Keywords
Gastric Cancer, Neutrophil-Lymphocyte Ratio, Platelet-Lymphocyte Ratio, Serum Albumin, Tumor Stage (MeSH)
This article is included in the Oncology gateway.
Gastric Cancer, Neutrophil-Lymphocyte Ratio, Platelet-Lymphocyte Ratio, Serum Albumin, Tumor Stage (MeSH)
Gastric cancer occupies fifth place among commonly diagnosed neoplasms and third place in mortality worldwide.1–3 Stomach cancer has important geographical, ethnic, and socioeconomic differences. Although risk factors are described, the main factors are environmental (Helicobacter pylori, diet, overweight, and obesity) as well as due to host (genetic factors).4
According to Globocan, during 2018, in Peru, 5731 new cases of gastric neoplasm were diagnosed, falling in third place of frequency, just below breast and prostate cancer. Furthermore, 4606 of deaths were registered, placing it in first place for mortality.5
The prognostic molecular, genomic factors and other high complex instruments are currently not available in all hospital centers in economically emerging countries. For this reason, this study seeks to determine the clinical factors associated with tumor stage in gastric cancer that are of easy access and low cost, in order for them to help us identify survival and an adequate treatment.
The inflammation caused by tumors can promote the tumoral growth, invasion, angiogenesis, and even metastasis. This is why inflammatory markers, such as neutrophil–lymphocyte ratio (NLR) and platelet–lymphocyte ratio (PLR) have shown to be useful in multiple neoplasms.6,7
Furthermore, the systemic inflammatory response induced by tumors may exacerbate malnutrition in patients. Which may promote tumor progression.6 For this reason, albumin has also been considered as an independent prognostic factor for many malignant diseases,8 since low levels of albumin in plasma reflect a chronic inflammation.9
Some researchers have reported that inflammatory markers such as, PLR, and serum albumin may be useful in the prognosis of patients diagnosed with gastric cancer.7,10–12 However, this is still in dispute.
Therefore, our objective was to determine the clinical-inflammatory factors associated with tumor stage of gastric cancer of the oncology service of Hospital María Auxiliadora during the years 2018–2020.
The research design is an observational, analytical and cross-sectional study.
It was carried out in male and female patients treated in the oncology service of Hospital María Auxiliadora, Lima, Peru, during the years 2018 to 2020. Inclusion criteria were all patients ≥18 years who had at least a hemoglobin and serum albumin test at the moment of diagnosis of gastric cancer. Exclusion criteria were patients with diagnosis of cancer of the gastroesophageal junction or another diagnosis (gastric lymphoma and others), clinical records that don’t have pathological report by biopsy of gastric cancer or incomplete clinical histories.
A simple random probability sampling of patients was carried out with a population size of 166 patients, and a confidence Interval of 95% and error of 0.05, and a final sample of 110 patients, data was obtained with power size at 90%.
The sample size calculation was carried out with an NLR risk in exposed of 83% and risk in not exposed of 51%; according to the study published by Huaman et al.10
The data obtained from the clinical factors associated with tumor stage in patients with gastric cancer were collected using the data collection form approved by the Institute of Biomedical Sciences of the Universidad Ricardo Palma and the Ethics committee of the University, which consisted of four parts:
• Identification: Medical record number, age, sex, weight, height, body mass index (BMI)
• Clinical stage of gastric adenocarcinoma: criterion T, criterion N, criterion M
• NLR/PLR: number of neutrophils, number of platelets, number of lymphocytes
• Serum albumin
The database continued to be filled in until the expected sample size was completed, checking in detail if there were incomplete data, poorly filled in, or any other cause that could have affected and altered their integrity. Subsequently, there was a review by the Institute's medical advisers and finally they were analyzed (see Figure 1).
To avoid selection bias: all patients included were confirmed with a diagnosis of gastric cancer according to a pathological report by a biopsy. To avoid information bias, all the laboratory studies (hematological and biochemical) of the patients were carried out in the same hospital laboratory under the instruments, same calibration techniques, reference values and international and national standards according to the guidelines of the Ministry of Health of Peru for a level III Health Hospital with the purpose of obtaining an exhaustive selection of biomarkers according to the objectives of the present study.
The quantitative variables were NLR, PLR and serum albumin. The quantitative variable of discrete reason was age. The ordinal quantitative variable was BMI. The qualitative variables were the criteria T, N, M and the clinical stage.
The dependent variable was the tumor clinical stage according to TNM for gastric cancer, for further analysis they were grouped into localized (stage I–II) and advanced (III–IV), the independent variables were the NLR, PLR, and serum albumin, whose cut-off points for NLR and albumin were 2.44 and 3.5, respectively, taking into account the studies by Sun et al.,7 Huamán et al.11 and Lian et al.13; while, for PLR the cut-off point was 284.4 according to the study by Hirahara et al.6 in China, which allows us to classify into high and low values. Possible confounding variables are sex, age and district of origin (sociodemographic variables).
A clinical and laboratory data collection sheet previously mentioned was used as the instrument to collect data from medical record.
Data were analyzed using STATA version 14 (RRID:SCR_012763) and SPSS version 22 (RRID:SCR_019096) programs.
In descriptive statistics, the quantitative variables were presented in measures of central tendency (mean or median) and dispersion measures (standard deviation or interquartile range) prior evaluation of distribution of values. Bar graphs were used for qualitative variables and box and whisker plots were used for quantitative variables.
For inferential statistics, we worked with 95% confidence with a statistical significance of p < 0.05. The Chi-squared and Poisson statistic tests were used for the analysis of categorical variables. The numeric variables were analyzed with a Student’s t-test (parametric distribution data). The PR values (prevalence ratio) were obtained from absolute frequencies of clinical stage, NLR, PLR and serum albumin variables.
This study was approved by the Research and Ethics Committee of the Human Medicine Faculty of the Universidad Ricardo Palma, Lima-Peru, with committee code PG-54-2020 on November 13th, 2020.
All procedures performed in this study preserved fundamental rights and the integrity of patients who were research subjects, guaranteeing the confidentiality of the obtained data.
This investigation was developed within the context of the VII thesis dissertations, according to the previously published methodology14 and based on the thesis “Clinical factors associated to tumor stage of gastric cancer of the oncology service of Hospital María Auxiliadora during the years 2018-2020”.15
In the Office of Statistics and Informatics, the numbers of the medical records of patients diagnosed with gastric cancer among the years 2018-2020 were 166, 15 had other diagnoses, 9 were duplicate medical records, 17 without pathological report and 15 incomplete histories that did not meet the inclusion criteria. 110 medical records met the inclusion and exclusion criteria for statistical analysis and results (see Figure 1).
The percentage of patients who were female was 55.45%. The average age in the study was 53 years (range 27–96 years). Furthermore, we can observe that the most frequent histological type of gastric cancer was the intestinal type 62.72% (Table 1). The frequency of signet-ring cell adenocarcinoma was 34.5%.
| Age | ||
|---|---|---|
| Average | 63.727 | |
| Standard deviation | 13.8471 | |
| Minimum age | 27 | |
| Maximum age | 96 | |
| Pathological anatomy | Frequency | Percentage |
| Intestinal adenocarcinoma | 69 | 62.72% |
| Diffuse adenocarcinoma | 41 | 37.28% |
| Total | 110 | 100% |
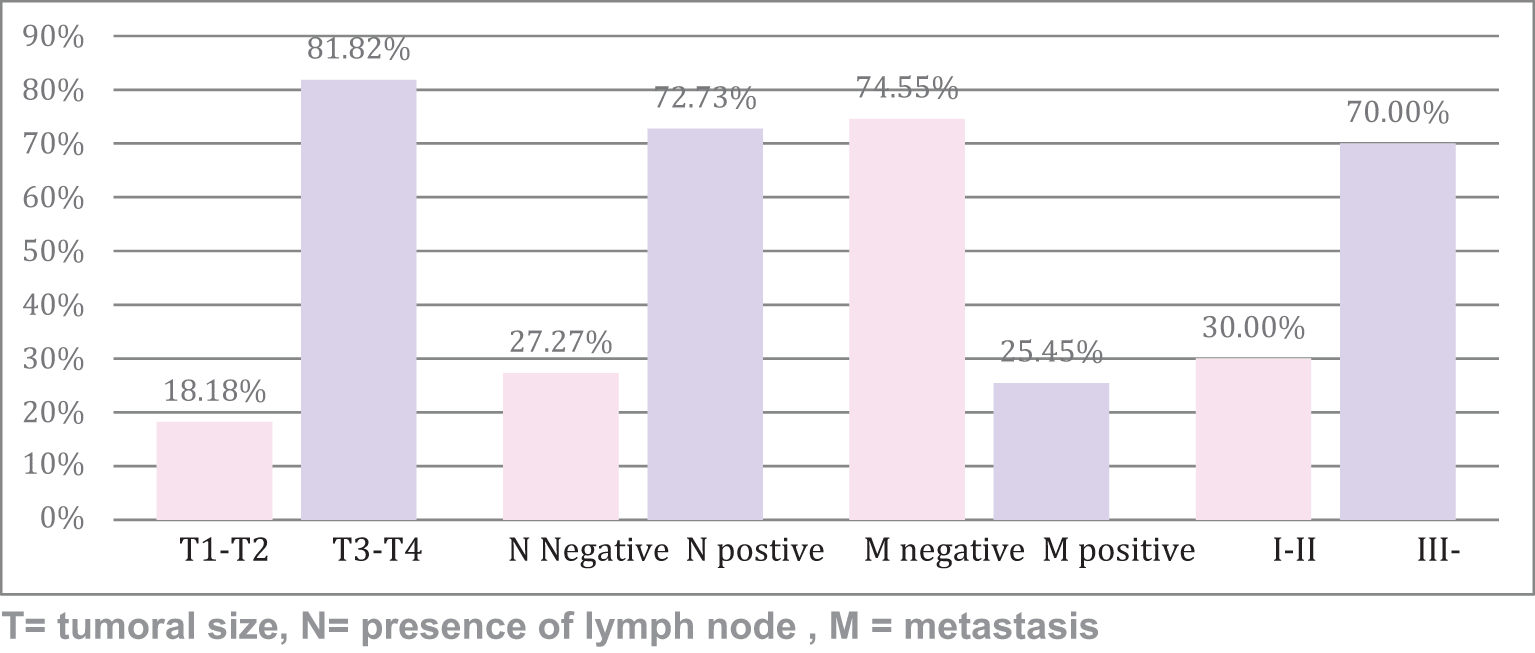
Regarding the TNM classification and clinical stage, in Figure 2, we demonstrate that 81.8% had a tumoral size criteria of T3–4 at the time of cancer staging. Likewise, 72.7% presented positive lymph nodes and 25.5% presented metastasis. Finally, 77% presented an advanced clinical stage (EC III–IV).
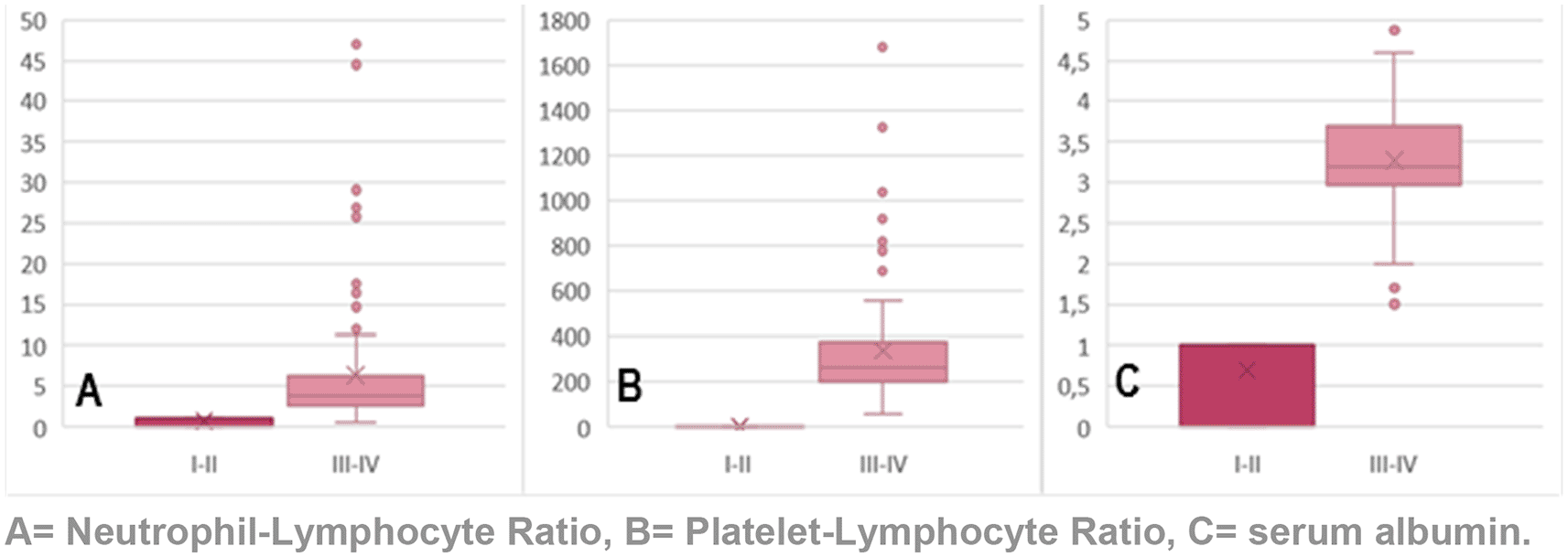
In Figure 3, we observe the value distribution of NLR, PLR, and serum albumin according to clinical stage. As far as NLR, in the early clinical stage (I–II), the average was 2.75, and in advanced clinical stage the average obtained was 9.50. With respect to PLR, in the early clinical stage (I–II), the average was 168.52, while in the advanced clinical stage (III–IV) the average was 409.93. Lastly, regarding serum albumin, in the early clinical stage (I–II), the average was 3.7, while in advanced clinical stages (III–IV), the average was 3.1. These measures were evaluated with the Student’s t-test, where a significant difference was shown (p < 0.001).
In the bivariate analysis, patients with NLR greater than 2.44 were approximately 6-times more likely to have the presence of advanced gastric cancer (III–IV), compared with those with NLR lower than 2.44. Likewise, patients with PLR greater than 2448.4 were 2.5-times more likely to have a neoplasm of advanced stage (III–IV) than those with PLR lower than 248.4, and finally, those with albumin values <3.5 were 3-times more present in advanced stages compared with those with normal values. All these results were statistically significant (p < 0.001) (see Table 2). We did not find a statistically significant association regarding age and sex.
In Table 3, in the multivariate analysis of clinical factors associated to tumor stage of gastric cancer, we observed that patients with NLR greater than 2.44 were 4.11-times more likely to have prevalence of gastric cancer in advanced clinical stage compared with those who had NLR less than 2.44. This result is statistically significant (p < 0.05). PLR and albumin were not statistically significant.
Gastric cancer is associated with a high morbidity and mortality rate in Peru and worldwide,5 which is why an adequate assessment that allows us to establish a prognosis and make correct therapeutic decisions is necessary.
Within the immunologic and pathogenic process, gastric cancer is growing in areas of elevated inflammation, involving platelets, neutrophils, and lymphocytes. The inflammatory markers can be used as a tool for the prognosis of said neoplasm.13
In our research work, we evidenced a greater frequency in those over 50 years of age, which is consistent with the international literature previously reported.16
The distribution of patients in our research was predominantly female at 55.4%, this result coincides with other studies,7 unlike other studies carried out in other contexts.16,17
With respect to pathological anatomy, the most frequent type of cancer based on the WHO classification18 was intestinal adenocarcinoma at 62.72% (tubular adenocarcinoma: 60.9% and papillary adenocarcinoma: 1.82%) and in second place was diffuse adenocarcinoma with 37.28% (with signet-ring cells: 36.37% and mucinous adenocarcinoma: 0.91%), results were similar to those already intentionally reported and mentioned by Reyes19 and Ramírez et al.,20 where intestinal type gastric adenocarcinoma versus diffuse was 68.3% versus 31.7% and 63.8% versus 25%, respectively.
Regarding clinical stage, we evidenced that 70% were in advanced stages (III–IV), while 30% corresponded to early stages (I–II). Similar to a study in Peru in 2019, reporting 70.8% in advanced stage (III–IV) and 29.2% were in early stages (I–II),11 confirming a diagnosis in late stages among the Peruvian population in both studies, as was mentioned in previous studies.13
In the bivariate analysis report, we evidenced that patients with a NLR greater than 2.44 were 6.88-times more prevalent to have gastric cancer in advanced clinical stage. This coincides with a study carried out by Jingxu Sun et al.7 where it was evidenced that NLR was greater in patients with gastric cancer in advanced stages compared with gastric cancer in early stages (OR: 2.76; 95% CI: 0.85–3.54; p < 0.005). Along the same lines, Zhang et al.21 in 2018, found that those who had NLR lower were in early clinical stages (p < 0.001). These findings suggest that NLR values are useful as predictive factors in advanced tumoral stages.
Regarding PLR, withing the bivariate analysis we evidenced that patients with values greater than 248.4 were 2.55-times more prevalent to have gastric cancer in advanced clinical stages. These findings were similar to those observed in the study carried out by Lian et al.,13 where high levels of PLR were associated with advanced stages of the tumor, metastasis to lymph nodes and a greater depth of tumor invasion (p < 0,005). In the same manner, Zhang et al. found that patients with high PLR had 1.17- and 1.99-times the risk of having an advanced clinical stage, respectively.21,22
We found that low serum albumin increased the prevalence of having a late clinical stage by 3.16-times. These results coincide with that reported by Huamán et al.,11 who evidenced that low serum albumin is a predictive factor for gastric cancer in advanced tumor stage (OR: 13.02 95% CI 1.78–5.36; p < 0.005).
In the multivariate analysis report, we observed that patients with a NLR greater than 2.44- had 4.11-times the prevalence of having advanced clinical stage gastric cancer (p < 0.02). Regarding PLR and albumin level, they did not reach a statistically significant relationship. These results suggest that NLR is a potentially independent predictive factor of gastric cancer in advanced clinical stage. These findings are supported by the retrospective cohort study carried out by Zhao et al.,23 who found an association with NLR (HR: 1.61; CI 95%: 1.032–2.525; p = 0.036) in the multivariate and by the retrospective longitudinal study published by Ramos-Esquivel et al.,24 where, in the multivariate, only the NLR was associated independently to a poor global survival (HR: 1.59; 95%CI: 1.15–2.28; p = 0.005).
While this study has limitations, such as being an observational, retrospective and unicentric study with a limited number of patients, however, poor global survival, the study population is representative of a referral hospital in the south of Lima, Peru with a statistical power greater than 90%. Furthermore, these inflammatory biomarkers are of easy access, economical and reproducible, with a potentially prognostic use in the clinical medical practice. Multicentric, prospective studies are necessary to confirm these results.
The inflammatory markers (NLR and PLR) and hypoalbuminemia were associated to advanced gastric cancer clinical stage by bivariate analysis. NLR resulted as an independent predictive factor in relation to the clinical stage of gastric cancer in this study.
Zenodo: Underlying data for ‘Clinical inflammatory biomarkers associated with the tumor stage of gastric cancer: retrospective analysis of a Hospital in Peru during the years 2018–2020’ https://doi.org/10.5281/zenodo.5781815
The project contains the following data:
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0)
We thank the Instituto de Investigaciones en Ciencias Biomédicas de la Universidad Ricardo Palma, the team and staff of admissions/statistics/archives: Miguel Chutas from Hospital María Auxiliadora and the research unit from the Oncology department of Hospital María Auxiliadora.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: solid organ malignancies chemotherapy,targeted therapy and immunotherapy
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 17 Jan 22 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)