Keywords
Cystectomy, Thromboembolism, Urinary Bladder Neoplasm, Neoadjuvant Therapy, Central Venous Access
This article is included in the Oncology gateway.
Cystectomy, Thromboembolism, Urinary Bladder Neoplasm, Neoadjuvant Therapy, Central Venous Access
Muscle invasive bladder cancer (MIBC) accounts for circa 25% of all urinary bladder cancer. The 5-year overall survival (OS) is approximately 58% after three cycles of cisplatin-based neoadjuvant chemotherapy (NAC) and radical cystectomy (RC).1 Both NAC and the risk of thromboembolic events (TEEs) has increased during the last decades. Evidence suggests that chemotherapy, in combination with the already hypercoagulable state induced by cancer, could contribute to thrombosis.2,3 It has also been suggested that placement of central venous access (CVA) before NAC could initiate thrombosis as well, with the potential mechanism being endothelial injury of veins.4 In a recent randomized controlled trial, it is also suggested that a much smaller caliber vein, where a CVA in the form of a peripherally inserted central catheter-line (PICC) is inserted, could be related to the risk of the device causing venous thrombosis. A five times higher risk (11%) of thrombosis from PICC-line was seen compared to (2%) the risk from totally implanted (PORTs), which are directly inserted in larger vessel, either the jugular or subclavian vein.5 To our knowledge, there are no clear guidelines on prevention of venous TEE during NAC before cystectomy. We report on three male patients with MIBC from the Swedish Northern health region, who all developed venous TEE after CVA placement, during NAC. The uniqueness of the report includes a combination of clinical information, images and existing sources on the subject presented here in a combined format.
A 71-year-old Caucasian, male patient presented with gross hematuria. His medical history included ischemic heart disease, anti-platelet medication and prostatectomy due to prostate cancer 10 years prior to the hematuria. His previous occupation was a laborer within the steel industry before retirement. He was staged cT3N0M0 after transurethral resection of bladder (TURB) and received a PICC-line via the basilic vein in the right arm, prior to first NAC-cycle with methotrexate/vinblastine/Adriamycin/cisplatin (MVAC). Within two weeks he developed neutropenic fever, septic infection and chest pain correlated to deep breathing. A computed tomography (CT) pulmonary angiogram (CTPA) displayed multiple pulmonary embolism (PE) on a lobar artery level, to the right middle and inferior lobe (Figure 1A,B). Physical examination showed no sign of swelling in upper or lower extremities and he remained respiratory and circulatory stable. He received venous anticoagulant treatment with anti-Xa; Tinzaparin, with a daily dosage of 16000 IE (equivalent to 144 mg) for a predetermined period of six months. He received the last NAC-cycle and the RC one month delayed (Table 1). According to the most recent follow-up the patient remains in remission (Table 1).
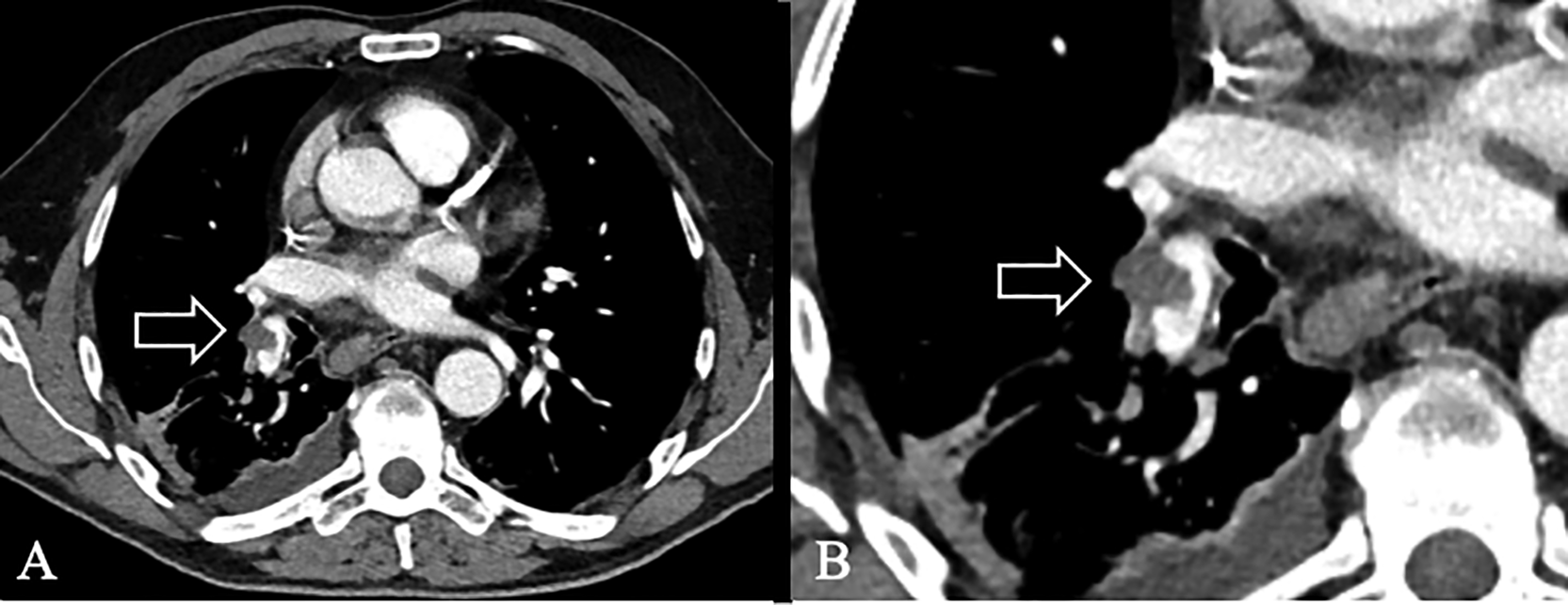
(A) CTPA of our first patient. PE was seen in the right lung on a lobar artery level, to the middle and inferior lobe. (B) Enhancement of PE in (A).
Date of diagnosis; muscle invasive bladder cancer. Age of diagnosis. cTNM stage (clinical tumor-node-metastasis). Histopathology of tumor. Smoking history in pack-years. Types of neoadjuvant chemotherapy (NAC); methotrexate/vinblastine/Adriamycin/cisplatin (MVAC), fluorouracil/mitomycin (FUMI). Number of NAC-cycles. Time from first cycle to thromboembolic event (TEE). Type of TEE. Anti-Xa Treatment; type of antithrombotic medication. Time from first NAC-cycle to radical cystectomy (RC). Radical cystectomy (RC) delayed due to TEE. pTNM stage (pathological tumor-node-metastasis: response outcome of primary tumor; stable disease; pT2-T4aN0M0, partial response; pTa, pTis, pT1.
A Caucasian, male patient aged 74, also presenting with gross hematuria, had anti-platelet medication due to ischemic stroke in 2018, and a well-treated HIV-infection. Before retirement, he had worked for a large telephone company. The tumor was staged cT2N0M0 and he also received PICC-line via the right basilic vein and MVAC. A routine CT-scan after two cycles displayed multiple PE in the right lung (Figure 2A). The patient had symptoms of fatigue and shortness of breath, but he was respiratory and circulatory stable and physical examinations showed no signs of swelling in upper or lower extremities. He received treatment with anti-Xa; Tinzaparin at the dosage of 16000 IE to be evaluated during a period of six months. Due to deteriorated health, RC was cancelled and alternative curable treatment was recommended from a multidisciplinary conference, including urologist and oncologist. The last MVAC cycle was replaced by fluorouracil/mitomycin to be followed by radical radiotherapy. Initial dosage was 2 Gray, increased gradually to target dose of 68 Gray during 34 sessions under a period of 50 days. Follow-up CTPA after two months detected no residual PE, but the initial symptoms persisted up to a year post-TEE. According to the most recent follow-up the patient remains in remission (Table 1).
A Caucasian male aged 69, was admitted due to suspected prostate cancer, but TURB revealed MIBC stage cT3N0M0. He had type 2 diabetes and essential hypertension. He worked in agriculture prior to retirement. The patient also received PICC-line via the right basilic vein and MVAC. After the second cycle, he developed neutropenic fever and back pain. CT scan displayed a centrally peripheral saddle PE (Figure 2B). Physical examination showed no sign of swelling in upper or lower extremities and besides symptoms such as fever, fatigue and pain, he was respiratory and circulatory stable. He received treatment with anti-Xa; Tinzaparin with the dosage of 12000 IE (equivalent to 192 mg) for a predetermined period of six months. He was fit to continue his third MVAC cycle one week post-TEE but RC was ultimately delayed one month. According to the most recent follow-up the patient remains in remission (Table 1).
Our patients all received TEE of venous type; PE within 12-30 days after CVA (Table 1). Evidence that NAC could be a contributing factor for thrombosis has been suggested.2,3 Significant higher TEE ORs before RC were seen in NAC-patients compared to NAC-eligible NAC-naïve.4 In Zareba et al, TEEs had connection with CVA and preoperative TEE was associated with shorter OS (Hazard ratio 3.26; 95% confidence interval:1.12–9.44; p = 0.03). NAC-patients also had a 3-fold higher incidence of TEE than NAC-naïve.3 In addition, there is a 70-fold higher risk of TEE during cancer in general.2 A potential connection between TEE and the placement of CVA with following endothelial damage has also been proposed by previous studies.3,4 In a recent randomized controlled trial, it is also suggested that a smaller caliber vein, where PICC-line is inserted, could be related to a five times higher risk of having venous thrombosis from the device, compared to from a CVA of totally implanted (PORTs), which are directly inserted in larger vessel. Recommendations are to use PORTs rather than PICC-line on NAC-patients. PICC-line is however less staff-demanding and more common.5 To our knowledge, there are no current guidelines on prevention of TEE before RC, even though evidence suggests that incidental TEE of venous origin can cause significant mortality and morbidity without proper treatment. The time from diagnosis to RC was significantly longer among NAC-patients compared to NAC-naïve (p < 0.001).3 Our patients had similar delays, including one cancelled RC (Table 1). Prolonged time from diagnosis to RC could potentially allow metastatic dissemination. Our first and second patient had anti-platelet medication prior to diagnosis, but none had specific venous anticoagulants such as anti-Xa. Studies on whether prophylaxis on thrombosis during NAC can reduce the risk of TEE or not, have shown conflicting results.2,3 But in patients with pancreatic cancer, dalteparin achieved an 85% risk-reduction from a 23% TEE incidence rate to 3.4%.2
Evidence from literature suggests that NAC and malignancy increase the risk for TEE in MIBC-patients. CVA induced thrombosis during NAC, from endothelial damage or insertion in small caliber veins, has also been suggested as a potential mechanism for TEE. There are no current guidelines on prophylactic venous anticoagulation treatment and results from previous studies are conflicting. Time before curable operation can be prolonged if TEE arises. Further prospective trials are warranted on prevention and for developing future guidelines pre-RC regarding TEE in NAC-patients.
All data underlying the results are available as part of the article and no additional source data are required.
Written informed consent for publication of clinical details and images were specifically obtained from the patients included in this report.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the background of the cases’ history and progression described in sufficient detail?
Yes
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Partly
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Yes
Is the conclusion balanced and justified on the basis of the findings?
Yes
References
1. Key N, Khorana A, Kuderer N, Bohlke K, et al.: Venous Thromboembolism Prophylaxis and Treatment in Patients With Cancer: ASCO Clinical Practice Guideline Update. Journal of Clinical Oncology. 2020; 38 (5): 496-520 Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Urology
Peer review at F1000Research is author-driven. Currently no reviewers are being invited.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 13 Jan 22 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)