Keywords
Snakehead fish, wound healing, hyperglycemia, fibroblast, neutrophil, vascular changes
Introduction: Wound healing is an integrated response to tissue injury. A hyperglycemic state can lead to delayed wound healing process. Snakehead fish (Channa striata) is native freshwater fish of South East Asia that contains high protein, albumin, and several micronutrients. The aim of this study is to evaluate the efficacy of snakehead fish extract on acute wound healing process in streptozotocin-induced hyperglycemic rats.
Methods: This study was an experimental trial on the hyperglycemic animal model. Thirty male Wistar streptozotocin-induced rats were divided into two groups which were then given snakehead fish extract (Pujimin Plus®) 81mg a day for 10 days after wound infliction in intervention group and carboxymethyl cellulose sodium (Na-CMC) in control group. On day 0, day 3, and day 10 after wound infliction, the histological changes (number of neutrophil and fibroblast, and vascular changes of the wounded tissues) of each group were analyzed. Assessments were also made on erythema and crust formation by the visual scores.
Results: Our study showed a significant increase in the number of fibroblasts on day 3 in the snakehead fish extract group compared to control group (40.33 ± 10.13 vs. 24.60 ± 10.25, p =0.04). There were no significant differences in vasculature and neutrophil numbers. The results also showed snakehead fish extract could decrease mean erythema visual score on day 3 (3.24 ± 0.25 vs. 3.64 ± 0.35) and decreased crust formation on day 5 (3.36 ± 0.75 vs 3.44 ± 0.83).
Conclusions: Snakehead fish extract has potential effect to accelerate the wound healing process by increasing fibroblast, decreasing erythema, and decreasing crust formation in streptozotocin-induced hyperglycemic rats.
Snakehead fish, wound healing, hyperglycemia, fibroblast, neutrophil, vascular changes
Editorial Note (20th October 2023): The F1000 Editorial Team has not yet received a new version of this article, as detailed in the Editorial Notes published on 16th June and 4th August 2023, despite repeated chasing. The F1000 Editorial Team will no longer be requesting a new version from the authors. Readers should be aware that the raw images for this article have been seen and verified by the F1000 Editorial Team, but have not been uploaded by the authors. Peer review activity remains suspended until the authors publish a new version of this article.
Editorial Note (4th August 2023): The F1000 Editorial Team has not yet received a new version of this article, as detailed in the Editorial Note published on 16th June 2023. The F1000 Editorial Team is actively contacting the authors to request the new version of the article. Peer review activity remains suspended until the authors publish a new version of this article.
Editorial Note (16th June 2023): Since publication, it has been brought to the attention of the Editorial Team that raw macroscopic and histological images were missing from the underlying data of this article. As per our data availability policy, the Editorial Team requested these images from the authors in March 2023. The authors provided these images, which have been verified. The authors have been requested by the Editorial Team to upload the raw images to a data repository and create a new version of the article to include the data DOI. Peer review activity has been suspended until the authors publish a new version of this article.
Prolonged hyperglycemic conditions can cause various complications, both macrovascular and microvascular. Patients tend to experience impaired wound healing processes, which can develop into chronically infected wounds. Chronically infected wounds will increase the risk of systemic infection leading to increase in the morbidity and mortality of patients. Therefore, it is necessary to have a good understanding of the wound healing process and appropriate interventions to support a good wound healing process.1,2 Physiologically, wound healing is achieved through four phases: hemostasis, inflammation, proliferation, and remodeling. All these phases occur in an integrated way and require an optimal environment and conditions. In hyperglycemia conditions, systemic inflammation leads to increase oxidative stress, impairs migration of leukocytes to the wound area, and inhibit extracellular matrix synthesis.2
Protein plays an important role in regulating the body's homeostatic processes. They are the constituent components of immune cells, cytokines, tissues, and enzymes that are involved in the physiological process of wound healing. Neutrophils, monocytes, and macrophages release pro-inflammatory cytokines and growth factors such as platelet-derived growth factor (PDGF), transforming growth factor-β (TGFβ), and fibroblast growth factor (FGF), which are involved in the activation of fibroblasts and epithelial cells. PDGF stimulates fibroblast functions and collagenase expression. TGFβ controls the regulation signal of extracellular matrix deposits, increasing the production of matrix proteins and downregulation of protease enzymes. In the wound area, there is an increase in the level of hypoxia-inducible factor (HIF) in cells. HIF will bind to specific DNA and stimulate specific transcription genes such as vascular endothelial growth factor (VEGF). VEGF is the main vascular growth factor in the entire angiogenesis process.3
Snakehead fish (Channa striata), a native freshwater fish in South East Asia, contains high protein and albumin which act as antioxidants and constitutive tissue components. Snakehead fish also contains micronutrients such as zinc, iron, calcium, and magnesium which have role in the immune system and wound healing process. Administration of snakehead fish significantly increased fibroblasts in oral mucosal wounds of Wistar rats.4
Although there have been several studies regarding the benefits of snakehead fish extract on wound healing in diabetics, the wounds in those studies have different sizes and conditions. The difference in type of wounds, wound size, food intake factors, and metabolic condition can be confounding in assessing the effect of snakehead fish extract on wound healing.4–6
We developed snakehead fish extract formula (Pujimin Plus®) as a protein and micronutrients source with an aim to reduce hypoalbuminemia among patients and improve the wound healing process. However, the exact mechanism of how this extract improves wound healing remains unclear.
Therefore, this study was conducted to see the effect of snakehead fish extract on healing acute wound tissue in hyperglycemic rats by analyzing macroscopic and microscopic changes in wound tissue.
This study used an animal experimental model.26 It was approved by the Health Research Ethics Commission in Faculty of Medicine, Hasanuddin University, Indonesia with number 209/UN4.6.4.5.31/PP36/2021 and registered in preclinicaltrials.eu with ID PCTE0000240.
Thirty healthy male Wistar rats (Rattus norvegicus) weighing 150–200 g and aged 8– 10 weeks were used in this study. The rats were kept at Molecular Biology and Immunology Laboratory, Faculty of Medicine, Hasanuddin University and housed in six plastic cages (five rats per cage) at room temperature (22 ± 2°C) for 1 week in a light-dark cycle of 12 hours with free access to food and water. They were fed a conventional rat diet with Comfeed AD II and had an unlimited supply of drinking water.
Hyperglycemia induction was performed by using intraperitoneal injection of Streptozotocin 8.5 mg. Blood glucose levels were checked 24 hours after streptozotocin induction, a level > 200 mg/dL confirmed hyperglycemia. Blood glucose level was re-checked prior to sample collection.7,8
Throughout the experimental study, every effort was made to minimize the pain and distress of the animals. Wound was inflicted after confirmation of hyperglycemic status. Prior to the wound infliction, the dorsal hair was removed using hair remover gel, the area was disinfected using a 70% alcohol swab and then allowed to dry. The rats were anaesthetized with ketamine (80-100 mg/kg) intraperitoneally. Wound was inflicted on the bare area of dorsal skin using a punch biopsy with a diameter of 8 mm to the depth of deep fascia.9,10
The rats were divided into 2 groups, intervention and control groups. After wound infliction, the rats in intervention group were given snakehead fish extract (Pujimin Plus®) at a dose of 81 mg/day by oral gavage for the next 10 days (Table 1). Those in control group were given carboxymethyl cellulose sodium (Na-CMC) solution as a placebo.11,12 Pujimin Plus® capsule is a patent trademark of snakehead fish extract with patent number IDM000301812.
The wounds were observed daily and photo documentation was done on days 0, 3, 5, 7, and 10. The lesions on each rat were rated by 3-blinded physicians as investigators. The observation was performed twice and assessed by a supervisor in order to reduce the reliability problem. The lesion were rated using the following 5-point-erythema score13: 0 = no red color at all, 1 = light red just visible, 2 = clearly red, 3 = dark red, but not whole area, 4 = dark red widespread. Likewise, 6-point-crust formation score14 was also followed: 0 = 100% crust formation, 1= 76 – 99% crust formation, 2 = 51–75% crust formation, 3 = 26–50% crust formation, 4 = 1–25% crust formation, 5 = 0% i.e. no crust formation.
Tissue samples were collected from 5 rats of each group on day 0, day 3, and day 10 after wound infliction. The rats were sacrificed using isoflurane inhalation (0.05 μg/kg/day) by placing the rat in a box and waiting for 5-10 minutes. The process of extracting wound tissue involved making an excision on the skin with a scalpel number 20 at a distance of 1.5 cm from all edges of the wound. Tissue samples were stored in a container containing neutral buffered formalin 10% and then transported to the Anatomical Pathology Laboratory of the Hasanuddin University Hospital for histological examination with hematoxylin and eosin (H&E) staining. H&E stain is a type of histological stain that clearly stains cell structures including the cytoplasm, nucleus, organelles, and extra-cellular components.25 Histological analysis was carried out by assessing the number of neutrophils, fibroblast, and vascular changes (arterioles and capillaries) in three fields of view with 40× magnification.
Descriptive statistics and bivariate analysis were performed using independent samples t-test for parametric and Mann-Whitney U test for nonparametric analysis. Descriptive statistics of rats were presented for body weight and blood glucose level after 24 hours of streptozotocin induction. Bivariate analysis was performed to assess the difference of the mean of wound healing level between groups (number of neutrophils and fibroblast, vascular changes, and macroscopical scores). The data were entered into statistical package for social sciences (IBM SPSS Statistics, RRID:SCR_019096) version 23.0 database for Windows. The p-values < 0.05 were considered statistically significant in all analyses.
A total of 30 male Wistar rats (R. norvegicus) were divided into 2 groups (snakehead fish extract group and control group). No rats were dropped out, nor did any rat die during the research. All rats experienced hyperglycemia with a target of blood glucose level >200 mg/dL after the first streptozotocin induction (Table 2). The body weight and blood glucose level 24 hours after streptozotocin induction were measured for both groups.
Macroscopic analysis was done by assessing the presence of erythema and the formation of crusts in wounds on day 0, 3, 5, 7, and 10. The highest mean of erythema visual score was found on day 3, which decreased day by day until no erythema was found (score 0) on day 10 in both the groups. The erythema scores of control group on day 3, 5, 7, and 10 were higher than the extract group, but there were no significant differences between the two groups (Table 3).
| Group | Day 3 | Day 5 | Day 7 | Day 10 |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| Control | 3.64 (0.35) | 2.66 (0.48) | 1.10 (0.49) | 0.00 (0.00) |
| Extract | 3.24 (0.25) | 2.50 (0.37) | 0.96 (0.52) | 0.00 (0.00) |
| p-value | 0.292 | 0.283 | 0.787 | 0.000 |
In general, the mean crust visual score of extract group was lesser than the control group. The increase of mean crust visual score of extract group occurred on day 3 (1.84±0.22) and the highest score was noted on day 5 (3.36±0.75), which then decreased on day 7 (1.04±0.29) until there was no crust formation (score 0) on day 10. Similarly, in control group, the highest mean crust visual score was noted on day 5 (3.44±0.83) which decreased on day 7 (1.18±0.27) until there was no crust formation on day 10. There was no significant difference between the two groups (Table 4).
Microscopic observations for counting the number of neutrophils, fibroblasts, and vascular changes [or angiogenesis processes (the growth of new blood vessels from existing vessels)] were performed at 40× magnification. As noted in Table 5, the average number of neutrophils in extract group reached the highest value on day 3 (54.47±6.27) then declined on day 10 (3.6±2.43). Similar to extract group, the highest mean of neutrophils in control group were observed on day 3 (59±21.27) then declined on day 10 (5.58±2.63). There were no significant differences in neutrophil numbers in both the groups.
| Histological view | Control Mean (SD) | Extract Mean (SD) | P value | |
|---|---|---|---|---|
| Neutrophils | Day 0 | 21.00 (5.48) | 19.67 (3.57) | 0.661 |
| Day 3 | 59.00 (21.73) | 54.47 (6.27) | 0.666 | |
| Day 10 | 5.58 (2.63) | 3.60 (2.43) | 0.279 | |
| Fibroblasts | Day 0 | 1.33 (0.71) | 1.87 (0.18) | 0.141 |
| Day 3 | 24.60 (10.25) | 40.33 (10.13) | 0.040* | |
| Day 10 | 44.83 (14.81) | 42.07 (6.39) | 0.715 | |
| Vascular changes | Day 0 | 6.33 (2.26) | 5.67 (2.50) | 0.670 |
| Day 3 | 13.53 (4.84) | 14.80 (3.50) | 0.648 | |
| Day 10 | 4.08 (2.52) | 3.87 (1.17) | 0.868 | |
The angiogenesis processes on day 3 increased to 14.80±3.50 in extract group and 13.53±4.84 in control group then decreased to 3.87± 1.17 in extract group and 4.08±2.52 in control group on day 10. There were no significant differences between both groups. Based on Table 5, the mean number of fibroblast cells in extract groups on day 0 and day 3 were higher than the control group. In extract group, observations on day 0 showed the mean number of fibroblast cells were 1.87±0.18, which then increased to 40.33±10.13 on day 3, and the highest number 42.07±6.39 was observed on day 10. The mean number of fibroblasts in extract group on day 3 had a significant difference compared to the control group (p=0.040). On day 0 and day 3, the mean number of fibroblasts in control group were lesser than the extract group with the value 1.33±0.71 and 24.60±10.25 respectively. On day 10, the mean number of fibroblasts in control group were higher than extract group but there was no significant differences (p=0.715).
Figure 1 shows the histological changes of the wound healing process over time. Histological images on day 0 were collected from wounded skin tissue of the rats after 2 hours of wound infliction. The clot was formed as a result of hemostasis process (Figures 1A-1B). On day 3, infiltration of inflammatory cells in wound tissue was seen, particularly neutrophils and several lymphocytes. More active fibroblasts migrated into the wound area of the extract group on day 3 compared to the control group (Figures 1C-1D). The proliferation phase represents a proliferation of both epithelial and dermal elements which results in re-epithelialization of the wound and laying of the primary extracellular matrix. On day 10, the number of inflammatory cells were less and the layer of the epidermis was thicker. The granular tissue was full of cells and arteries completely occupied the wound in both groups. The histological images showed a complete re-epithelialization with dense collagenous matrix (Figures 1E-1F).
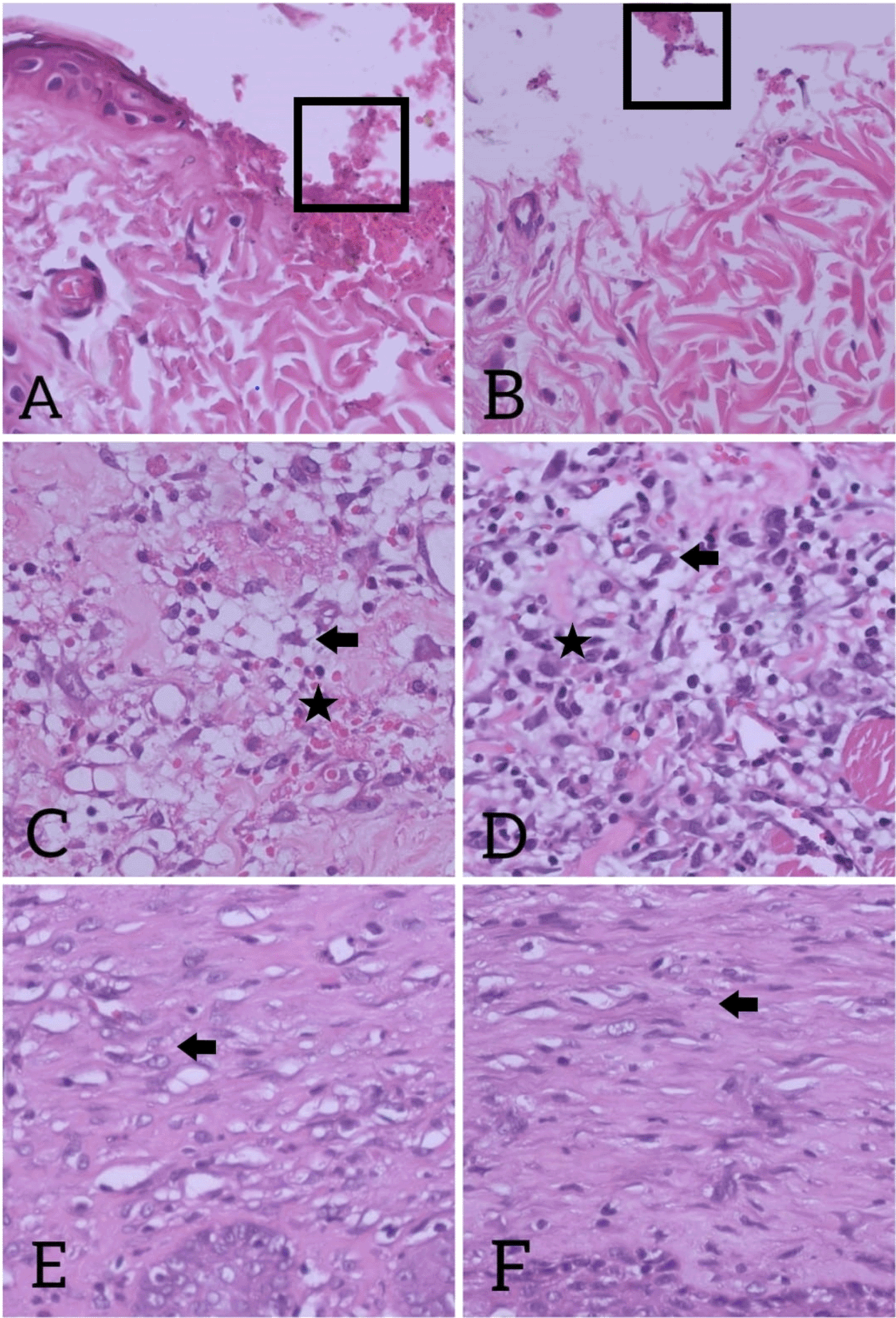
(A) Control group on day 0; (B) Extract group on day 0. Clotting blood (square) in wound area; (C) Neutrophil infiltration (star), lymphocyte infiltration, and fewer active fibroblast (arrow) in control group on day 3, (D) More active fibroblasts (arrow) with neutrophil infiltration (star), lymphocyte infiltration seen in intervention group on day 3; (E) In control group on day-10; (F) Fibroblast (arrow) with dense extracellular matrix seen in intervention group on day-10. H&E stain, magnification 40×. Fibroblast is spindle-shaped cells (flat and elongated) with oval-shape nucleus. The cytoplasm is basophilic and is stained in purplish blue by H&E stain. During stress, fibroblasts adapt to their environment and have the ability to respond and send local signals. On day 3 after wound creation (Figs. C and D), fibroblasts transformed to active forms with euchromatic nuclei and obvious cytoplasm. This form plays role in wound healing to sends local signal, synthesizes the extracellular matrix, and produces the structural stroma for tissue. On day 10 (Figs. E and F), in remodeling phase, fibroblasts became to inactive forms with small cytoplasm and reduced amount of rough endoplasmic reticulum. The extracellular matrix and stroma became denser in this phase.
Wound healing is the complex process that includes several phases such as hemostasis, inflammatory, proliferative, and remodeling. This whole phase requires optimal environment and conditions to be able to take place properly. In conditions of hyperglycemia, systemic inflammation occurs which increases the oxidative stress, impaired migration of leukocytes to the wound area, endothelial cell dysfunction, and decreased synthesis of extracellular matrices. This condition can interfere with the wound healing process.2 Snakehead fish (C. striata) extract has high nutritional value and has shown various benefits in increasing albumin levels, lowering inflammatory status, and aiding the wound healing process.12,15–17
In the inflammatory phase of the wound healing process, there is an increase in the number of neutrophils in the tissue. Neutrophils play a role in fighting infections through the phagocytosis of pathogens and foreign bodies as well as secreting inflammatory mediators such as Tumor Necrosis Factor Alpha (TNF-α) and Interleukin-1 (IL-1) that can attract and activate fibroblasts and epithelial cells. Neutrophils also produce high levels of protease (elastase and collagenase) that can clean up damaged extracellular matrix components.3 Generally, the number of neutrophils in a tissue begins to increase in the first 24–48 hours after injury, reaching a peak at 2–3 days and then decreasing thereafter through the process of apoptosis. In inflammatory conditions with infection, the number of tissue neutrophils can last longer and lead to an elongated wound healing process.18
Various studies have shown that micronutrients such as zinc, calcium, magnesium, manganese, and iron are required in the wound healing process. Micronutrient deficiency is associated with prolonged wound healing process and the occurrence of chronic wounds. Zinc plays a role in various stages of wound healing including platelet activation, polymorphonuclear influx, re-epithelial processes, angiogenesis, and extracellular matrix remodeling.19 Magnesium can improve insulin sensitivity and blood sugar regulation that play a role in the healing process of diabetic wounds.20 Calcium is a micronutrient that plays a role in the homeostasis of skin tissue in mammals. Locally, calcium can modulate proliferation, maturation, and cell motility through transduction signals and gene expression in the process of epidermal regeneration and dermal reconstruction in wound healing.21 Iron plays a role in the process of angiogenesis in wound healing.22
In this study, it was found that the number of tissue neutrophils in the control and groups increased on day 3 and decreased on day 10, although there were no significant differences in both groups, the average number of neutrophils in the control group on days 0, 3, and 10 were higher compared to the extract group.
In the proliferation phase, there are several processes that occur including matrix turnover to restore tissue structure and function, angiogenesis processes, and granulation tissue formations that lead to re-epithelialization processes. Migration and accumulation of fibroblasts in the wound area play a role in the movement from the extracellular matrix to the wound area.3
This study showed that the number of tissue fibroblasts in the control and extract group increased on days 3 and 10 with statistically significant differences on day 3 where the number of fibroblast cells of the extract group was higher than the control group. Similar results were obtained in a previous study that showed the administration of snakehead fish extract increased the number of fibroblasts and expression of fibroblast growth factor-2 (FGF-2) in the healing process of oral mucosa of the Wistar rats. Fibroblasts regulate angiogenesis via secretion of FGF-2.16 Snakehead fish extract contains zinc and calcium that can stimulate fibroblasts in tissue healing. Zinc plays an important role in the proliferation phase by stimulating fibroblast infiltration and extracellular matrix deposit.19 Calcium modulates proliferation and maturation of dermal structures in the wound area.21
The process of angiogenesis is stimulated by local factors including low oxygen pressure, low pH, and high lactate levels. VEGF is the primary vascular growth factor in the entire angiogenesis process.3,23 Iron grade has a positive effect on wound healing by increasing VEGF and HIF1-α and contains functional proteins that play a role in collagen metabolism and extracellular matrix by procollagen-proline dioxygenase.22
Our study showed that the number of vascular tissues in the control and extract groups increased on day 3 and decreased on day 10, despite no significant differences in both the groups. From this data, it can be concluded that snakehead fish extract does not affect the vascular tissue significantly in the wound healing process. Dose adjustment and assessment of anemia status in samples were required to assess iron requirements in rats, given the previous studies that assessed the effect of iron on wound healing using different doses, methods of application, and anemia status.22,24
Macroscopically the extract group had a smaller erythema visual score than the control group, especially on day 3. Even though not significant, the formation of crust in the extract group on day 3, 5, and 7 had smaller visual score compared to control group. These macroscopic results showed that the extract group had lesser erythema on day 3 and fewer crust formation compared to the control group.
The limitation of this study is lack of tissue examination days (Microscopic changes in skin tissue can occur rapidly, especially during the inflammatory and proliferative phase, so histological examination should be done on a regular basis). Therefore, more routine tissue examination is needed to better assess histological changes in the wound healing process. The longer duration of snakehead fish extract intake can affect the result of the study.
In hyperglycemic rats, oral administration of snakehead fish extract significantly increased the number of fibroblasts in the proliferation phase of the acute wound healing process. Although insignificant, snakehead fish extract can also decrease the number of neutrophils of wound tissue in the inflammatory phase of acute wounds. Macroscopically, decreased erythema and fewer crust formation were seen in intervention group compared to control group. Therefore, snakehead fish extract has potential benefits and can be used to support the healing process of acute wounds in hyperglycemic conditions.
Figshare: Underlying data for ‘The effect of snakehead fish extract on acute wound healing process in hyperglycemic rats’. https://doi.org/10.6084/m9.figshare.19146155.v1.26
The project contains the following underlying data:
Figshare: ARRIVE checklist for ‘The effect of snakehead fish extract on acute wound healing process in hyperglycemic rats’.
https://doi.org/10.6084/m9.figshare.19146260.v1.27
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
Nurpudji Astuti Taslim: Conceptualization, Funding Acquisition, Investigation, Supervision, Validation, Writing – Review & Editing; Caroline Prisilia Marsella: Writing – Review & Editing; Agussalim Bukhari: Validation, Writing – Review & Editing; Muhammad Husni Cangara: Validation, Writing – Review & Editing; Andi Makbul Aman: Validation, Writing – Review & Editing; Aminuddin: Validation, Writing – Review & Editing; Mardiana Madjid: Validation, Writing – Review & Editing.
We thank all contributors, especially Hasanuddin University Research and Community Services for support.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Ahmad Farouk Musa - Clinical Trials Shaik Farooq - Pharmacology
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Marine biology and Aquatic Resources Management; Fisheries and Marine Sciences
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 24 Mar 22 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)