Keywords
Epstein-Barr virus, diffuse large B cell lymphoma, central nervous system, cytology, magnetic resonance image
This article is included in the Oncology gateway.
Epstein-Barr virus, diffuse large B cell lymphoma, central nervous system, cytology, magnetic resonance image
Epstein-Barr virus (EBV) is a human gamma-herpes virus that has been linked to lymphomas and epithelial cell carcinomas, most of which are of B-cell derived lymphomas and less frequent are T and natural killer (NK) cell derived lymphomas.1,2 B cells are the main target of EBV infections. In infected B cells, EBV escapes from immunosurveillance by downregulating their viral gene expression, allowing the virus to establish life-long latency in memory B cells.2 Upon antigen stimulation, EBV upregulates gene products and drive infected-B cell proliferation and transformation into plasma cells. In immunocompetent individuals, EBV reactivation triggers humoral and cell-mediated immune responses mediated mainly by CD4+, CD8+ T cells and NK cells, which lead to the prompt clearance of reactivated EBV-positive B cells followed by the re-establishment of the steady state of EBV latent infection. However, in the immunosuppressive state of T cell dysfunction, including HIV infection or application of long-term and high dose of immune inhibition medicines in transplant recipients, lymphoproliferative diseases will occur and may eventually progress into lymphoma, commonly diffuse large B cell lymphoma (DLBCL).2
It is noted that EBV-positive DLBCL also arises in patients without any history of immunosuppression.1 In 2003, Oyama et al were the first to report 22 senile patients with EBV positive lymphoproliferative disorders, who had no evidence of immune impairment. Nine out of the 22 patients were diagnosed with DLBCL.3 Later, EBV-positive DLBCL with no immune deficiency was observed in young patients, even though very rare.4 Compared with EBV negative DLBCL, EBV positive DLBCL patients showed worse clinical courses and outcomes.5
Here we present a young patient with EBV positive primary central nervous system (CNS) DLBCL together with primary Sjögren’s syndrome (PSS). Prior to entering the late stage of the disease, her brain lesions were predominantly located in cranial nerves and other superficial structures. She died four and a half months after symptom onset. The rapid deterioration of her condition was recorded by clinical and dynamic brain magnetic resonance imaging (MRI) changes.
A 40-year-old previously heathy woman was admitted to our hospital with main complaints of dizziness for two months, headache and face numbness on the left side for one and a half months, and weakness in the legs and urinary retention for 10 days. The patient was firstly aware of dizziness, nausea, and left ear tinnitus. Within three weeks, she developed a consistent distending headache, face numbness on the left side, and walk imbalance. She had no fever.
The patient’s brain MRI taken in a local hospital showed contrasted lesions involving right facial nerve and cerebellum peduncle, and left trigeminal nerve and trigeminal ganglia (Figure 1A and B). Routine blood tests were normal. The level of blood angiotensin converting enzyme was in the normal range. Serum antibodies against HIV, syphilis, and hepatitis C were all negative. Serum anti-autoimmune antibodies against SSA and Ro-52 were positive whereas those against SSB and double-stranded DNA (dsDNA), and antinuclear antibodies (ANA) and anti-neutrophil cytoplasmic antibodies (ANCAs), et al. were negative. The serum levels of tumor markers, alpha-fetoprotein (AFP), carcinoembryonic antigen (CEA), cytokeratin fragment 19 (Cyfra21-1), carbohydrate antigen 19-9 (CA199), and cancer antigen (CA125) were all in the normal range. Cerebral spinal fluid (CSF) test showed 72*106/L of WBC with 99% of mononuclear cells, 247 mg/dl (normal 15-45) of protein, 0.3 mmol/L (normal 2.5-4.5) of glucose, and normal chloride level. IgG synthesis within 24 hours was 93.21 mg/24 h (normal 0-9). CSF oligoclonal bands were absent. CSF etiological explorations for tuberculosis, fungi, Lyme’s disease, and brucella were all negative. The CSF cytology revealed some enlarged cells with atypical nucleus and visible nucleolus in the background of numerous lymphocytes. CSF flow cytometry and immunophenotyping did not identify atypical cells. Neither T cells with an abnormal phenotype nor monoclonal B cells were found by blood flow cytometry and immunophenotyping.
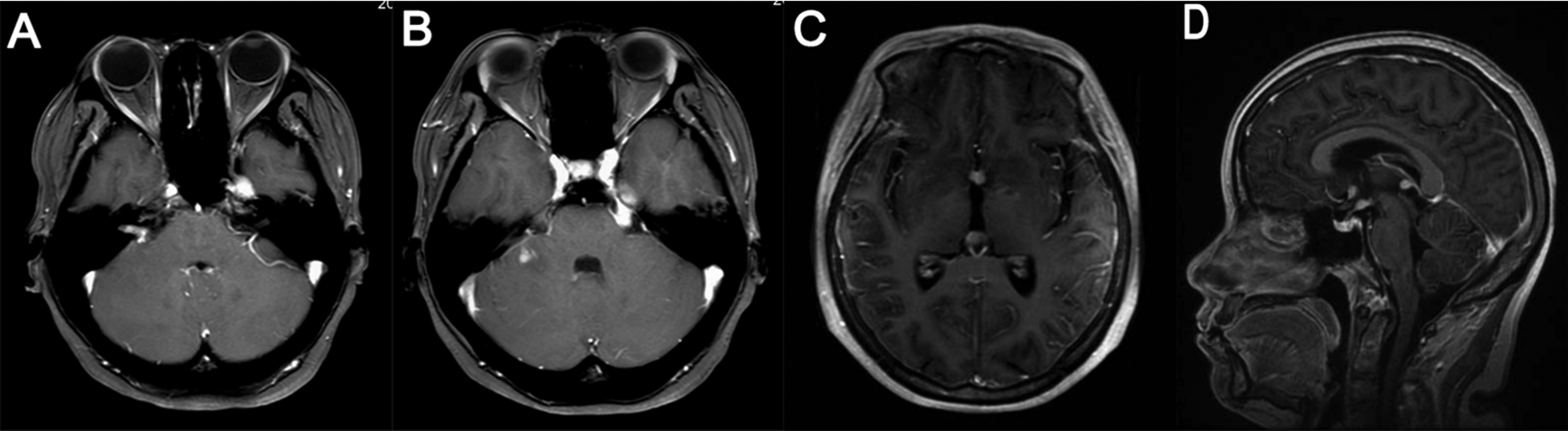
Three weeks after symptom onset, brain MRI showed enlarged left trigeminal nerve, trigeminal ganglia and right facial nerve, and a small patchy lesion in right cerebellum peduncle, all the lesions were hyperintensity on T2 FLAIR and obviously enhanced (A and B). On the 80th day, brain MRI showed enlarged and contrasted lesions in left trigeminal nerve, right facial nerve, bilateral optical nerves, mamillary body and pineal body, in addition to the contrasted lesions in right cerebellum peduncle and slightly contrasted lesions in meninges (C and D).
Tumor cells were highly suspected, and then explored extensively. Chest CT showed a small node in right upper lobe and enlarged lymph nodes in right axillary. Tumors were not observed by gastrointestinal endoscopy. 18-fluorodeoxyglucose (FDG) PET-CT of the whole body showed remarkably increased glucose metabolism in the right cerebellar peduncle, right facial nerve, left trigeminal nerve root, intraspinal canal tissues on T10 to L1 level, and axillary lymph nodes on the right side. Aspiration biopsy of right axillary lymph node demonstrated lymphadenosis with EBV-encoded small RNA (EBER) negative in situ hybridization.
To release the patient’s headache, 20% mannitol and glycerin fructose were given intravenously. Her condition continuously deteriorated and she developed double vision, urinary retention, and weakness in both legs.
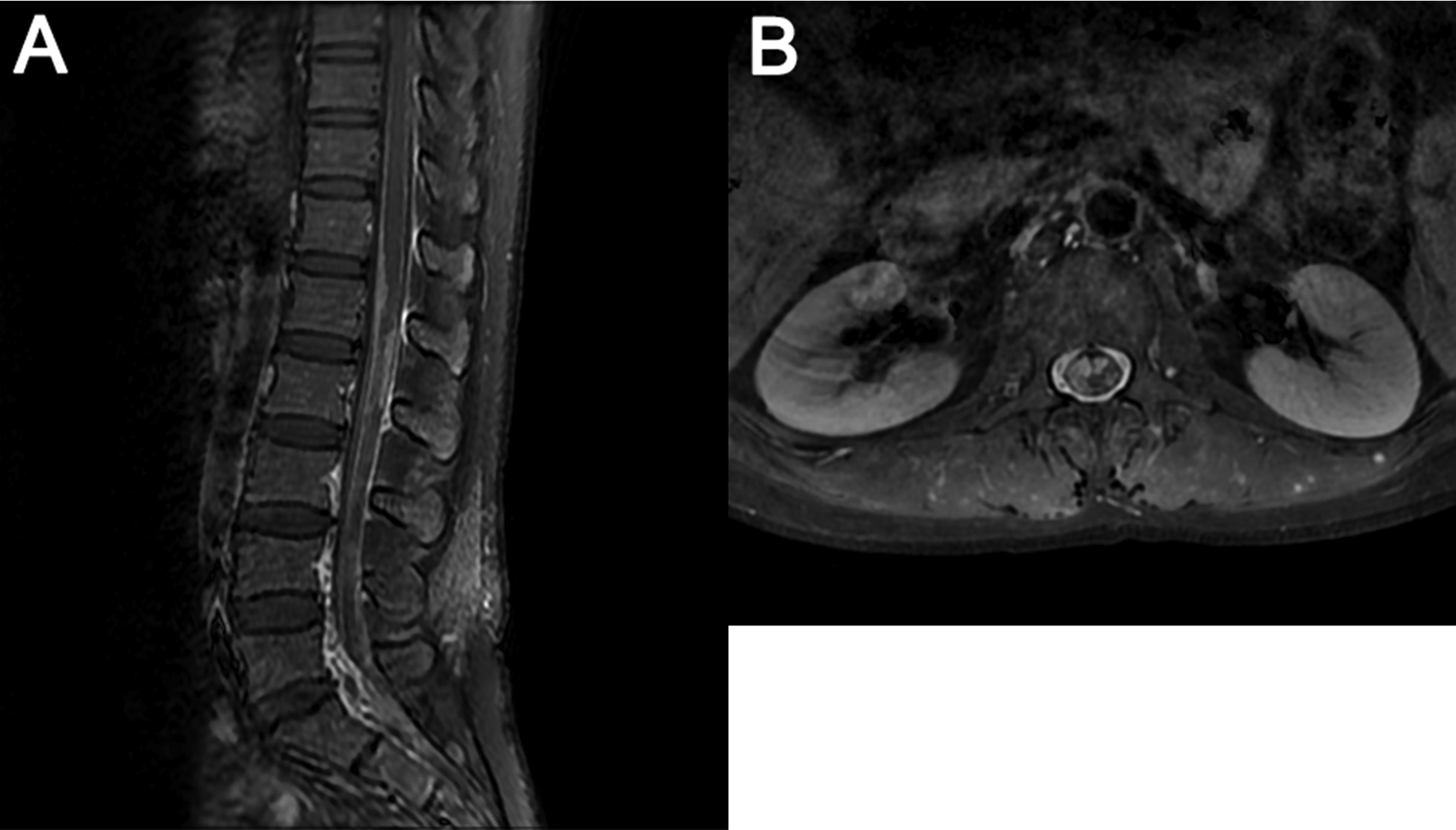
Two months after symptom onset, the patient was transferred to our hospital. On admission, neurological examinations revealed diplopia, limited movement of right eyeball abduction, pain sensation impairment on the left side of face, shallow nasolabial sulcus on the right, reduced muscular strength of IV and disappearance of tendon reflex on lower limbs. She showed no signs of meningeal irritation. Electrophysiological examination detected slowing of bilateral motor and sensory nerve conduction in the lower limbs and reduced amplitude on the left tibial nerve. Lumber puncture revealed high pressure over 350 mmH2O, glucose of 0.2 mmol/L, and protein of 257 mg/dl. Again, CSF cytology showed a lymphocytic reaction and large atypical cells. The patient’s conditions worsened rapidly and impaired vision acuity and right pyramid signs were observed. Her brain MRI on the 80th day showed new lesions with obvious enhancements in optic nerves, pituitary stalk, anterior commissure, mammillary body, pineal body, and meninges (Figure 1C and D). Lumber spinal MRI indicated enhancement in meninges between the T12-L3 level and the cauda equine roots (Figure 2).
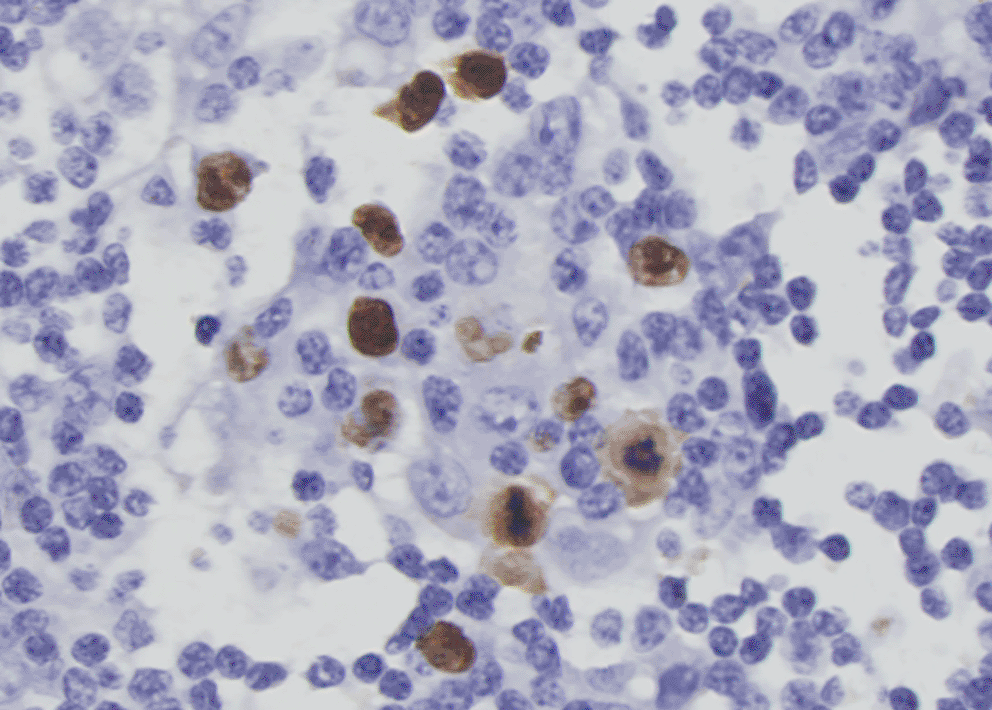
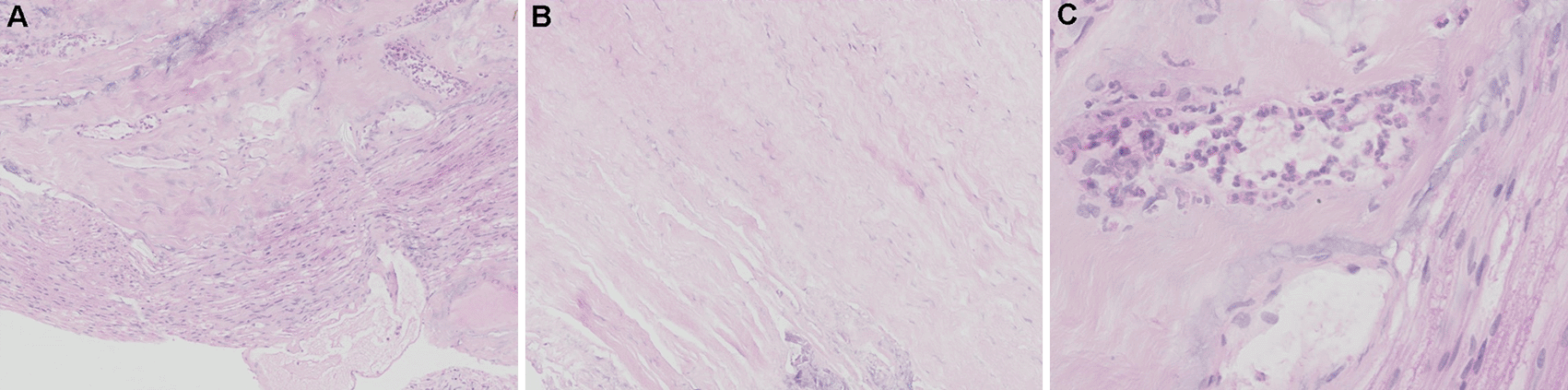
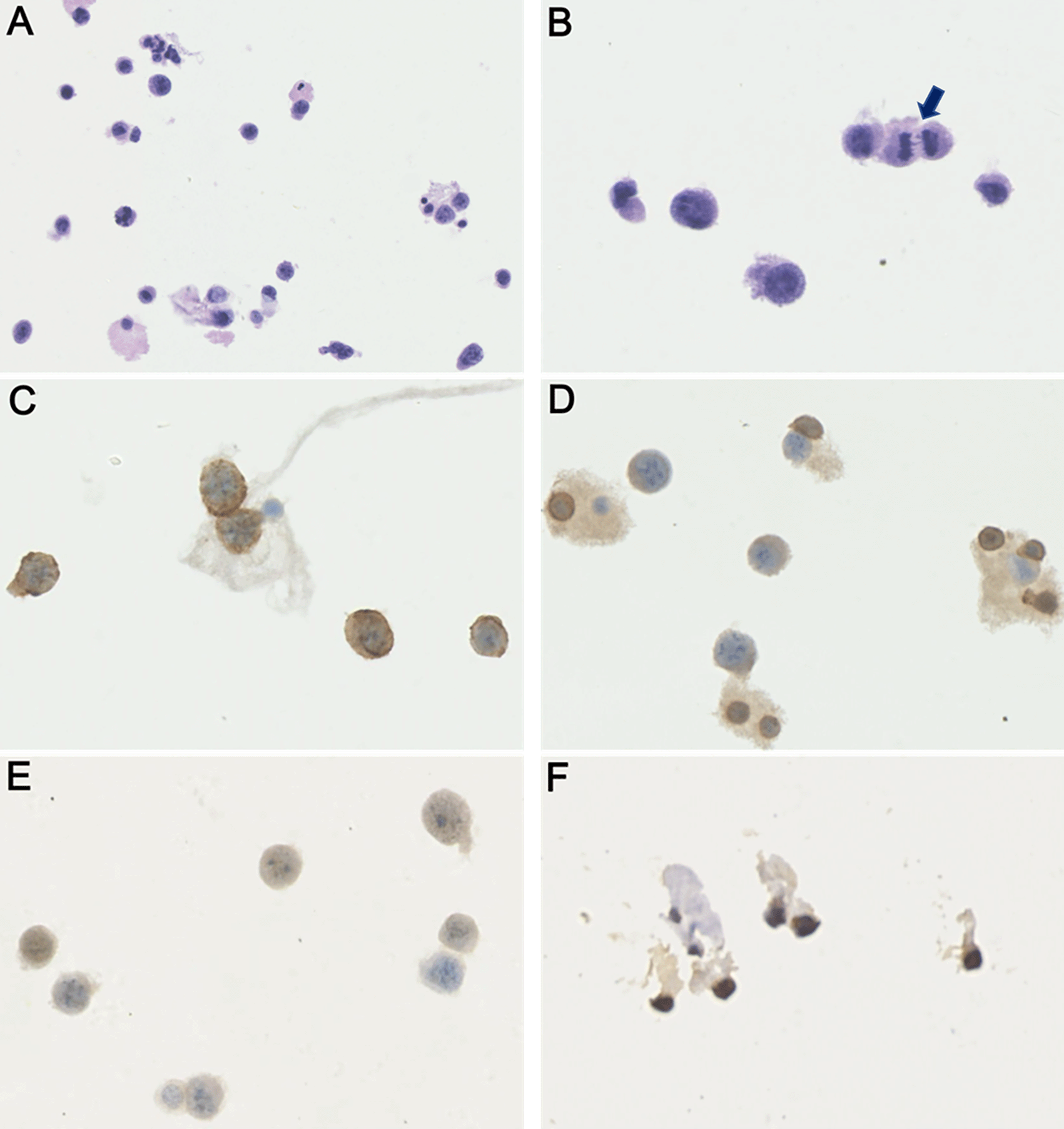
More investigations of etiology were conducted. A whole axillary lymph node biopsy on the right side showed reactive lymphoproliferation and scatter EBER positive cells by in situ hybridization, with the number reached 50/HPF in a local area (Figure 3). No angiocentric lesion was observed. EBV-related lymphoproliferation in the right axillary node was diagnosed. EBV infection evidence was obtained in serum and CSF. Blood tests for EBV showed VCA IgM (-), VCA IgG (+), VCA IgA (+), EA IgG (+), EA IgA (-), and EBNA1 IgG (+). Blood EBV DNA copy numbers detected by PCR was lower than 500 copies/ml. Next-generation sequencing of CSF identified 5 sequences of EBV dsDNA. EBV-positive lymphoproliferation disorder in CNS was up to considered. On the 90th day, biopsy of lesions within lumber spinal canal was performed. Pathological analysis did not identify tumors in the meninges nor nerve roots. Heavy adhesion of thickened and degenerated cauda equine roots, and hyperplasia and degenerated fibrous tissues surrounded by enlarged small vessels with intravascular abundant eosinophil cells were observed, indicating chronic infection (Figure 4).
After the biopsy, unfortunately, the patient presented fever, strong headache, worsened weakness of the right leg, and a loss of sensation below left knee. CSF disclosed WBC of 351/mm3, 58.7% poly-nucleus cells, and increased number of atypical cells. Mepem was given intravenously at a dose of 1g every 8 hours for 2 weeks. Although CSF WBC returned to 85/mm3 with 6% poly-nucleus cells two weeks later, the patient deteriorated rapidly.
Four months after symptom onset, the patient became somnolent. The brain MRI showed new contrasted lesions in the left hippocampus and temporal lobe, and in the bilateral cerebellums, in addition to the enlargement of previous lesions (Figure 5). CSF test revealed numerous large, atypical cells undergoing mitosis which were positive for CD20, Mum-1, Cymic and ki67 and negative for CD3 and CD10 immuno-staining, and over 80% of these atypical cells were also positive for EBER by in situ hybridization (Figure 6). CSF level of interleukin-10 (IL-10) was 578.0 pg/ml. As uniform large, atypical cells were exclusively positive for B-cell markers without a polymorphic background rich in T cells, and no characteristic angiocentric lesion of lymphomatoid granulomatosis was present in dissected lymph node, and lumbar meninges and nerve roots,6 EBV-related DLBCL became a high-priority diagnosis. PCR probing IgH gene rearrangement performed on EBV-positive lymph nodes as well as lumbar meninges and nerve roots showed negative results for clonality. Combined with brain MRI and FDG PET-CT findings, and no tumor identified in the biopsy tissues outside of CNS, primary CNS DLBCL was diagnosed. Meanwhile, primary Sjögren’s syndrome (PSS) was considered by the results of positive Schirmer’s test (3 mm/5 min in right eye) and dry mouth (reduced salivary flow rate of 0 ml/min), combined with serum positive antibodies against SSA and Ro-52. The patient was treated with rituximab 500 mg intravenously infusion weekly and lenalidomide 25 mg orally once a day, together with 1 g methylprednisolone intravenously therapy for three days followed by 500 mg/day for another three days. The patient quickly progressed to coma and respiratory failure. Half a month after initiation of the above treatment, she died. The immediate family refused autopsy of the deceased patient.
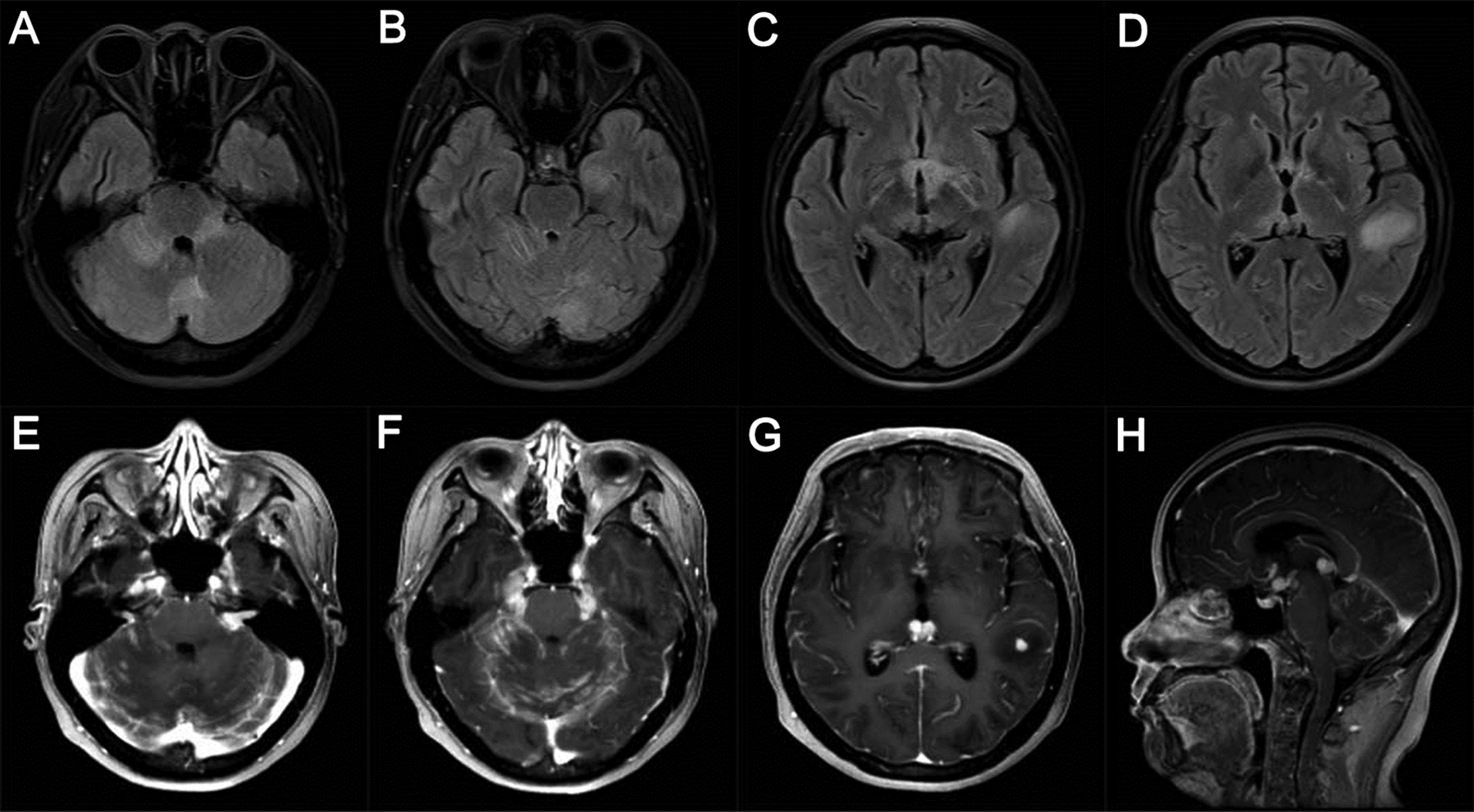
Brain MRI presented multiple lesions in bilateral cerebellum peduncles, left hippocampus, temporal, and occipital lobes, in addition to the continuously enlarged lesions in bilateral optical nerves, pituitary stalk, facial nerves, trigeminal nerves and trigeminal ganglia, maxillary body, anterior commissure and pineal body. The parenchymal lesions were hyperintensity on T2 FLAIR (A-D). All the lesions, together with cerebral meninges and cerebellar tentorium, were apparently enhanced (E-H).
Here we present a young patient with extremely aggressive EBV-related CNS DLBCL. As no evidence of DLBCL was found outside of the CNS, primary CNS DLBCL was diagnosed. The rapid progression of the disease was recorded by dynamic changes in brain MRI, starting from multiple lesions in the left trigeminal nerve, and right cerebellar peduncle and facial nerve to optical nerves, mammillary body, pineal body, meninges, and finally to the bilateral cerebellum and cerebral lobes. The predominant involvement of superficial structures of the brain in early stages of disease gave rise to the suspicion of metastatic carcinoma, sarcoidosis, tuberculosis, and other diseases. Coupled with consistent finding of atypical cells at every time point of CSF cytology, the disease was initially suspected to be metastatic carcinoma. However, no tumor outside CNS was found upon comprehensive explorations. It seemed that the acute CNS infection post lumbar intraspinal canal biopsy induced a lesion outbreak. Eventually, EBV-positive large B lymphoma cells were identified in the CSF.
The superficial brain location of DLBCL in cranial nerves or mammillary body closing to subarachnoid space, may increase the diagnostic probability by CSF cytology, but will likely result in serious functional damage induced by brain biopsy. For this reason, we performed lumbar intraspinal canal biopsy instead of brain biopsy. Unexpectedly, the biopsy mainly revealed chronic inflammatory radiculitis, but no tumor cells. Combined with the finding of five EBV DNA sequences in CSF, chronic EBV infected polyradiculitis was considered.
The overall incidence of neurological inflammatory complications of EBV infection has been estimated to be at 1-5%, including encephalitis, meningitis, encephalomeningitis, myelitis, cranial neuropathy, and radiculopathy.7 The prevalence is greatly elevated in EBV-infected patients with immunodeficiency. This patient presented several stages of EBV-related disorders in the nervous system, chronic radiculitis, and brain DLBCL, implying the process from EBV infection to lymphoma. We recognized that the unfortunate bacterium infection in the CNS after the biopsy exacerbated the disease. It is likely that the acute infection stimulated the growth and proliferation of EBV-related DLBCL by reactivating EBV.
It is well known that EBV positive lymphoma is rare in patients without immunodeficiency.1,8 Roughly 5-10% of systemic DLBCL patients are positive for EBV infection, with a significantly higher incidence in patients with immunodeficiency, rare in competent old patients.3,5 EBV-related DLBCL in the elder hosts could be due to aging-induced deterioration of immune states termed “immuno-senescence”.1 Compared with non-EBV related DLBCL, EBV-related systemic DLBCL exhibits a high rate of extranodal lesions in various sites, including the stomach-intestine, skin, and bone marrow, leading to lower sensitivity to chemo-immunotherapy, and worse prognosis.1,3,5
Until now, very few studies with small number of cases reported EBV-related primary CNS DLBCL in patients without immunodeficiency. Among these cases, patients over 50 years old are almost exclusively documented. In primary CNS lymphoma, incidence of age-related EBV-positivity is 10-14% in Asian9 and 5-7% in Western countries.10 Although no distinct pathological differences between EBV-positive and -negative systemic or primary CNS DLBCL have been shown, EBV positive CNS DLBCL patients tend to present more rapid deterioration and worse outcome. The medium lifetime expectancy after symptom onset is 26 months for EBV negative primary CNS DLBCL patients versus merely four months for EBV positive primary CNS DLBCL patients.9 One of the underlying mechanisms is that the upregulation of NF-κB and JAK/STAK signaling triggered by the EBV-specific latent membrane protein 1, leads to rapid growth of DLBCL.11
In addition to DLBCL, our young patient also suffered from PSS. PSS is a systemic autoimmune disease with heterogeneous clinical phenotypes ranging from characteristic exocrine gland dysfunction to broad extraglandular manifestations. About 5% of patients with PSS develop B-cell non-Hodgkin lymphomas, most commonly mucosa-associated lymphoid tissue lymphoma, followed by nodal marginal zone lymphoma and DLBCL.12 There may be a common mechanism of the chronic activation of B cells that underlies the development of PSS and lymphoid malignancies. For example, the overproduction of the B cell growth factors, BAFF and IL14, play important roles in the development of PSS-associated lymphomas.11 EBV infection results in an increased risk of EBV associated lymphoma; the risk of lymphoma is raised by PSS as well, which may lead to the risk accumulation.
A limitation of our study is that the diagnosis of CNS DLBCL was reached by extensive CSF examination, not by tissue biopsy. After lumbar intraspinal canal biopsy, the patient’s condition deteriorated rapidly, and her husband refused brain biopsy and final autopsy.
In summary, the young patient presented with primary CNS DLBCL and chronic inflammatory radiculopathy and spinal meninges, indicating the process from chronic EBV infection to DLBCL in nervous system. Intracranial acute bacterium infection probably exacerbated the progression of CNS DLBCL. Repeated CSF cytology, multi-parameter flow cytometry, and/or PCR for IgH gene rearrangement are necessary for the identification of CNS DLBCL entities where tissue biopsy is unavailable.13
Written consent for the publication of the report and any associated images was obtained from the patient’s husband.
All data underlying the results are available as part of the article and no additional source data are required.
The authors are grateful towards Professor Jingwen Jiang, Department of Neurology, Beijing Hospital for his careful review of this paper. The authors also appreciate the help from Professor Cheng Sha and Ao Pei, Department of Neurosurgery, Beijing Hospital, for the biopsy of lumbar intraspinal canal.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
At the request of the author(s), this article is no longer under peer review.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)