Keywords
case report, COVID-19 vaccine, adverse reactions, before-after study, statistic interrupted time analyses, fever, anorexia
This article is included in the Emerging Diseases and Outbreaks gateway.
Background: In Japan, more than 1,000 participants died shortly after receiving the coronavirus disease 2019 (COVID-19) vaccine, but the causal relation between the injection and death remains uncertain. Methods: Applying long-term personal vital care data for 28 months for an elderly patient, I investigated and evidenced adverse reactions after the first dose of the COVID-19 Pfizer vaccination. Results: The precise, detailed, and continuous data statistically clarified the long-term fevers associated with no meals or drinks. Interrupted time series analysis showed significant and fluctuating increases of body temperatures, pressures, and pulses, although solely long-term plots showed an abrupt and timely increase in these vital data after the vaccine. Conclusions: Anorexia was fatal, and newly reported in the present care records since the patient received the first dose of the COVID-19 vaccine.
case report, COVID-19 vaccine, adverse reactions, before-after study, statistic interrupted time analyses, fever, anorexia
The conclusion, incorporating medically appropriate terms, was revised by me without involving a medical co-authorship, which was deemed impossible.
Medically suitable terminology was applied, particularly in the Abstract, alternating with unsuitable terms.
The end of the Introduction was revised, replacing it with medically appropriate terms.
Half of the Patient Information was modified using medically appropriate terms.
The second sentence of the Methods section was adjusted with more suitable terminology.
The timeline was extensively modified, incorporating more appropriate terms.
"Tongue coat" was replaced with "lingual coating."
Unsuitable English, including terminology, was revised in the Discussion.
References need to be provided for "cytokine storm," including the newly cited source: Fajgenbaum DC, June CH: Cytokine Storm. N Engl J Med 2020; 383: 2255-73. doi: 10.1056/NEJMra2026131.
The statement "This case report was supported by funding from the Japan Society for the Promotion of Science" appears peculiar as the funding does not seem to be for medical research, but it is retained for technical reasoning.
To read any peer review reports and author responses for this article, follow the "read" links in the Open Peer Review table.
According to the descriptions by the World Health Organization (2021):
“Like any vaccine, coronavirus disease 2019 (COVID-19) vaccines can cause mild to moderate, short-term side effects, such as a low-grade fever or pain or redness at the injection site, fatigue, headache, chills, diarrhea, and allergic reaction. Most reactions to vaccines are mild and go away within a few days on their own. More serious or long-lasting side effects to vaccines are possible but extremely rare. Vaccines are continually monitored for as long as they are in use to detect rare adverse events and implement approaches to limit their occurrence.”
To date, except for anaphylactoid reactions, blood clots, myocarditis (Nassar et al., 2021) or pericarditis (Ashaari et al., 2021), and Guillain-Barré syndrome (McKean and Chircop, 2021; Kanabar and Wilkinson, 2021), adverse reactions to the COVID-19 vaccines are mild or moderate, occur shortly after vaccination and are not associated with more serious or lasting illness (Medicines & Healthcare products Regulatory Agency UK, 2021). Similarly, the reactions peaked within one day, although in rare cases lasted a week (Menni et al., 2021). The likelihood of accepting the vaccine were lower when the probability of serious adverse reactions such as paralysis was 1/100,000 in contrast to 1/million or 1/100 million (Kaplan and Milstein, 2021).
The present research, which incorporates elements of “implementation research” (an integrated concept that links research and practice to accelerate the development and delivery of public health approaches; Theobald et al., 2018) is connected to evidence-based intervention. It does not fall under the strict definition of a clinical study associated with a clinical double-blind study and is presented as a case report. I applied the statistical concept of interrupted time series analysis to an elderly participant who received the first dose of the COVID-19 Pfizer vaccine on June 2, 2021. While this analysis is typically applied to populations, the concept remains the same even for a single participant. In Japan, over 1,000 participants died shortly after receiving COVID-19 vaccine injections until September 2021, but the causal relationship between the injection and death remains uncertain (Ministry of Health, Labour and Welfare, Japan). Herein, I report previously unreported serious side effects, including unexpected adverse reactions, based on thoroughly monitored reliable long-term care records.
The participant was diagnosed with dementia in 2015 and a femoral neck fracture on 1 March 2019, and when the fracture was almost repaired in a hospital, the participant moved to the Geriatric Health Services Facility, on 2 April 2019. This facility is staffed by with physicians, nurses, physical therapists, occupational therapists, nutritionists, and care workers.
The Japanese Government (Ministry of Health, Labour and Welfare) decided that healthcare workers and people over 65 years were the first to be vaccinated, and elderly participants were initially included as targeted persons. The participant was 90 years old and died on 25 August 2021, 28 weeks after the first vaccination. The second injection was planned on 25 June was cancelled due to the severe side effects that presented immediately after the first injection. The initial nursing care level was 2 (partial assistance) but was changed to level 5 (bedridden) on July 20, 2021.
The personal vital data and other related information were continuously monitored from April 2019 to August 2021, including the day of the vaccine, June 2, 2021. For example, the night shift care worker checked the digital data every hour, and the “Care Records” were precise and detailed. These records include timetable entries for three meals (e.g., expressed as main dish 6/10, side dish 9/10, where 10/10 = complete meal, 0/10 = no meal), drinks (ml), body temperature, blood pressure, pulse, saturation of percutaneous oxygen (SpO2), urination, defecation, and physical and medical examinations. The data summary can be viewed on a computer screen in the facility. It’s important to note that when the individual has a fever, the temperature is measured several times a day, and the last monitored temperature (not the maximum) appears on the PC screen as the default setting.
Contrasting the questionnaire survey among participants (Suehiro et al., 2021) and the multinational network cohort with electronic health records and health claims data (Li et al., 2021), the concept of the analysis is also applicable to a single participant. Because data from the million participants with mild to moderate and short-lasting side effects were privately measured and lacked long-term records, the present high-quality data, even for a single participant, are valid for scientific analyses.
This research used a method of statistical analysis that involves tracking a long-term period before and after a point of intervention, in order to assess the intervention’s effects. According to Ferron and Rendina‐Gobioff (2005) and others, “the time series refers to the data over the period, while the interruption is the intervention, which is a controlled external influence or set of influences. The effects of the intervention are evaluated by changes in the level and slope of the time series and the statistical significance of the intervention parameters”. For this case report, the intervention is the COVID-19 Pfizer (Comirnaty) vaccination, given on 2 June 2021.
I created a figure illustrating vital data tracking over a long-term period (28 months) using the Care Records for the patient (1,500-page hard copies in Japanese; Figure 1; created using Excel 2008 for Mac). Additionally, I generated a figure depicting vital data tracking over a short-term period (6 months) from the summary records (28-page hard copy in Japanese) produced by the care records for the patient (Figure 2; created using Excel 2021) for the application of interrupted time series analyses. Table 1 provides a summary of the statistical analyses.
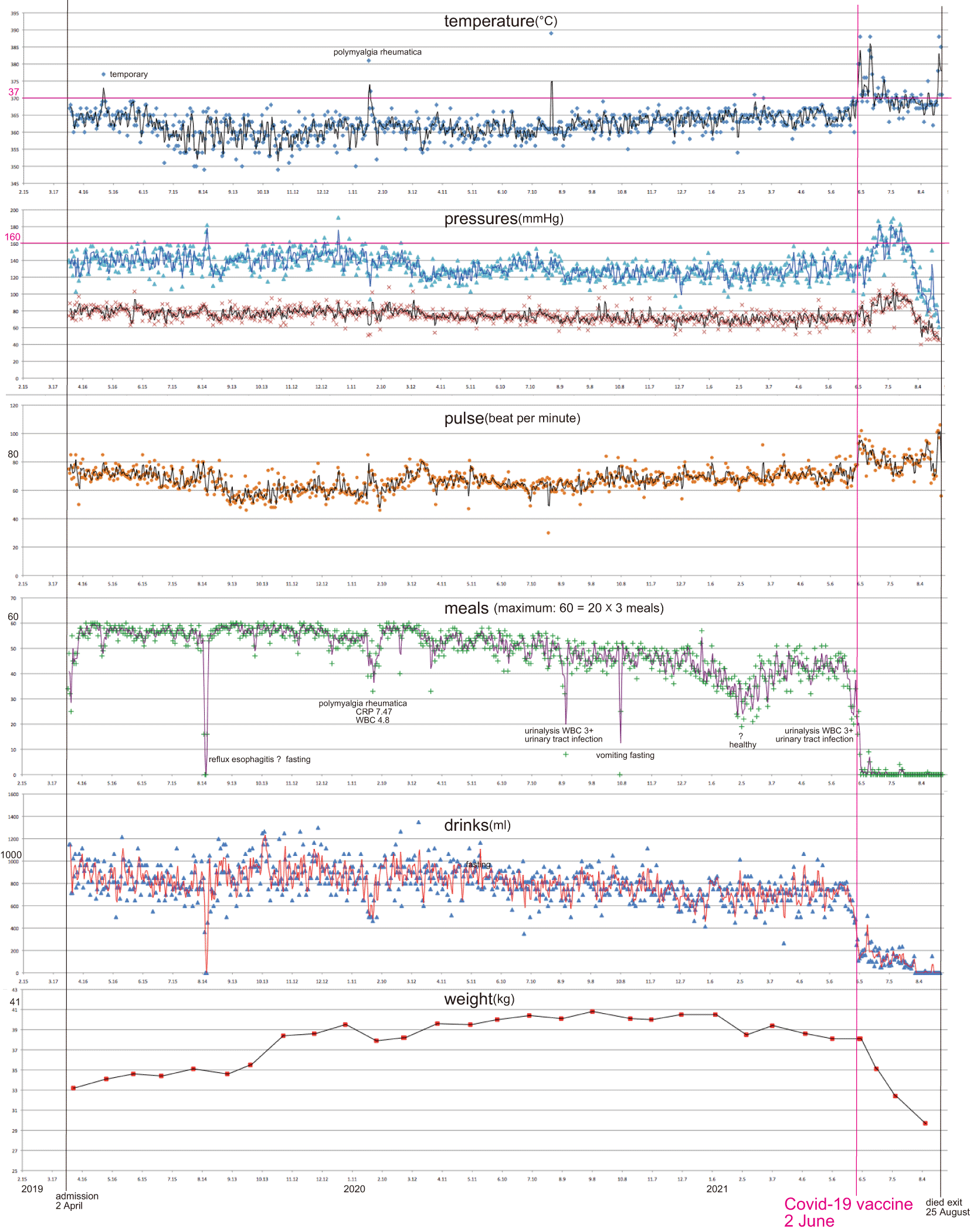
The COVID-19 vaccine was injected on 2 June 2021 (intervention), but the second injection planned on 25 June was cancelled. 5 pre-injection disorders are also shown.
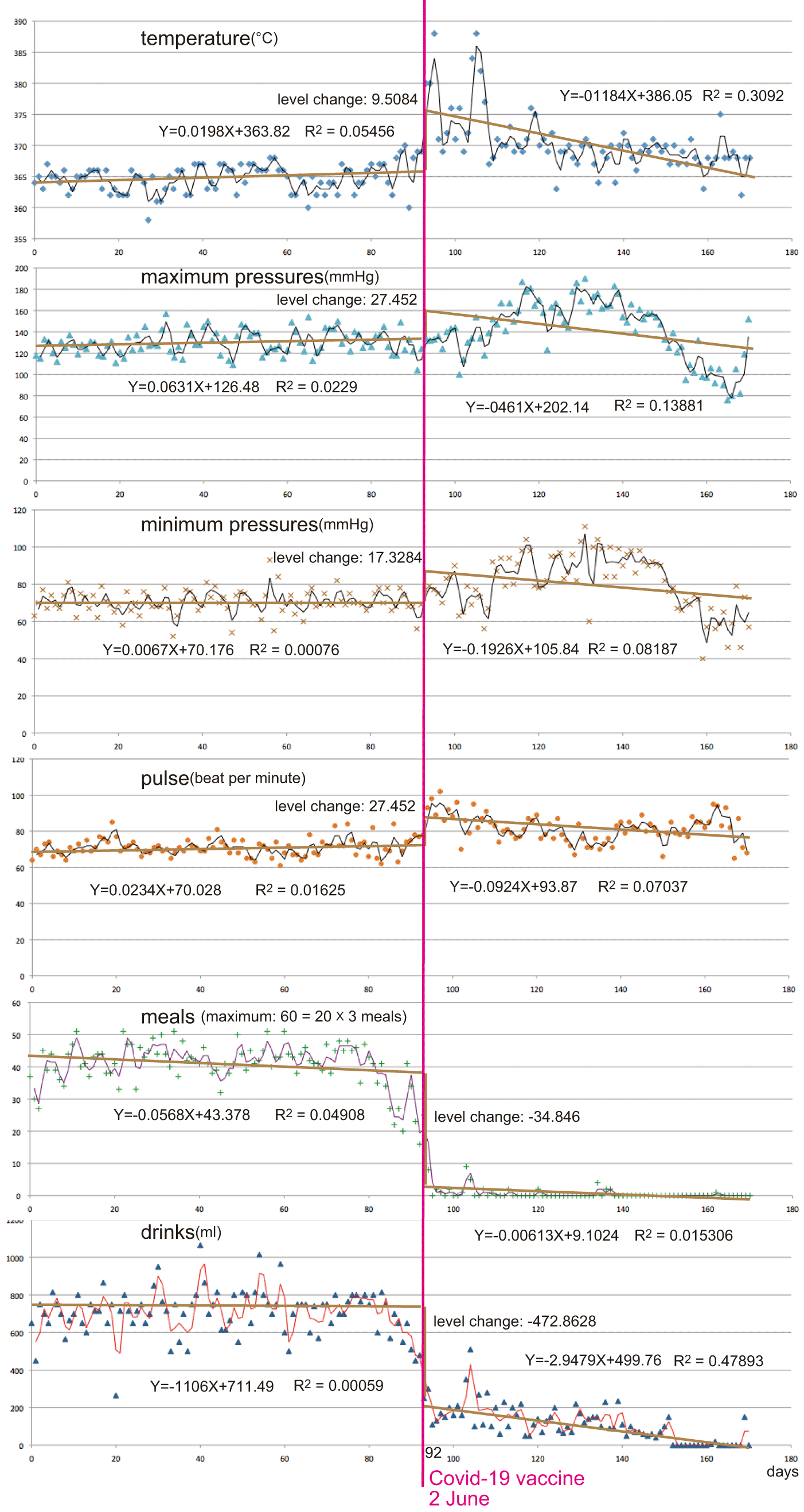
The last one-week plots were omitted by body temperature disturbance. The regression line with the equation, coefficient of determination (R2), and level change by the interruption are shown.
The last one week of data were omitted by body temperature disturbance.
For body temperature for example, an interrupted time-series analysis approach was utilized. A linear regression model was fitted to describe the magnitude of change in temperatures in transitioning from one phase to another and the trend of temperatures at any specific time segment. Parameters of interest included: baseline error trend; immediate change in daily temperatures from the last observation in the pre-implementation phase to the first observation in the implementation phase; change in the slope of temperature trend from pre-implementation to implementation; immediate change in daily temperatures from the last observation in the implementation phase to the first observation in the post-implementation phase; temperature trend in the post-implementation phase; and estimated reduction in daily temperatures into the implementation phase (Elsaid et al., 2013).
Vital records covering 28 months (Figure 1) revealed a drastic and violent fluctuation, with a significant increase in body temperature, maximum and minimum pressures, and pulse. Additionally, there was a substantial decrease in both the intake of meals and drinks, and weight (eventually leading to no meals and drinks; the patient lost 1/4 of body weight) after the COVID-19 vaccination. This was in contrast to the pre-injection “steady state,” which had persisted for more than two years with sporadic, short-term disorders (detailed in Figure 1).
Vital records limited to the last 6 months (180 days; Figure 2) illustrate the pre-injection horizontal regression line, a significant positive level change for temperature, pressures, and pulse, and a negative level change for meals and drinks, post-injection - regression line. The R2 value and other statistical parameters (Table 1) suggested that the injection strongly influenced and affected the vital system, inducing a critical phase.
Table 2 presents detailed care records for the period of June 2nd – June 14th, extracted from the full care records (Osozawa, 2021). During this time, the patient’s temperature increased and remained elevated until death. The patient also experienced hallucinations and a decreased mental state, along with reduced SpO2 levels. Another visual hallucination was observed on 17 June. Lingual coating was observed on 18 June. The meal dinner (including breakfast and lunch) was on 2 June, the day of vaccination, after which the patient developed anorexia and did not eat, contrasting with the pre-vaccination period where the patient usually had complete meals and drinks.
Pre-injection disorders are illustrated in Figure 1, and they were short-term, recovered from, and of course, unrelated to the vaccination.
Abrupt and severe disorders that manifested just after the COVID-19 vaccination were evidently causally related to the vaccination (Figures 1 and 2; Table 1). Death was probably caused by these adverse reactions, particularly due to the absence of meals or drinks for nearly three months.
The fevers observed just after the injection in early June were likely a consequence of an adverse reaction to the vaccine (Table 2). The subsequent fevers in mid-June and early August were potentially causally related (Figures 1 and 2; Table 1).
CRP (C-reactive protein) increased from 0.11 mg/dl on 18 February 2021 (pre-injection) to 1.25 on 22 June and 1.12 on 9 July 2021 (post-vaccination; associated fever), and the leukocyte count decreased below the reference values. Thrombocytopenia frequently associated with critical thrombosis (Ministry of Health, Labour and Welfare; Fueyo-Rodriguez et al., 2021; Waraich and Williams, 2021; Sessa et al., 2021) was not found in the patient, but neutropenia reported by Charan et al. (2021) was probable. An increased CRP over 0.3 (< 0.3: normal level) suggested moderate class inflammation and might have been related to the following reported symptoms: fever flash on June 2; leg pain on June 3; nausea on June 4; visual hallucinations (15 dancing girls are visible; deceased husband is visible) on June 4 and 17; lingual coating on June 18; and tongue swelling on June 21. Fevers exceeding 38°C tended to be associated with SpO2 95 (note that SpO2 was minimal at 94 on June 6) and implied lung dysfunction and pneumonia. Polymyalgia rheumatica in early 2020 (see Osozawa, 2021) had a much higher CRP of 7.4 (c.f., Parperis and Constantinou, 2021), which later decreased within the reference values and was unrelated to the present case. These disorders were possibly associated with cytokine release (or storm; Fajgenbaum and June, 2020).
Significant increases in blood pressure and pulse to higher levels can be considered adverse reactions of injection (Figures 1 and 2; Table 1), presumably caused by inflammation. Inflammation was possibly associated with belching and nausea (and partial chills and fatigue; diarrhea was uncertain due to the dosage of magnesium oxide), which might have affected the appetite, reduced it, and led to anorexia. Note that the participant retained chewing and swallowing abilities, as dentists and physical therapists regularly checked and trained. Thus, the anorexia nervosa triggered by the vaccination reduced the patient’s weight to 3/4 of the pre-vaccination weight.
Another concern was the dosage of the vaccine (0.3 ml/participant in the present case of the COVID-19 Pfizer (Comirnaty) vaccine). The amount is the same for both men and women and Japanese and American individuals. The mean weight of an American woman was 77 kg in 2015-2016 (CNN), but the patient’s weight was only just over half of 77 kg at 40.5 kg (so the vaccine dosage should have been 0.15 ml). Therefore, I am concerned about the risk of overdosage and its potential to increase adverse reactions, particularly in the Japanese population. The Centers for Disease Control and Prevention recently (November 2021) decided that the dose should be 0.1 ml/US children less than 11 years old.
The long-term vital records provided excellent data on adverse reactions, including abnormally prolonged fevers, high blood pressures, pulses, and severe anorexia, ultimately leading to death.
Zenodo: Table S2: In Anorexia as a new type of adverse reaction caused by the COVID-19 vaccination: a case study applying detailed personal care records. https://doi.org/10.5281/zenodo.5778025 (Osozawa, 2021)
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the background of the case’s history and progression described in sufficient detail?
Partly
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Partly
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
No
Is the case presented with sufficient detail to be useful for other practitioners?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Geriatric Pharmacovigilance, Geriatric Neuropsychiatry
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 2 (revision) 04 Jan 24 |
|
|
Version 1 04 Jan 22 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)