Keywords
Sugar substitutes, Dental caries (DC), Sucrose, Maltitol, Chocolates, Cariogenic potential, Salivary pH, sugar free
Sugar substitutes, Dental caries (DC), Sucrose, Maltitol, Chocolates, Cariogenic potential, Salivary pH, sugar free
Dental caries (DC) is a chronic disease with multifactorial etiology. Though various factors may lead to the development of DC, diet is considered as the major contributory factor.1 Sugars in the diet constitute an important etiological factor in DC.1 In a study conducted by Moynihan and Kelly, a relationship between the amount of sugar consumed and the development of DC among subjects of all age groups was identified.2 It has been shown that restricting the intake of free sugar to <10% of daily energy intake decreases the risk of DC throughout one’s life.3 Furthermore, the risk for caries is greater if sugars are consumed at a higher frequency.4 Among frequently used sugars, sucrose has been considered as a potential cariogenic substrate, owing to large amounts of acid production after fermentation by the oral bacteria, particularly Streptococcus mutans and Lactobacillus rhamnosus.5
The pH of saliva is normally between 6.7 and 7.4. A drop in pH below the critical pH value of <5.5 has been related to the development of DC.6 A study by Lingström and Birkhed has evaluated the plaque pH after intake of starchy snack foods such as potato chips, crackers, among others.7 However, the effect of chocolates, which are frequently consumed by children and teenagers (and sometimes even by adults)8 has not been investigated adequately.
The factors which influence the relative cariogenicity of chocolates include their composition, texture, solubility, retaining capacity, and ability to stimulate salivary flow.8 The composition of chocolate can widely impact its cariogenicity.8 Chocolates contain sugars, viz. sucrose, and lactose, hence their ability to cause DC. Cocoa is one of the ingredients of chocolates that is present in different concentrations. It has been reported that the higher the concentration of cocoa, the lower the cariogenic potential of the chocolate.9
Low-calorie sweeteners (LCS) are commonly used as sugar substitutes, which contain practically no calories. They are considered noncariogenic, i.e., they cannot be fermented by oral bacteria, and hence do not cause tooth decay.10,11 Several studies and reviews have examined and confirmed the noncariogenic nature of LCS.1,12–15 In addition, the United States Food and Drug Administration (USFDA) has approved the claim for sweeteners that they “do not promote tooth decay”.16
Steviol glycosides are one of the LCS that are natural and plant-based and are stable at high temperatures (up to 200°C) as well as varying pH values, i.e., acidic to alkaline pH 3–9.17 Moreover, they have been found to be noncariogenic when tested for their cariogenicity in vivo.18 Polyols like maltitol, sorbitol, and xylitol are widely used in sugar-based confectionaries to replace sucrose, which have helped in reducing the number of calories and are deemed as “safe for teeth”.19 Maltitol is a sugar alcohol (a polyol) used as a sugar substitute that is almost as sweet as sucrose (approximately 90%) and has nearly identical properties, except for browning, but its caloric value amounts to 2–2.4 kcal/g.20,21
Owing to the wide consumption of chocolates by both adults and children and the rising incidence of DC,8 we intended to conduct an open-label study on healthy human subjects to evaluate the safety and efficacy of maltitol-based sugar-free chocolates on salivary pH, through measurement of salivary pH and growth of S. mutans.
An open-label study was carried out on healthy human subjects to evaluate the safety and efficacy of Maltitol-based sugar-free chocolates. This dental study for Maltitol-based sugar-free chocolate was carried out to substantiate the claim of “no tooth decay” in accordance with the USFDA 21 CFR Part 101 for food labeling, health claims, dietary noncariogenic carbohydrate sweeteners, and DC. The study protocol was approved by the Institutional Ethics Committee dated 19th November 2018.
The study was approved by the Riddhi Medical Nursing Home Institutional Ethics Committee on 19th November 2018. The parents and/or legal guardians of the subjects were informed about the investigation and treatments and written consent was obtained.
A total of 15 healthy human volunteers including adults and children were screened and enrolled in the study after obtaining their written informed consent. All subjects who met the study criteria were enrolled in the study. The inclusion criteria were an age: between 2–55 years old (both inclusive); sex: male and/or nonpregnant/nonlactating females; and subjects with good general health. The exclusion criteria were: pregnant female subjects or females planning to get pregnant during the study duration; subjects with orthodontic bands, partial removable dentures, history of gastrointestinal problems, tumor(s) of the soft or hard tissues of the oral cavity, advanced periodontal disease, history of allergies to any oral care/personal care consumer products or their ingredients and carious lesions which require immediate restorative treatment; subjects having used antibiotics anytime during the three months prior to entry into the study; subjects who consume alcohol, smoke cigarettes, or consume any other form of tobacco; subjects on any prescription medicines that might interfere with the study outcome; subjects with dental prophylaxis during the previous two weeks before baseline examinations.
Each volunteer made two visits to the hospital:
• Visit 1: Screening phase
In the screening phase, various parameters were assessed which included physical examination, dental examination, demographic evaluation, and medical history.
• Visit 2: Enrollment/evaluation/end of the study phase (Day 0 and Day 1)
During the second visit, the screened subjects were housed until at least 4 hours post-dose. Day 0 was the enrolment day where the subjects underwent dental examination. Here, the subjects were assigned numbers and dinner was served. On Day 1 the saliva sample was collected for the measurement of S. mutans colonies and salivary pH measurement was done using a digital pH meter. After the baseline assessments, the subjects were given the test product for consumption (as per age groups). A saliva sample was collected at 0 and 4 h after consumption of the test product. This was followed by salivary pH measurement using the digital pH meter at 0, 1, 2, and 4 h after consumption of the test sample. The details of the study methodology have been elaborated in Figure 1.
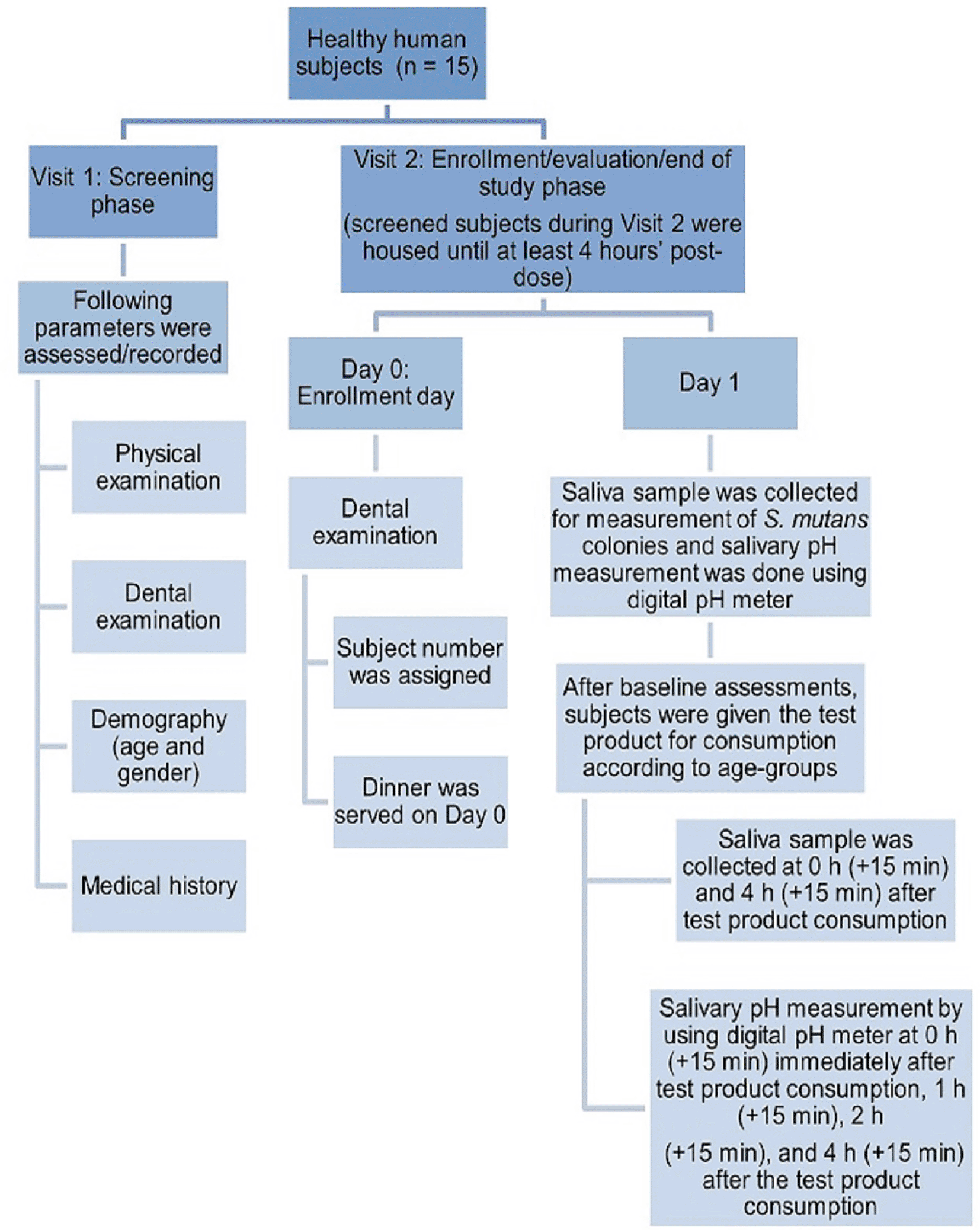
Subjects were instructed to refrain from oral hygiene, i.e., flossing, brushing, gargling with mouthwash, a day before the screening visit (Visit 1) and for 48 h before baseline reading (Visit 2). Visit 2 was approximately two weeks apart from the initial screening visit. Morning breakfast was given to the subjects after all the assessments were done, i.e., after 4 h assessments were completed. Adverse events assessment and ConMed was recorded (if any) at 4 h after the test product consumption. Subjects were informed to brush their teeth after 4 h post-dose assessments. S. mutans: Streptococcus mutans.
The test product Sugar Free D’lite chocolate has no added sugar (sucrose); it consists of a minimum of 18% cocoa powder, a minimum of 51% cocoa solids, permitted emulsifiers, and flavoring substances. Sucrose was completely replaced with sweeteners maltitol (minimum 45%) and steviol glycoside (0.05%).
Each subject was asked to consume the test product (cut into pieces from an 80 g slab), by chewing, as per their age:
Continuous variables such as age and instrumental readings were summarized with descriptive statistics (mean, standard deviation [SD], number of subjects [n], median, and range), and categorical variables such as gender, race were described as count (n) and percentage (%) of the subjects. All the statistical analysis was performed using two-sided tests, at a 5% level of significance, using Statistical Analysis System (SAS) 9.4 (SAS Institute Inc., NC Cary).
All the subjects (n = 15) having a mean age of 13.3 years completed both phases of the study and were included for analysis. Of all subjects, nine were females.
There was a statistically significant increase in the salivary pH from the baseline at 0 h measurement (p = 0.0181). There was no significant difference from the baseline pH at any other time points, subsequently, 1 h (p = 0.4643), 2 h (p = 0.7512), and 4 h (p = 0.1904) [Figure 2] and [Table 1]. Clinically, it can be appreciated that the salivary pH remained within the normal range (6.2–7.6) after consumption of the test product at 0 h for four hours post-consumption. There was no decrease in the pH of saliva up to the critical pH value (5.5–5.7), indicating that the test product does not lead to DC.
Compared to the baseline, there was a significant reduction in the S. mutans colonies, both at 0 h and 4 h measurement (p = 0.0001) [Figure 3] and [Table 2]. Clinically, this indicates that consumption of the test product does not promote the growth of S. mutans.
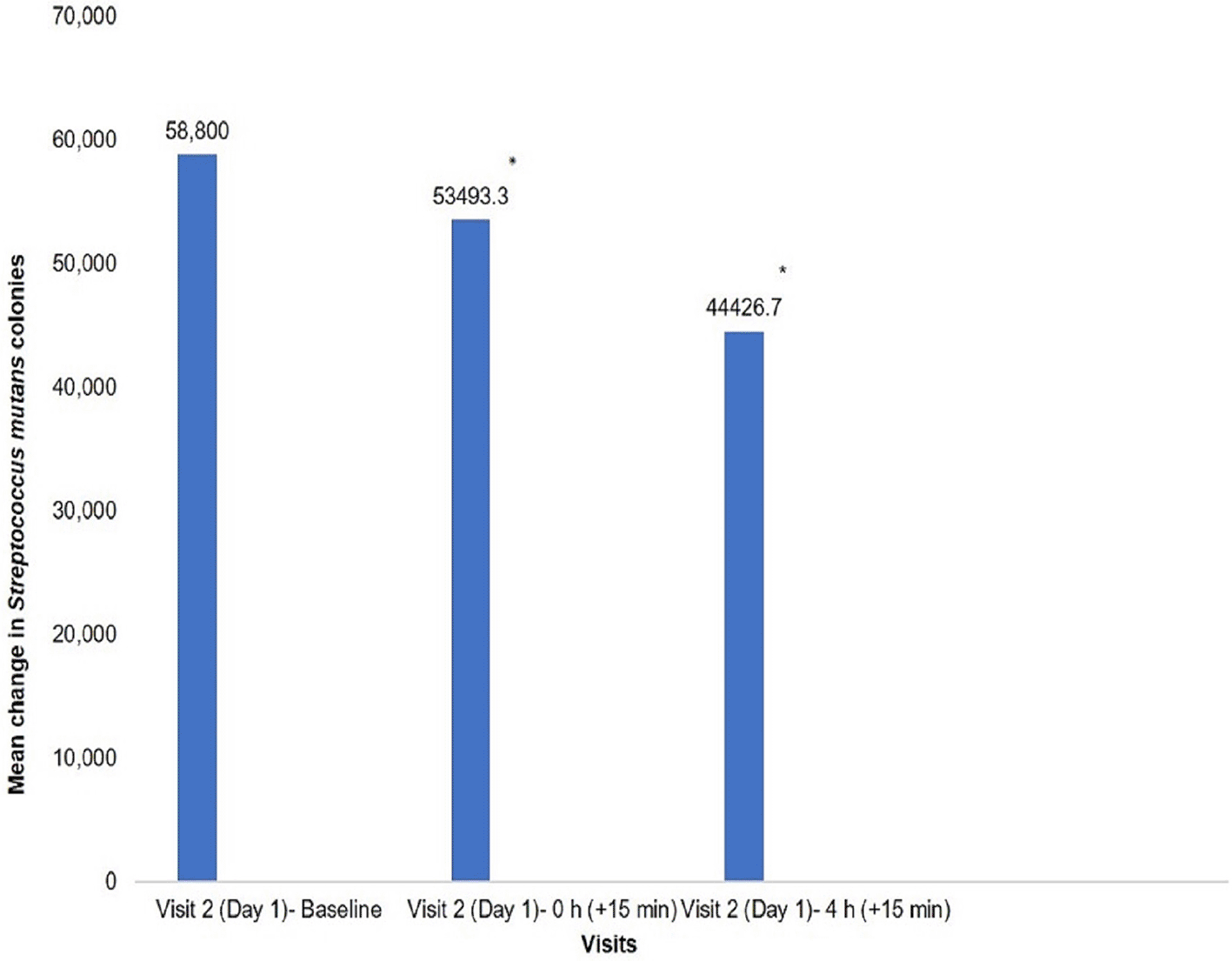
*Indicates statistically significant difference from baseline.
The purpose of this study was to determine the effectiveness and safety of maltitol-based sugar-free chocolates on reducing factors associated with DC. It was observed that the test product resulted in a statistically significant increase in the salivary pH level (p = 0.0181), which clinically indicates that salivary pH remained within the normal pH range of 6.2–7.6 after consumption of the test product. More importantly, there was no decrease in the salivary pH up to the critical value of 5.5–5.7. This implies that the test product may not lead to DC. Alongside the increase in salivary pH, there was a statistically significant reduction in S. mutans colony formation (p = 0.0001), which indicates that there was no effect of the test product on promoting the growth of S. mutans from baseline to 4 h after consumption of the test product. Thus, the test product does not support the growth of S. mutans and may not lead to DC.
Gopinath and Arzeanne, 200622 discovered that saliva flow rate, pH, viscosity, and buffering capacity were significantly lower (p < 0.01) in the high-caries group as compared with the control group. Thus, the saliva test is recommended for people with a higher risk of caries.22 A study by Thabuis et al. among 258 children (aged 13–15 years) investigated the cariogenic potential of maltitol- and xylitol-based chewing gums vs. gum-based chewing gums and compared these to a control group (negative control) that did not receive any gum. It was observed that maltitol- and xylitol-based chewing gums showed higher plaque pH (areas under the curve, p ≤ 0.05) that led to a decrease in plaque growth. This was paralleled by a significant reduction (p ≤ 0.05) in four cariogenic bacterial species, i.e., S. mutans, Streptococcus sobrinus, Actinomyces viscosus, and Lactobacillus in the dental plaque when compared with the control group.23 Further, an increase in salivary flow, pH, and better resistance to sucrose challenge was observed in the maltitol- and xylitol-based chewing gum groups when compared with the gum-based group.24
Tooth decay occurs at an acidic pH around 5 or below. Microorganisms of the mouth convert sugars into acids, resulting in the decrease of salivary pH. In the presence of these acids, the enamel of teeth is degraded, leading to tooth decay.25 Thus, the noncariogenic carbohydrate sweeteners are convenient when used as sugar substitutes, unlike sugars that are cariogenic.1,25,26
There are a few limitations to our study, such as its small sample size and evaluation of short-term effects of maltitol-based sugar-free chocolates. Further studies with larger sample populations and investigating the long-term effects are warranted.
This dental study for maltitol-based sugar-free chocolates aimed to substantiate the manufacturer’s claim of “no tooth decay” in accordance with the guidelines enunciated by the USFDA 21 CFR Part 101 for food labeling, health claims, dietary noncariogenic carbohydrate sweeteners, and DC. Based on the current study results, it can be concluded that these maltitol-based chocolates may not cause tooth decay or lead to DC. No adverse event was reported in this study, neither by the dentist nor by the subjects or their parent/legal guardian/caregiver in the case of children subjects. Hence, maltitol-based sugar-free chocolates containing maltitol are safe to consume and may not promote DC.
Figshare: Raw data for change in the salivary pH and growth of Streptococcus mutans, https://doi.org/10.6084/m9.figshare.19248248.27
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)