Keywords
Anti-inflammatory; acute; subchronic; toxicity; 1.3 bis (p- Hydroxyphenyl)urea
Anti-inflammatory; acute; subchronic; toxicity; 1.3 bis (p- Hydroxyphenyl)urea
in abstract there are some information that added based on reviewer suggestion and in pictures there are additional information
To read any peer review reports and author responses for this article, follow the "read" links in the Open Peer Review table.
Inflammation is a normal protective response caused by an injury or tissue damage, through physical trauma, damaging chemicals, or invasion of pathogenic microorganisms.1 The phenomena observed in this process include microvascular damage, increased capillary permeability, and leukocyte migration to inflammatory tissue.2 Also, the treatment of inflammation is generally based on limiting the operation of tissue damage, which occurs in the affected areas. The modern drugs commonly used are often non-steroidal anti-inflammatory drugs (NSAIDs), which have detrimental side effects to the body, such as gastric ulcers.3 Anti-inflammatory is a term for agents or drugs working against the process of inflammation. These are divided into two categories, namely steroid and non-steroidal anti-inflammatory drugs, with both having several side effects. The steroid anti-inflammatory drugs are found to often cause gastric ulcers, decreased infection immunity, osteoporosis, muscle and fat tissue atrophies, increased intraocular pressure, and diabetes. Mean- while, bleeding gastric ulcers, kidney disorders, and anemia, are the side effects of non-steroidal drugs.4 One of the modified p-aminophenol compounds is 1,3-bis (p-hydroxyphenyl) urea, which estimated to have more potent analgesic activity and fewer hepatotoxic side effects than acetaminophen. This is due to having an atomic charge (-0.110) that binds liver cells, which is less favourable than paracetamol (-0.107). In previous studies, the results of the in-silico and in-vivo tests indicated that this compound had analgesic potentials, based on the inhibition of the cyclooxygenase (COX-2) enzyme.5 Also, the in-silico test showed that 1,3 bis(p-hydroxyphenyl) urea had higher binding activity in COX-1 (1CQE) and TNF-α (2AZ5), compared to the control, dexamethasone, and diclofenac. This indicated that the compound had the potential of being developed as an anti-inflammatory agent.6
The anti-inflammatory action of non-steroidal drugs is mainly the inhibition of prostaglandin synthesis by cyclooxygenase-2 (COX-2). This is involved in the production of prostaglandins during the processes of inflammation. Except for COX-2-selective drugs, all non-steroidal analgesics are found to inhibit the isoforms of COX (COX-1 and COX-2).7 Furthermore, COX-2 is a mediator playing a significant role in the process of inflammation. This is responsible for the characteristic symptoms of inflammatory actions.8 Although anti-inflammatory and antipyretic drugs are heterogeneous compounds often chemically unrelated (although most of them are organic acids), they still have common specific therapeutic activities and side effects. These compounds are often known as NSAIDs (non- steroidal anti-inflammatory drugs), with the inhibition of cyclooxygenase being generally considered as a significant mechanism aspect. This is because the main therapeutic effect of NSAIDs originates from their ability to inhibit prostaglandin formation.9,10 Based on this study, the anti-inflammatory activity was tested on rats by measuring the percentage of inflammation inhibition. This was conducted after the administration of 1,3 bis(p-hydroxyphenyl) urea, which was previously induced by carrageenan solution intraplantar (using a plethysmometer) and histological analysis with hematoxylin-eosin staining, for the observed accumulation of neutrophils. These toxicity effect tests are conducted based on the safety of drugs, indicating that the effectiveness and efficiency of analgesics should be confirmed before being suitable for use. Also, 1,3-bis(p-hydroxyphenyl) urea has reportedly not been further studied for safety. The analysis of toxicity effect was carried out on acute and subchronic activities, using the OECD (Organization for Economic Co-operation and Development) guidelines. This indicated that an acute test was carried out by administering 1,3-bis(p-hydroxyphenyl) urea for 24 h and observed for 14 days. However, subchronic analysis was performed on rats for 28 days, with continuous observations carried out for 42 days on the satellite group.11,12
The performance of this study was conducted based on the guidelines and approval of the Ministry of Health and the Ethics Committee. In addition, all operations were approved by the Animal Research Ethics Committees (AREC) of the University of Sumatera Utara’s Faculty of Mathematics and Natural Science, Biological Department, with the approval number 0423/KEPH-FMIPA/2021, 14 July 2021. All animals were placed in a temperature controlled room with free access to air and food on light/dark cycle 12/12 hours. For all experiments, each group was handled separately. All procedures and veterinary care were carried out at room temperature (20-22 C), and special care. This was done to avoid environmental disturbances that might affect the animal's response. The anti-inflammatory test animals were sacrificed by neck dislocation, while the toxicity test animals were sedated first because the blood was taken intracardially. This technique was performed on animals that were anesthetized with ketamine at the end of the test and generally if large amounts of blood are needed and the rats for which blood is drawn are sacrificed and then necropsied for organs. After anesthesia and euthanasia, surgery was performed and the syringe was directly inserted into the heart and withdrawn slowly. This technique was done without having to open the chest cavity, by accessing the heart from the left side of the chest, feeling/ignoring the heartbeat then inserting the needle between the 3rd and 5th ribs, then slowly gathering it. The principle of euthanasia in test animals before being sacrificed was for them to be anaesthetized first. Animals were handled with care without causing any fear, then animals were sacrificed with one of the techniques in a separate place from other animals, and no living animals were kept in the vicinity. Euthanasia was carried out by competent personnel, and was accompanied by a confirmation process to confirm death. On the night before the animals were sacrificed, the animals were fasted prior to haematological, biochemical and urinalysis examinations. All the carcasses of the test animals were then buried in the ground at a depth that is safe from the risk of excavation, among others, by wild animals around the burial site.12
The experimental animal used was a male Wistar (Rattus norvegicus L.) (150-200 g) with an age of approximately two months. Based on the anti-inflammatory activity test, 35 rats were used and divided into seven groups. According to the acute toxicity test, 35 female rats were preliminarily and primarily divided into four and two groups, respectively, each consisting of 5 members. Meanwhile, 30 female and 30 male rats were divided into six groups during the subchronic test. Each group also consisted of 10 rats (five male and five female rats). These animals were obtained from the Pharmacology Laboratory of the Faculty of Pharmacy, University of Sumatera Utara.
The 1,3-bis(p-hydroxyphenyl) urea was obtained from Hari Purnomo.5
The animals were provided with 1,3-bis(p-hydroxyphenyl) urea, at doses of 12.5, 25, 50, 100, and 200 mg/kg BW. This was administered at approximately 1 h before the 1 carrageenan induction, which was injected through the intraplantar route into the right hind leg of the rat, at 0.1 ml. As a control measure, diclofenac sodium was similarly administered. Using a plethysmometer, the foot volume was immediately measured before (time 0) stimulation, at selected time intervals of 30, 60, 120, 180, 240, 300, and 360 mins. The results were expressed as variations in foot volume (ml), which were relatively calculated to the basal capacity.13 At the end of the test, the rats were sacrificed and their tissue was taken at the site of inflammation on the soles of the rats, then performed with Hematoxylin-Eosin (HE) preparations to observe the number of neutrophils. These were calculated and analyzed using a light microscope with 400 times magnification. In addition, each preparation was observed in 6 view fields, with the average being calculated.
At the end of the study, the rats were sacrificed, and then the inflammatory foot tissue was taken for histological preparations with hematoxylin-eosin staining. The tissues were separated in a 10% formalin buffer solution. The hematoxylin-eosin staining procedure was carried out at the Anatomical Pathology Laboratory, Faculty of Medicine, University of Sumatera Utara. The process for making histopathological preparations: The tissue will be immersed in a 10% formalin buffer solution at room temperature. The tissue is cut to remove the rest of the formalin is done with running water. The dehydration process was carried out with 70%, 80%, and 90% absolute ethanol. Then proceed with the purification times for 1 hour. Planting process Method: the sample is immersed in liquid paraffin at a temperature of 60 – 70°C for 2 hours. Print and allow to freeze, and then paraffin blocks are cut using a microtome with a line thickness of 5-7 m. After a good cut, the piece is attached to the glass object. The incision organ attached to the slide is immediately placed on a heating surface with a temperature of 56-58°C for approximately 10 seconds so that the organ stretches and sticks to the slide while being adjusted not to wrinkle or fold the organ. Then prepare to be stored at room temperature for staining. Staining was done using hematoxylin-eosin. First, the preparations were soaked with xylene solution for the deparaffination process for 12 minutes each. Then the dehydration was carried out by soaking the practices in 70%, 80%, 90% ethanol, and absolute ethanol for 5 minutes and washed with running water. Then soaked in a solution of hematoxylin for 5 minutes, washed with running water, and stained with eosin. Then, they were immersed in 70%, 80%, 90%, and absolute ethanol for 10 minutes, respectively. Finally, put in xylene for 12 minutes. The preparations were observed under a microscope.14
This test followed the OECD guidelines, in which the dose and animals were administered according to the required standard, namely 35 female rats, divided into seven groups of 5 rats each. The rats were separated from each cage to facilitate observation. The distribution of the groups of preliminary acute toxicity test animals, namely, Group I: Control, given CMC Na 0.5%, Group II: given the test preparation at a dose of 5 mg/kg BW, Group III: 50 mg/kg BW, Group IV: 300 mg/kg BW, Group V: 2000 mg/kg BW. The leading test group for acute toxicity is Group I: given a test preparation at a dose of 2000 mg/kg BW, Group II: 5000 mg/kg BW. In this analysis, a preliminary test is initially performed to determine the appropriate dose for the primary test.11,12 This analysis was orally provided using a probe for 24 h daily. Moreover, the observation of clinical symptoms was carried out during the initial 24 h after treatment. Dead rats were also counted from the treatment until the next 24 h towards a 14-day observation. On the 15th day, the rats were dissected to weigh their vital organs, accompanied by histopathology.
OECD Test Guideline 408 for measuring chemicals is used to perform sub-chronic toxicity analysis. Subchronic toxicity tests using test animals, namely 30 male rats and 30 female rats, were divided into six male treatment groups and six female treatment groups, namely Group I: Control, given CMC Na 0.5%, Group II: Treatment, given the test preparation at a dose of 50 mg/kg BW, Group III: 500 mg/kg BW, Group IV: 1000 mg/kg BW, Group V: CMC-Na satellite group 0.5%, Group VI: Satellite group 1000 mg/kg BW. This subchronic analysis was conducted by orally providing the test preparations to the animals at a single dose for 28 days. During these periods, toxic symptoms, mortality, and body weight were weekly observed. For the satellite group, observations were conducted for a total of 14 days, to assess the process of recovery from toxic effects.11
The tail, arterial, and heart arteries were used to collect blood for hematological and biochemical clinical parameters, which were assessed using the plasma. Meanwhile, ALT (alanine aminotransferase), AST (aspartate aminotransferase), creatinine, and BUN (blood urea nitrogen) levels were measured in the serum.
The results were expressed as a Mean increase in foot diameter SD. Using a one-way ANOVA method, the data were evaluated with the statistical significance determined at P 0.05, when compared to controls. Furthermore, the toxicity data were analyzed using the Statistical Package for Social Sciences (SPSS) version 22.0, which included the Kolmogorov- Smirnov normality test, a one and two–way analysis of variance (ANOVA), and Post Hoc Tukey HSD. These were used to determine significant differences (p >0.05) across test groups.
Pecentage of inflammation Based on the changes in the volume of the rat paw inflammations, the average percentage was calculated.38 This led to the creation of a percentage graph, based on the average of the rat's feet. Analysis of rat paw inflammation volume showed 1,3-bis(p-hydroxyphenyl) urea could suppress inflammation after induction of carrageenan-1%. The percentage analysis of inflammation is shown in Figure 1. In the negative control group, there was a very significant increase in leg volume compared to the other groups. This becomes a reference for comparing the results achieved by other groups. Meanwhile, the test group showed the effect of the compound given on animals. The group that was given diclofenac sodium 2.25 mg/kg BW experienced the highest decrease in inflammation percentage then {1.3 bis (p-hydroxyphenyl)urea} at doses of 200 mg, 100 mg, 50 mg, 25 mg, and 12.5 mg. It also showed a significant difference in results with 0.5% Na-CMC (p < 0.05), which indicates that this group of compounds has anti-inflammatory activity. At doses of 12.5 and 25 mg/kg BW at 60-360 minutes, the urea 1,3-bis(p-hydroxyphenyl) group showed significant differences with the diclofenac sodium group (p < 0.05). Meanwhile, the 50, 100, and 200 mg/kg BW groups did not show a significant difference in results with the diclofenac sodium category, between 60-360 minutes. This indicated that the 1,3-bis(p-hydroxyphenyl) urea group at 50, 100, and 200 mg/kg BW had an anti-inflammatory activity that was not different from that of diclofenac sodium. This led to the creation of a percentage graph, based on the average of the rat's feet. Analysis of rat paw inflammation volume showed 1,3-bis(p-hydroxyphenyl) urea could suppress inflammation after induction of carrageenan-1%. The percentage analysis of inflammation is shown in Figure 1. In the negative control group, there was a very significant increase in leg volume compared to the other groups. This becomes a reference for comparing the results achieved by other groups. Meanwhile, the test group showed the effect of the compound given on animals. The group that was given diclofenac sodium 2.25 mg/kg BW experienced the highest decrease in inflammation percentage then {1.3 bis (p-hydroxyphenyl)urea} at doses of 200 mg, 100 mg, 50 mg, 25 mg, and 12.5 mg. It also showed a significant difference in results with 0.5% Na-CMC (p < 0.05), which indicates that this group of compounds has anti-inflammatory activity. At doses of 12.5 and 25 mg/kg BW at 60-360 minutes, the urea 1,3-bis(p-hydroxyphenyl) group showed significant differences with the diclofenac sodium group (p < 0.05). Meanwhile, the 50, 100, and 200 mg/kg BW groups did not show a significant difference in results with the diclofenac sodium category, between 60-360 minutes. This indicated that the 1,3-bis(p-hydroxyphenyl) urea group at 50, 100, and 200 mg/kg BW had an anti-inflammatory activity that was not different from that of diclofenac sodium.
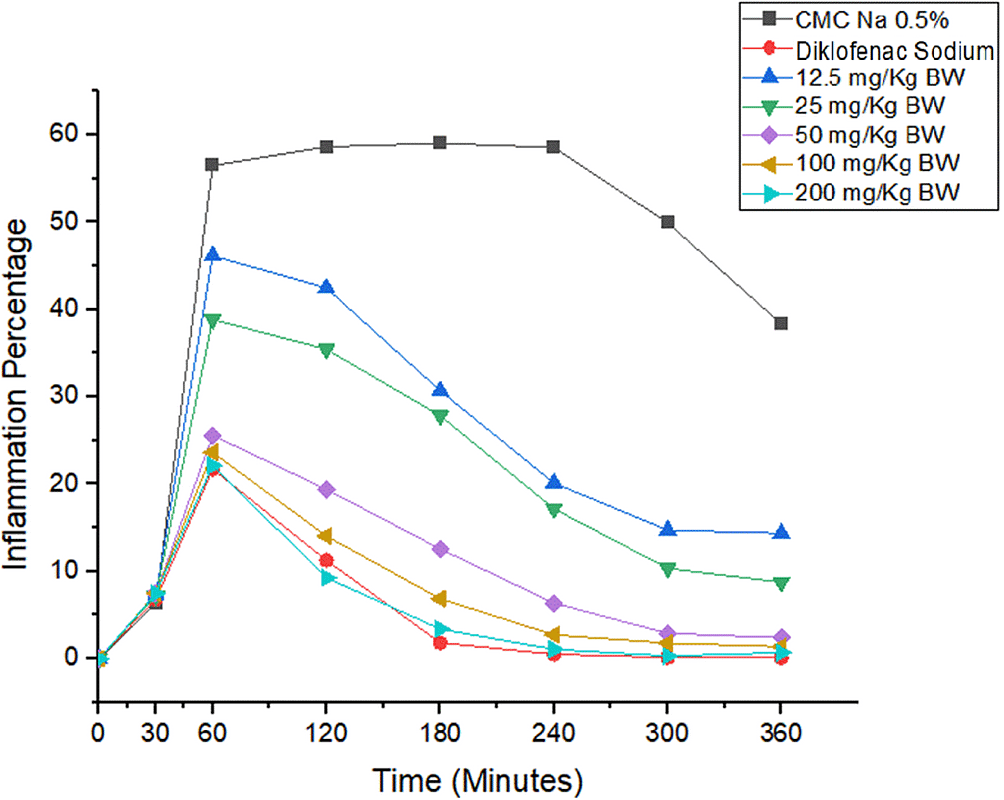
*p < 0.05 significantly different from the negative control group (CMC Na 0.05%). ^p < 0.05 significantly different from the positive control group (Diclofenac Sodium).
The results showed a neutrophil decrease in all the groups provided with 1,3-bis(p-hydroxyphenyl) urea and diclofenac sodium compared to CMC Na 0.5% experienced increase in the number of neutrophils. Observations of the number of neutrophils are presented in Figure 2.
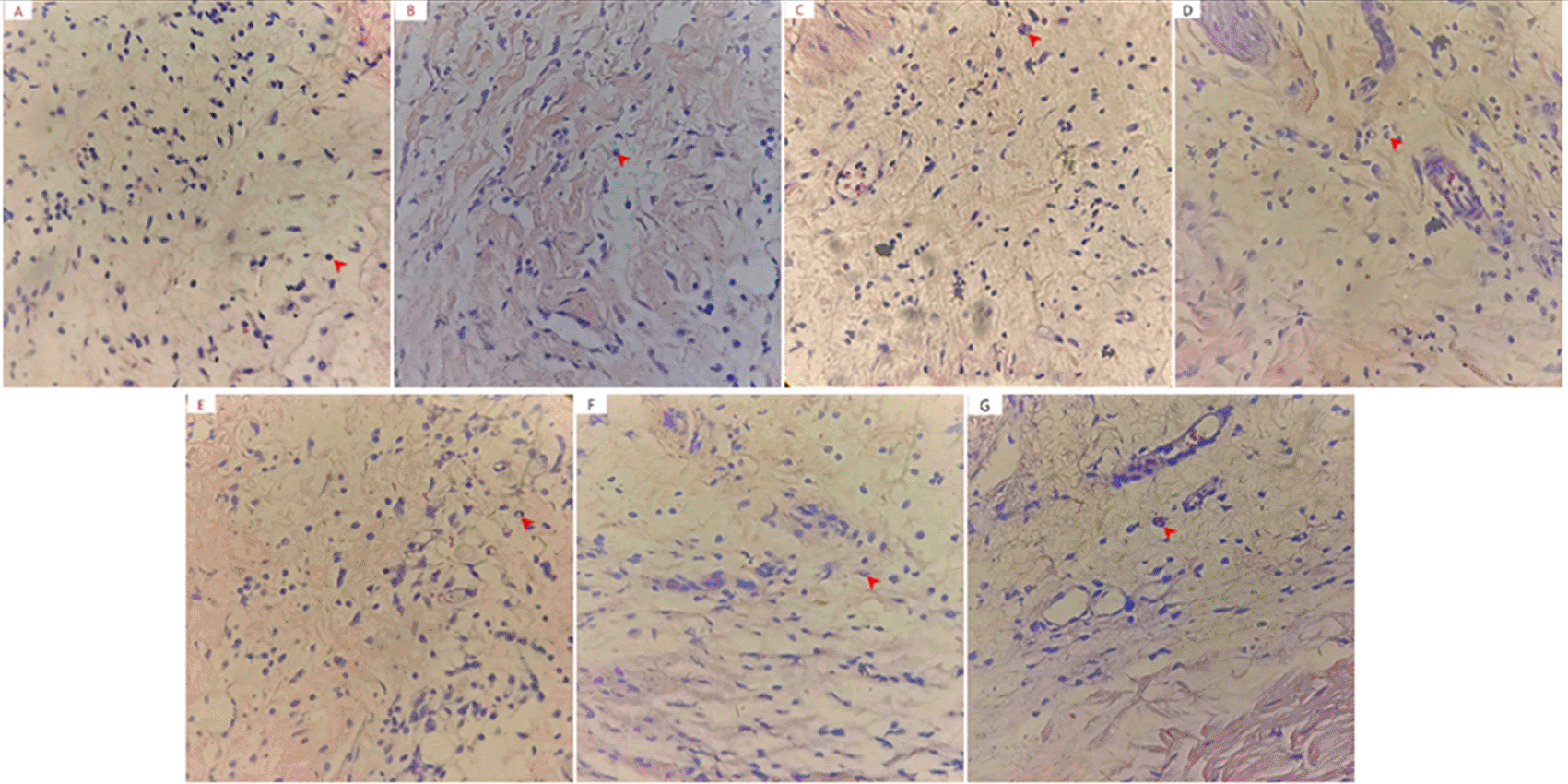
Statistical results showed a significant difference in the number of neutrophils between the analytical and negative control groups. From the Tukey HSD mean difference, the doses of 50, 100, and 200 mg/kg BW were not significantly different from diclofenac sodium (p > 0.05). The graph of the average neutrophil numbers in mouse paws is presented in Figure 3. The functions of neutrophils are to phagocytize foreign bodies and bacteria, increasing and decreasing when inflammation occurs or not. These were the first cells to reach the wound area, peaking at 24-48 h. In a 3-day observation process, the neutrophils were found to rapidly decrease after the third day, leading to activation by larger macrophage cells utilizing.15–17
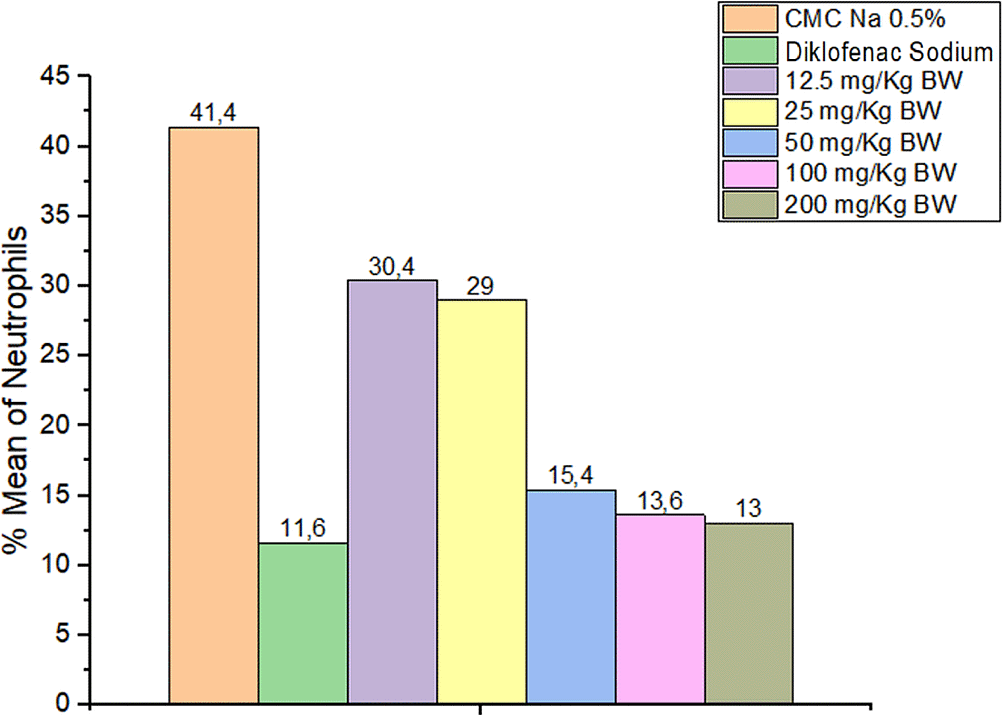
*p < 0.05 significantly different from the negative control group (CMC Na 0.05%). ^p < 0.05 significantly different from the positive control group (Diclofenac Sodium).
In the conditions of acute response, these cells caused degranulation and lysis of neutrophils. This was critical because lysosomal granules contained hydrolytic and proteolytic enzymes in addition to ROS source catalysts (specifically NADPH and myeloperoxidase). Therefore, damages to various biological molecules were observed when neutrophil lysis and these enzymes spilled into the tissue. In addition, live neutrophils are an essential factor in the inflammatory response.18,19
In a preliminary acute toxicity study, 1,3-bis(p-hydroxyphenyl) urea did not cause death or signs of infection at a dose of 2000 mg/kg BW, leading to the continuation of the primary test. Similar results were also observed at 5000 mg/kg BW, as each group did not show a significant change in body weight during the experimental period (Table 1). On pathological examination, no signs of organ toxicity were also observed when compared to the control group (Figure 4).
| Group | N | Gender | Days to | ||
|---|---|---|---|---|---|
| 0 | 7 | 14 | |||
| CMC Na 0.5% | 5 | Female | 162.4 ± 4.39 | 164.6 ± 45.5 | 172 ± 4.24 |
| 2000 mg/kg BW | 5 | Female | 164 ± 2.73 | 170.2 ± 3.70 | 174 ± 6.96 |
| 5000 mg/kg BW | 5 | Female | 165.5 ± 2.90 | 171.8 ± 4.32 | 179.2 ± 3.11 |
Based on Table 2, no animal was found dead during the administration of 1,3-bis(p-hydroxyphenyl) urea at 2000 and 5000 mg/kg BW for 14 days. According to the OECD (2008), the LD50 value was more significant than 5000 when a maximum dose of 5000 mg/kg BW provided no death, leading to its practical induction into the nontoxic criteria. This was due to death being a state of challenge or inability to survive,11 indicating that the LD50 1,3-bis(p-hydroxyphenyl) urea > 5000 mg/kg BW.
On the 15th day of surgery, observations were carried out on the animal organs based on macro pathology. The results showed several significant changes concerning the colour, surface, and consistency of the liver, kidneys, heart, spleen, and lungs. The colour change was one of the parameters for the occurrence of toxic effects, to obtain information on the toxicity of the test substance.19 The macropathological observations of the liver showed that the colour and surface were dark red and smooth, with the consistency being supple in all groups. According to Dorland,20 a normal liver was dark red and slightly hard when pressed. For the kidney, no changes were observed in the control and administration group 1,3-bis (p-hydroxyphenyl) urea, as the organ remained brownish-red, smooth, and chewy, based on the colour, surface structure, and consistency. Kidneys have a great capacity to bind chemical compounds, as colour change is one of the parameters for the occurrence of toxic effects.19 According to the macropathological examination of the heart, there was no change in the colour, surface structure, and consistency of the administration group, compared to the control, i.e., brownish red, smooth, and chewy. This indicated that the administration of 1,3-bis(p-hydroxyphenyl) urea did not affect the organ. Based on the spleen organs, there are no changes were observed in all groups, as the colour, surface, and consistency were dark-red, sharp, and chewy, respectively. The normal spleen is often found to be dark-red to blackish-blue, accompanied by a pointed or crescent-shaped border. Meanwhile, the damaged organs commonly experience swelling, as well as dark brown/black colour and blunt edges.21 In addition, the observation of the lungs in all groups indicated a pink colour, smooth surface, and chewy consistency. These observations are shown in Table 3.
The histological examination40 of the organs was carried out to determine the relationship between the symptoms that occurred with their structural exposure to the test compound. This indicated that the microscopic observations of the liver, kidney, heart, spleen, and lungs were used to show the degree of damage caused by the administration of 1,3-bis (p-hydroxyphenyl) urea for 14 days in test animals. Figure 4 showed that the histopathology in the control and test groups of 2000 and 5000 mg/kg BW were normal, as hepatocytes were radially distributed in the liver lobules with no apparent signs of hydropic degeneration or necrosis. Moreover, the damaged liver microscopically experienced changes, which were characterized by central vein bleeding and hepatocyte necrosis. This hydropic degeneration (necrosis) is a morphological change based on the enzymatic progressive degradation in injured cells. It is often characterized by the changes and destruction of the nucleus.22 In the microscopic observation of the kidney tissue, the control and administration groups were still in normal condition. According to the cardiac histology examination, no damage was observed in the heart muscle cells, which showed normal myocytes and myofibrils. For the spleen tissue, the control and the administration 1,3-bis(p-hydroxyphenyl) urea groups showed that the parenchymal arrangements of the white and red pulps were normal. This indicated that the administration of the compound did not affect the microscopic appearance of the spleen. In addition, the observation of the lungs showed that the tissue was still normal, as there was no inflammatory cell infiltration, edema, or congestion.
Based on the relative weight ratios of the liver, kidneys, lungs, spleen, and heart, no significant relationship was observed between the control and treatment groups (p > 0.05). This indicated that the compound 1,3-bis(p-hydroxyphenyl) urea did not affect the ratio of organ and body weights. The results are also presented in Table 4.
The hematological parameters tested for the acute toxicity of 1,3-bis(p-hydroxyphenyl) urea in rats include1: Hemoglobin (HB),2 Hematocrit (HCT),3 White Blood Cells (WBC),4 Red Blood Cells (RBC),5 Platelets,6 Mean Corpuscular Volume (MCV),7 Mean Corpuscular Hemoglobin (MCH),8 Mean Corpuscular Hemoglobin Concentration (MCHC),9 Eosino- phils (EOS),10 Monocytes (MON), and11 Basophils (BAS). Using a One-Way Anova method and a Post Hoc Test (Tuckey HSD), the results showed that there was no significant difference between the control and treatment groups (p > 0.05), as HB, HCT, WBC, RBC, Thrombo, MCV, MCH, MCHC, EOS, MON, and BAS = 0.412, 0.633, 0.894,
0.986, 0.575, 0.392, 0.572, 0.204, 0.266, 0.495, and 0.498, respectively. This indicated that 1,3-bis(p-hydroxyphenyl) urea did not affect the hematological values between the control and treatment groups. In addition, a good physiological condition of the body was often characterized by an optimal blood profile and components.23,30 The results are also presented in Table 5.
The blood biochemical parameters observed were total protein, direct bilirubin, SGOT, SGPT, ALP, urea, and creatinine. Blood biochemical levels can be seen in Table 6. The results of measuring levels of total protein, direct bilirubin, SGOT, SGPT, ALP, urea, and creatinine were then analyzed using the One Way ANOVA method, followed by Post Hoc Test in the form of the Tuckey HSD test, indicating that there is a significant difference (p > 0.05) in total protein and urea p > 0.05) between the control and test groups. Mean-while, in the Direct Biliburin parameter, p = 0.991 (p < 0.05); SGOT p = 0.542 (p > 0.05); SGPT p = 0.347 (p > 0.05), ALP p = 0.052 (p > 0.05); and creatinine p = 0.409 (p > 0.05) did not show a significant difference. However, according to Charles River Laboratories (1998), blood chemistry parameters in acute toxicity testing are still within normal limits.
| Group | N | Gender | CMC Na 0.5% | 2000 mg/kg BW | 5000 mg/kg BW |
|---|---|---|---|---|---|
| Total Protein (g/dL) | 5 | Female | 6.30 ± 0.18 | 6.69 ± 0.12* | 6.77 ± 0.07* |
| Direk Bilirubin (mg/dL) | 5 | Female | 0.03 ± 0.01 | 0.03 ± 0.92 | 0.03 ± 0.01 |
| SGOT (U/L)/AST | 5 | Female | 110.2 ± 31.6 | 92 ± 18.91 | 99.8 ± 3.70 |
| SGPT (U/L)/ALT | 5 | Female | 35.6 ± 3.84 | 30.4 ± 3.85 | 35.6 ± 9.81 |
| ALP (U/L) | 5 | Female | 145.6 ± 5.09 | 165.4 ± 32.88 | 170 ± 4.30 |
| BUN (mg/dL) | 5 | Female | 23.72 ± 0.37 | 19.28 ± 2.96* | 19.14 ± 0.69* |
| Creatinine (mg/dL) | 5 | Female | 0.35 ± 0.04 | 0.37 ± 0.08 | 0.33 ± 0.02 |
The observations of toxic symptoms were carried out in each group and compared with controls. The symptoms observed included tremor, diarrhea, drooling, weakness, fur, skin, and eye mucosa alterations, as well as animal movements such as backward and stomach motions. Based on the subchronic test, the dosages at 50, 500, and 1000 mg/kg BW, as well as satellite 1000 mg/kg BW, showed no toxic symptoms. This indicated that no relationship was observed between the doses and toxic effects. According to the doses provided, several substances were found to cause unwanted side and toxic effects.24
Based on this study, no test animals experienced weight loss during the subchronic analysis. This indicated that all treatment and control groups showed weight gains. According to Table 8, there was a significant bodyweight difference (p < 0.05) between the male and female rats on the 0 (0.011), 7th (0.003), 14th (0.000), 21st (0.000), 28th (0.000), 35th (0.000), and 42nd (0.000) day, respectively. However, there was no significant difference between the control and treatment groups after the administration of 1,3-bis(p-hydroxyphenyl) urea, at a significance level of 0.096, 0.239, 0.196, 0.070, 0,058, 0,085, and 0.062 for 0, 7, 14, 21, 28, 35, and 42 days, respectively. These indicated that the 28-day administrations did not affect the bodyweight of rats. Bodyweight is known to influence toxic effects, where mass loss is a simple and sensitive index of toxicity.19 In addition, rapid and severe weight loss is often a source of health problems, due to lack of foods and drinks, illness, or specific harmful indications.25
Based on the subchronic analysis, the microscopic evaluation of the liver at 1000 mg/kg BW experienced the changes characterized by central vein bleeding, although hepatocytes did not experience necrosis. The gap between these plates contained the products of the hepatic sinusoids. These elements are irregularly dilated tissues, where only one endothelial layer is broken.26,27 However, the liver tissue was not different from the control satellite group at 1000 mg/kg BW. This indicated that the microscopic evaluation found that female rats had an inflammatory infiltrate at a dose of 1000 mg/kg BW. Based on the kidney, no difference was observed from the control satellite group at a dose of 1000 mg/kg BW. Furthermore, the treatment group's spleen microscopic examination was comparable to the control group, while the heart provided a normal result. The microscopic examination of the lung tissue at 50 and 500 mg/kg BW also showed no inflammatory cell infiltra-tion, edema, or congestion. The inflammatory cell infiltration suggests a response to the body's natural defense system against foreign invaders. This is due to their response to injuries through elimination, reduction, or containment of wound agents on a local level.20 The circulatory and metabolic disturbances in the lungs and other organs also caused congestion, which is a passive accumulation of blood in the pulmonary arteries, occurring due to inflammation or heart failure. This microscopically manifests as capillary dilations (widening) in the alveolar septum. Also, it is defined as an increase in blood volume or bodily component.27,28 In the control satellite group, the lung tissue was not different at a dose of 1000 mg/kg BW, indicating that the toxic effects caused by the 28-day administration of 1,3- bis(p-hydroxyphenyl) urea were reversible when stopped. This was because the cells reverted to normal when the stimulus was removed. However, cell death occurred due to the continuity of excessive doses, leading to the impossibility of reversibility.28,29 Figure 5 showed that the histopathology in the control and test groups of subchronic test.
Based on the relative organ weight ratio, there was a significant difference between the males and females, concerning the masses of the liver and lungs (p < 0.05). Meanwhile, there was no significant difference in the kidney, spleen, and heart. This indicated that no significant difference was observed between the control and treatment groups (p > 0.05). Also, the results showed that 1,3-bis(p-hydroxyphenyl) urea had no significant effect on the ratio of organ and body weights. This relative organ weight is shown in Table 9.
Based on Table 10, the hematology measurements were statistically analyzed using the Two-Way Anova method and Post Hoc Test (Tuckey HSD). The results indicated that MCH and BAS showed a significant difference (p < 0.05) between male and female animals. However, there was no significant difference between the control and test groups (p > 0.05), where Hb, HCT, WBC, RBC, Thrombo, MCV, MCH, MCHC, SEN, EOS, and BAS = 0.813, 0,058, 0.475, 0.583, 0.180, 0.589, 0.670, 0.238, 0.995, 0.134, and 0.377, respectively. Based on these results, 1,3-bis(p-hydroxyphe- nyl) urea did not affect the hematological value of animals between each group.
| Parameter | N | CMC Na 0.5 % | 50 mg/kg BW | 500 mg/kg BW | 1000 mg/kg BW | Satelit CMC Na 0.5% | Satelit 1000 mg/kg BW | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | F | M | F | M | F | M | F | M | F | M | F | ||
| Hb (g/dL) | 5 | 15.06 ± 1.29 | 15.06 ± 1.22 | 14.58 ± 0.83 | 13.6 ± 1 | 14.42 ± 0.86 | 14.1 ± 0.31 | 14.66 ± 1.21 | 15.08 ± 1.15 | 14.82 ± 1.23 | 14.44 ± 0.95 | 14.44 ± 0.92 | 15.3 ± 1.51 |
| HCT (%) | 5 | 40.63 ± 1.44 | 40.78 ± 3.38 | 46.66 ± 2.21 | 41.38 ± 3.27 | 40.24 ± 0.87 | 39.4 ± 1.09 | 41.02 ± 2.23 | 44.44 ± 3.74 | 42.16 ± 3.67 | 39.04 ± 2.18 | 40.9 ± 2.33 | 39.7 ± 4.91 |
| WBC (103/μL) | 5 | 5.44 ± 3.03 | 2.65 ± 1.5 | 3.61 ± 0.76 | 3.45 ± 2.06 | 2.9 ± 1.06 | 3.41 ± 1.61 | 3.96 ± 1.64 | 3.21 ± 2.01 | 3.93 ± 1.78 | 2.42 ± 1.62 | 2.78 ± 0.72 | 2.316 ± 0.52 |
| RBC (106/μL) | 5 | 7.89 ± 0.86 | 7.89 ± 0.24 | 7.83 ± 0.73 | 7.29 ± 0.18 | 8.16 ± 0.79 | 7.72 ± 0.39 | 7.74 ± 0.67 | 7.97 ± 0.31 | 7.73 ± 0.28 | 7.50 ± 0.60 | 7.88 ± 0.64 | 7.86 ± 0.62 |
| Trombo (103/μL) | 5 | 685 ± 30.41 | 813.2 ± 121.11 | 822.4 ± 110.17 | 852.2 ± 78.36 | 839.6 ± 116.77 | 901.6 ± 124.77 | 828.6 ± 81.13 | 831.4 ± 115.7 | 838.6 ± 120 | 822.8 104 | 784.4 ± 80.3 | 854.2 ± 80.6 |
| MCV (fL) | 5 | 57.7 ± 0.12 | 53.16 ± 2.96 | 53.2 ± 2.98 | 55.06 ± 2.63 | 53.64 ± 3.12 | 53 ± 1.72 | 53.4 ± 3.75 | 53.32 ± 2.93 | 53.22 ± 3.49 | 55.66 ± 4.43 | 51.18 ± 1.82 | 57.04 ± 1.25 |
| MCH (pg) | 5 | 17.62 ± 0.49 | 18.96 ± 0.96 | 18.02 ± 0.45 | 18.52 ± 0.55 | 18.36 ± 1.15 | 18.54 ± 0.65 | 17.96 ± 0.48 | 18.34 ± 0.93 | 18.78 ± 0.88 | 18.48 ± 0.98 | 17.94 ± 0.33 | 18.34 ± 0.50 |
| MCHC (g %) | 5 | 35.74 ± 1.26 | 36.12 ± 1.88 | 36.08 ± 2.29 | 35.7 ± 1.77 | 33.88 ± 1.33 | 35.14 ± 1.71 | 34.44 ± 1.51 | 34.34 ± 0.83 | 35.7 ± 1.51 | 34.86 ± 0.86 | 34.20 ± 0.92 | 33.34 ± 0.65 |
| EOS (%) | 5 | 0.78 ± 0.48 | 1.1 ± 0.34 | 0.28 ± 0.1 | 0.94 ± 0.37 | 0.52 ± 0.35 | 0.76 ± 0.32 | 0.98 ± 1.02 | 0.84 ± 0.26 | 2.28 ± 1.35 | 1.38 ± 0.86 | 2.1 ± 1.74 | 1.04 ± 0.63 |
| MON (%) | 5 | 1.4 ± 0.91 | 2.28 ± 1.52 | 1.4 ± 0.90 | 2.1 ± 1.2 | 1.9 ± 1.43 | 2.08 ± 0.89 | 1.98 ± 0.84 | 2.0 ± 1.00 | 1.76 ± 1.01 | 2.02 ± 1.11 | 1.72 ± 1.42 | 2.02 ± 0.85 |
| BAS (%) | 5 | 0.34 ± 0.31 | 0.12 ± 0.08 | 0.28 ± 0.31 | 0.18 ± 0.3 | 0.36 ± 0.28 | 0.002 ± 0.04 | 0.32 ± 0.13 | 0.14 ± 0.21 | 0.52 ± 0.21 | 0.28 ± 0.25 | 0.38 ± 0.27 | 0.24 ± 0.21 |
Using a two-way ANOVA and Tukey Post-hoc Test, the results showed that there was no significant difference (p > 0.05) in total protein, Direct Billiburin, ALT, AST, creatinine, and BUN levels between the control and the treatment groups (Table 11). However, a significant difference was observed in Billiburin Direct, SGOT, SGPT, ALP, urea, creatinine, between all groups. This was based on the use of a Two-Way Anova method and Post Hoc Test (Tuckey HSD test). Meanwhile, there was no significant difference (p > 0.05) in the Total Protein parameter at 0.323. In comparing hematological levels between male and female animals, there was a significant difference (p < 0.05) in Total Protein, SGOT, ALP, and Urea parameters. According to Charles River Laboratories,23,30 the hematological levels were still within normal limits in the subchronic toxicity tests. This indicated that 1,3 bis (p-hydroxyphenyl) urea did not affect the levels of total protein, Billiburin Direct, SGOT, SGPT, ALP, urea, and creatinine in male and female rats.
The p-aminophenol compound is a product of aniline metabolites; whose toxicity is more minor compared to ortho and meta-aminophenols. It has a strong antipyretic anal-gesic action and is too toxic to be used as a drug. Therefore, some modifications were made to reduce its toxicity. Based on these modifications, changes were made to the amine and phenol groups, to obtain more potent and less toxic compounds.31 These modified compounds, including 1,3 bis (p-hydroxyphenyl) urea, were found to have analgesic ac-tivities that were 1.96 times more potent than paracetamol.32 When the alkyl group of paracetamol was replaced with low ring lipophilicity (log P less than 1.8), analgesic activity was still observed. This provided anti-inflammatory activity when increased between 1.8 to 4.4. An example is a benzylic compound, an ester of aspirin with paracetamol (logP = 1.97), which has analgesic and anti-inflammatory activities.33 Also, paracetamol has deficient anti-inflammatory activity, due to being hydrolyzed into a p-aminophenol compound, which binds to arachidonic acid to block the inflammation pathway.31 The addition of urea (logP = -1.4) also allows the synthesis of 1,3-bis(p-hydroxyphenyl) urea, which has more potent analgesic and lower anti-inflammatory activities, compared to diclofenac sodium. In previous studies, in silico results, this compound has COX-1 (1CQE) and TNF-α (2AZ5) binding activity which plays a role in the inflammatory process. However, in this study, we did not specifically look at the effect of cyclooxygenase selectivity compounds on cox 1 and cox 2 and this is our consideration for further research.
The action of 1% carrageenan (0.1 ml, i.pl.) also induced significant leg edema in this study, with an average rise in pain at 1 h after administration. Based on this study, the test group 1,3-bis(p-hydroxyphenyl) urea showed different results than the CMC Na (p 0.05). At 0 h, there was no significant difference between 1,3-bis(p-hydroxyphenyl) urea and sodium diclofenac groups (p > 0.05). Meanwhile, there was a significant difference between the Na-CMC groups (p 0.05) between 1-5 h. This indicated that the test group had anti-inflammatory activity at 1-5 h. The results also showed a marked accumulation of neutrophils in the legs, with the least occurrence observed in the diclofenac sodium and 1,3 bis (p-hydroxyphenyl) urea groups, at 200, 100, and 50 mg/kg BW. Neutrophils are cells carrying out the phagocytosis of foreign bodies when they rapidly increase. This indicates that inflammation occurs and lasts longer when there is no infection. During an injury, these cells are always the first to occur at the damaged tissues at approximately 24-48 h. After the third day of infection, neutrophils rapidly increase within the injury and are more activated by macrophage cells.15,16 Based on an acute response, these cells caused degranulation and lysis through a fatal enzyme. This was because neutrophil lysosomal granules contained hydrolytic and proteolytic enzymes, in addition to ROS source catalysts (mainly NADPH and myeloperoxidase). Therefore, damages to various biological molecules were observed when lysis and enzymes were spilled into the tissue. In addition, live neutrophils are an essential factor in the inflammatory response.17,18
Although 1,3-bis(p-hydroxyphenyl) urea increased body weight when compared to the control group, it was still not significant. This was because clinical signs and symptoms were sensitive indications of poisoning. In this study, the animals were regularly monitored and assessed based on clinical signs and body weight. In addition, rapid and severe weight losses were often signs of a health problem, due to lack of food and drink consumption, certain disorders, or toxic symptoms.34 According to this study, the effects of 1,3-bis(p-hydroxyphenyl) urea on the liver, lungs, kidneys, and liver were indicated by hematological and biochemical markers. In the acute and subchronic toxicity tests, there was no mortality after the administration of the extract at 5000 and 1000 mg/kg BW, respectively. On high-dose treatment, histopathological studies indicated damages to the liver and lungs. The satellite group also showed that all toxic symptoms, as well as biochemical and pathological disruption responses, were reversible when treatment was terminated. In addition, several previous studies indicated that the length and intensity of lethal drug exposure influenced the form and toxicity of a specific sensation.35 When these metabolic responses were sustained, they became adaptive, leading to pathogenic and biochemical problems.36
In the tests carried out when viewed from the size of the dose, diclofenac sodium had better anti-inflammatory properties, namely at a dose of 2.25 mg/kg BW compared to this compound which required doses above 50 mg/kg BW. However, considering that the toxicity test showed no symptoms of toxicity and death up to a dose of 5000 mg/kg BW in the acute toxicity test, and in the subchronic toxicity test for 28 days, the administration of this compound had few symptoms of toxicity and the symptoms were irreversible. Meanwhile, based on reports, diclofenac sodium can cause death in rats at a dose of 53 mg/kg BW in acute toxicity testing.37 This compound has anti-inflammatory potential with less toxicity if developed.
Authors should discuss the results and how they can be interpreted from the perspective of previous studies and of the working hypotheses. The findings and their implications should be discussed in the broadest context possible. Future research directions may also be highlighted.
Based on this study, the results showed that 1,3-bis(p-hydroxyphenyl) urea had an anti-inflammatory effect in rats through various experiments. Long-term administration of this compound at 50, 250, 500, and 1000 mg/kg BW did not generate lethal effects, according to the acute and subchronic toxicity tests. In line with this, no deaths were ob-served in the acute and subchronic analyses, at 5, 50, and 300 mg/kg BW, as well as 50 and 500 mg/kg BW, respectively. In addition, hematological and biochemical markers were within normal ranges. At high dosages (5000 and 500/1000 mg/kg BW for the acute and subchronic tests), the occurrence of toxic effects was observed on the organs. However, these effects were reversible, as observed in the satellite group.
Zenodo: The dataset of ‘Anti-Inflammatory Activity and Toxicity Evaluation of 1,3-bis(p-Hydroxyphenyl)urea. https:// doi.org/10.5281/zenodo.5886208.38
This project contains the following underlying data:
• Raw data of Acute Body Weight.xlsx (Raw data of body weight of acute toxicity)
• Raw data of Acute of Relative Organ Weight.xlsx (Raw data of relative organ weight of acute toxicity)
• Raw data of Biochemistry of acute toxicity.xlsx (Raw data of biochemistry of acute toxicity)
• Raw data of Biochemistry of subchronic toxicity.xlsx (Raw data of biochemistry of subchronic toxicity)
• Raw data of Hematological of Acute toxicity.xlsx (Raw data of hematological of acute toxicity)
• Raw data of Hematological of subchronic toxicity.xlsx (Raw data of hematological of subchronic toxicity)
• Raw data of Percentage of inflamation.xlsx (Raw data of percentage of inflamation in feet of rat)
• Raw data of Subchronic Body Weight.xlsx (Raw data of body weight of acute toxicity)
• Raw data of Subchronic of Relative Organ Weight.xlsx (Raw data of relative organ weight of acute toxicity)
• Figure of The organ of acute toxicity.pdf (Figure of the organ in acute tocicity)
• Figure of The organ of subchronic toxicity.pdf (Figure of the organ in subchronic tocicity) Zenodo: Original unedited histological image https://doi.org/10.5281/zenodo.5921241.39
Zenodo: Original unedited microscopic observation image https://doi.org/10.5281/zenodo.6076466.40
Zenodo: Preparation and synthesis of 1,3-bis(p-Hydroxyphenyl) urea. https://doi.org/10.5281/zenodo.5769528.41
Zenodo: ARRIVE checklist for “Anti-inflammatory activity and toxicity evaluation of 1,3- bis(p-Hydroxyphenyl)urea”, https://doi.org/10.5281/zenodo.6075572.42
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: cardiovascular disease
Is the work clearly and accurately presented and does it cite the current literature?
No
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 20 Sep 22 |
||
|
Version 1 13 Apr 22 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)