentamicin.48 In adult in-patients with nonsevere CAP, the following empirical treatment regimens were recommended: combination therapy with a beta-lactam (ampicillin+sulbactam 1.5-3 g every 6 h, cefotaxime 1-2 g every 8 h, ceftriaxone 1–2 g daily, or ceftaroline 600 mg every 12 h) and a macrolide (azithromycin 500 mg daily or clarithromycin 500 mg twice daily; strong recommendation, high quality of evidence), or monotherapy with a respiratory fluoroquinolone (levofloxacin 750 mg daily, moxifloxacin 400 mg daily; strong recommendation, high quality of evidence). The third option for adults with CAP who have contraindications to both macrolides and fluoroquinolones is: combination therapy with beta-lactam (ampicillin+sulbactam, cefotaxime, ceftaroline, or ceftriaxone, doses as above) and doxycycline 100 mg twice daily.13,14
Cefotaxime may also be used prophylactically before surgery to prevent surgical infections. Enterobacteriaceae is particularly sensitive to cefotaxime and thus the drug is capable of treating MDR strains of Enterobacteriaceae.49 A favorable characteristic of cefotaxime, compared with the other cephalosporins, is that it does not cause a notable occurrence of coagulopathies and pseudocholelithiasis.44 Cefotaxime can readily cross the blood-brain barrier when administered intravenously and may treat gram-negative infections resistant to previous generations of cephalosporins.50
A study was performed on children aged 0-18 years admitted to the paediatric ICU who received intravenous cefotaxime (100-150 mg/kg/day, interval 6-8 h) as an antibiotic. The maximum cefotaxime doses (200 mg/kg/day, interval 6 h) proved adequate for MICs ≤0.5 mg/L across the whole age range against the majority of pathogens; these doses can be combined with therapeutic drug monitoring (TDM) to improve exposure after the start of treatment in critically ill children.51
A German study group led by Bruch and Kujath evaluated twice a day dosing of cefotaxime in patients with postoperative pneumonia. Of the 88 (n=88) patients without serious underlying diseases who received 1 g every 12 h dosage regimen in this study, all were clinically cured along with the eradication of all pathogens. In the cohort with severe infection/severe underlying disease receiving 2 g every 12-h of cefotaxime, the overall clinical success rate (cure plus improved) was 98.4%. Results supported the use of cefotaxime as a feasible alternative for empiric monotherapy to manage nosocomial pneumonia in surgical-service patients who were non-neutropenic or on long-term artificial ventilation.52 A similar study which evaluated twice-a-day daily dosing of cefotaxime (2 g every 12 h) in patients with severe nosocomial pneumonia documented this regimen of cefotaxime as adequate and appropriate. It was suggested that a combination of cefotaxime with aminoglycosides be reserved for cases with suspected multi-resistant Gram negative bacterial infections.53
In a randomized, multicentric trial, use of cefotaxime and ceftriaxone in adult patients with nosocomial pneumonia was proven 80% clinically and 93% bacteriologically successful with cefotaxime compared to ceftriaxone.7 In terms of adverse events the ceftriaxone group reported a higher incidence of gall bladder sludge and diarrhoea.
One Canadian trial compared cefotaxime and ceftriaxone in patients with nosocomial LRTI. It was inferred that cefotaxime at 1 g IV every 8 h was noninferior to ceftriaxone at 1 g i.v. every 12 h, with a trend for a lower likelihood of side effects, reinfections, and superinfections.54
In a multicentric clinical trial, adult patients diagnosed with nosocomial pneumonia and not receiving mechanical ventilation were treated randomly with monotherapy with cefotaxime or the antibiotic combination routinely used in a clinical set-up. The cure rate was 79% in the cefotaxime group and 71% in the group receiving antibiotic combinations; this difference was statistically significant (p ≤ 0.03). The trial concluded that monotherapy with cefotaxime is a regimen with better results, hinting at superiority of cefotaxime as an empirical therapy for nosocomial pneumonia.55 One more study was conducted on 46 adults hospitalized with pneumococcal pneumonia in Spain. The common underlying diseases in these patients included heart disease, COPD/asthma and diabetes mellitus. When resistance pattern of the pneumococci to antibiotics was studied, it was reported to be 15%, 11% and 6% with penicillin, erythromycin and cefotaxime respectively.56
Rylski conducted a study on 100 patients with respiratory infections like bronchopneumonia, acute bronchitis and chronic bronchitis who underwent fibre optic bronchoscopy. 48% of these patients tested positive for bacterial flora (aerobic, anaerobic and mixed bacterial flora). Cefotaxime was one of the antibiotics to which high sensitivity was reported in antibacterial susceptibility reports.57
In another publication, Konishi et al. reported 129 patients with respiratory tract and other infections were treated with cefotaxime at a dose of 0.5 to 8 mg of daily through the intravenous route for two to 61 days. Cefotaxime was documented to be efficacious in 87.1% (108/124) of cases. The study concluded that cefotaxime is effective against LRTIs.58 All the reported trials suggest that cefotaxime has the lowest resistance pattern towards pneumococci and is much safer as it doesn’t lead to encephalopathy, CIIHA, or cholelithiasis, and is much more effective in treating infections as it also has the potential to cross the blood-brain barrier.
Cefotaxime administration and dosing require adjusting in geriatric populations, patients with decreased renal function, and hepatic dysfunction. CBC should also be monitored with cefotaxime use.27 Cefotaxime is an FDA Pregnancy Category B drug. Cefotaxime use in pregnancy has not been studied clearly and should be used with caution. Toxicity may result in convulsions, dyspnea, hypothermia, cyanosis, reversible encephalopathy, and death. Mortality has occurred with dosages of 6000 mg/kg/day.44
LRTIs are responsible for substantial morbidity and mortality across the globe. LRTIs caused by various organisms can often be complicated by emerging antimicrobial resistance. Strategies to promote the timely and appropriate use of effective antibiotics should be implemented to reduce symptom duration, complications and mortality associated with LRTIs. Cefotaxime, demonstrates good efficacy and safety in the management of LRTIs including CAP, hospital-acquired/nosocomial-acquired pneumonia, acute exacerbation of pneumonia and acute bronchitis caused by both Gram-positive as well as Gram-negative bacteria. Despite the usage of cefotaxime in LRTIs for several decades, significant resistance has not been reported and the drug remains a reasonable option in managing these infections in current times and has much fewer adverse effects reported than its main competitor drug ceftriaxone. Also, the cost of treatment is low compared to other third-generation cephalosporins. Finally, the most important aspect is patient education, to ensure they continue the full course of antibiotics to eradicate the pathogen, to improve infection cure rates and avoid the development of any resistance or treatment failures.
The writing of this article was supported by a medical writer at Medwiz Healthcare Communications Private Ltd.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the topic of the review discussed comprehensively in the context of the current literature?
Partly
Are all factual statements correct and adequately supported by citations?
Partly
Is the review written in accessible language?
Yes
Are the conclusions drawn appropriate in the context of the current research literature?
Partly
References
1. Smith C, Petty B, Hendrix C, Kernan W, et al.: Ceftriaxone Compared with Cefotaxime for Serious Bacterial Infections. Journal of Infectious Diseases. 1989; 160 (3): 442-447 Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: infection disease, meta-analysis
Is the topic of the review discussed comprehensively in the context of the current literature?
No
Are all factual statements correct and adequately supported by citations?
Yes
Is the review written in accessible language?
Yes
Are the conclusions drawn appropriate in the context of the current research literature?
No
References
1. Kiedrowska M, Foryś WJ, Gołębiewska A, Waśko I, et al.: Antimicrobial resistance among Haemophilus influenzae isolates responsible for lower respiratory tract infections in Poland, 2005-2019.Eur J Clin Microbiol Infect Dis. 2022; 41 (6): 961-969 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: pediatric pulmonology, LTRI, immunology, respiratory viruses, bronchiolitis, pneumonia,
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 30 Jan 23 |
read | |
|
Version 1 23 Mar 22 |
read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)