Keywords
Dementia, Alzheimer's Disease, Mendelian Randomization, vascular risk factors, meta-analysis, systematic review
Although observational studies demonstrate that higher levels of vascular risk factors are associated with an increased risk of dementia, these associations might be explained by confounding or other biases. Mendelian randomization (MR) uses genetic instruments to test causal relationships in observational data. We sought to determine if genetically predicted modifiable risk factors (type 2 diabetes mellitus, low density lipoprotein cholesterol, high density lipoprotein cholesterol, total cholesterol, triglycerides, systolic blood pressure, diastolic blood pressure, body mass index, and circulating glucose) are associated with dementia by meta-analysing published MR studies. Secondary objectives were to identify heterogeneity in effect estimates across primary MR studies and to compare meta-analysis results with observational studies.
MR studies were identified by systematic search of Web of Science, OVID and Scopus. We selected primary MR studies investigating the modifiable risk factors of interest. Only one study from each cohort per risk factor was included. A quality assessment tool was developed to primarily assess the three assumptions of MR for each MR study. Data were extracted on study characteristics, exposure and outcome, effect estimates per unit increase, and measures of variation. Effect estimates were pooled to generate an overall estimate, I2 and Cochrane Q values using fixed-effect model.
We screened 5211 studies and included 12 primary MR studies after applying inclusion and exclusion criteria. Higher genetically predicted body mass index was associated with a higher odds of dementia (OR 1.03 [1.01, 1.05] per 5 kg/m2 increase, one study, p=0.00285). Fewer hypothesized vascular risk factors were supported by estimates from MR studies than estimates from meta-analyses of observational studies.
Genetically predicted body mass index was associated with an increase in risk of dementia.
Dementia, Alzheimer's Disease, Mendelian Randomization, vascular risk factors, meta-analysis, systematic review
We have made few revisions based on reviewer feedback. The background section has been expanded to clarify the purpose and rationale of the study. In particular we highlighted the advantages of using Mendelian randomization over observational studies to infer causality. We have also added a discussion on the potential effects of cohort overlap on our results and how sensitivity analyses were conducted to mitigate this concern. Additionally, minor edits were made throughout the manuscript to enhance clarity and improve the overall flow of the text.
See the authors' detailed response to the review by Ahmet Turan Isik
Higher measured mid-life blood pressure, mid-life and late-life hyperlipidaemia, mid-life obesity and diabetes are associated with the development of dementia in observational cohort studies.1 If this association is causal, risk factors for vascular diseases (e.g. diabetes and hypertension) may be responsible for around 40% of the cases of dementia worldwide.2 However, the effect sizes observed in cohort studies are often larger than those seen in randomized trials of vascular risk interventions aimed at reducing cognitive decline or dementia.3 This may be because cohort studies are limited by residual confounding, reverse causation, differential loss to follow-up, or selection biases.4,5 More data from less biased designs are needed to triangulate the causal effects of vascular risk factors on the development of dementia.
Mendelian randomization (MR) studies provide a means of overcoming these limitations by using genetic variants as instrumental variables to infer causality. MR is less susceptible to confounding and reverse causation than observational studies because genetic variants are assumed to be randomly assigned at meiosis.6 As such, MR can be thought of as a “natural” randomized control trial. MR studies are subject to different biases from observational studies. Their validity rests on three key assumptions: (i) the genetic variant has a known association with the risk factor of interest; (ii) the genetic variant is not associated with a known confounder; and (iii) the genetic variant affects the outcome only through the risk factor of interest. As most genetic instrumental variables are only modestly associated with their exposures of interest, MR gives an unbiased but imprecise estimate.7 Meta-analysis of MR studies could mitigate this imprecision. This approach has previously refined estimates of the effect obesity on vascular diseases.8
This study aims to determine whether genetically predicted vascular risk factors are associated with the risk of developing dementia by conducting a meta-analysis of existing MR studies. We also estimated the heterogeneity of effect estimates across different MR studies for a given risk factor within the same outcome cohort. The results were then compared to findings from meta-analyses of observational studies to assess discrepancies and enhance our understanding of causality. To ensure the robustness of our findings, potential cohort overlap between studies was addressed through careful study selection and sensitivity analyses.
We used the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline to report this study.9 A protocol has been developed and made available online (Supplementary Material 3).10 Minor amendments in study methodology have been made since its publication. Specifically, 1) the quality assessment questionnaire was shortened from 11 questions to 10 questions; 2) we applied a Bonferroni correction to account for the assessment of multiple risk factors; 3) MR meta-analysis results were compared with observational studies. No ethical approval was required.
We searched on OVID, Scopus and Web of Science, covering 13 databases: Medline, Embase, AMED, PsycINFO, BIOSIS Citation Index, Web of Science core collection, Current Contents Connect, Data Citation Index, Derwent Innovations Index, KCI-Korean Journal Database, Russian Science Citation Index, SciELO Citation Index, and Zoological Record. The search looked for a combination of dementia, and Mendelian randomization (Supplementary Material 1, Table 1). No risk factors were specified in the search query. We forward searched by screening for all referenced articles in the retrieved articles using Google Scholar. The final search was performed on 22nd October 2021.
We included published or pre-print studies that used inverse-variance weighted (IVW) two-sample MR with a poly- or oligo-genetic instrument for type 2 diabetes mellitus, low density lipoprotein (LDL) cholesterol, high density lipoprotein (HDL) cholesterol, total cholesterol, triglycerides, systolic blood pressure, diastolic blood pressure, body mass index (BMI), or plasma glucose. Included studies reported a causal estimate value with an odds ratio (OR), hazard ratio (HR), risk ratio (RR) or β-coefficient by an absolute value of per unit increase, and associated 95% confidence interval (CI) or standard error. When interquartile range was reported, we estimated the standard deviation as interquartile range/1.35.11
We excluded studies that were duplicates, not written in English, or where no full text was available. We included only one estimate from each cohort per risk factor. Where more than one study had been carried out using a cohort, we included the highest quality study; where the quality of the studies was similar, we included the study with the most recent exposure GWAS. The outcomes were all cause dementia or late-onset Alzheimer’s disease (LOAD). Uncertainties were resolved by discussion with two other reviewers (WNW and RMW).
A quality assessment tool was developed by synthesizing three published guidelines for assessing MR studies12–14 ( Table 1). Studies that did not satisfactorily address items 3-5, which describe the three core assumptions of MR, were excluded.
2SMR = 2-sample Mendelian Randomization, has independent outcome and exposure samples. 1SMR = 1-sample Mendelian Randomization.
For each study, we extracted: GWAS source, ethnicity, number of single nucleotide polymorphisms (SNPs) used as instrumental variables for each risk factor, case/control sample size, effect estimates and units, and measures of variation were extracted for each study. When multiple analysis approaches were used to generate an effect estimate, the value generated using the most SNPs without compromising pleiotropy was used (linkage disequilibrium r2 < 0.2). Effect estimates that included IVs mapping to the APOE locus, which has a known association with dementia were excluded. For studies with missing data, the authors were emailed twice and the study was excluded if no reply was received. The full details are available in Supplementary Material 2, S1 & S2.42
Effect estimates and measures of variation were standardized into common units for each risk factor. The effect estimates were pooled using a fixed effects model to generate an overall estimate for each risk factor of interest. Cochrane Q and I2 statistics were used to assess heterogeneity across studies. Analyses and plots were executed with the Metafor package (version 2.4-0) in R (version 4.0.3).15 A Bonferroni correction was applied to maintain a 5% family-wise error rate, yielding a significance threshold of 0.05 divided by n risk factors assessed (p = 0.05/9).
We performed sensitivity analysis by meta-analysing using alternative eligible studies, which were excluded in our primary meta-analysis due to outcome cohort overlap or the use of a superseded exposure GWAS. We substituted the studies with IGAP (2013) as the outcome cohort, with another study with the same outcome cohort with either the lowest or highest estimate in attempt to determine any significant change in overall effect estimate. Statistical significance was determined by applying a Bonferroni correction, as described above.
Search strategy
We searched on OVID, Scopus and Web of Science for a meta-analyses of cohort studies estimating the association between vascular risk factors with later dementia (Supplementary Material 1, Table 2).
Study selection
We selected one representative meta-analysis of observational studies for each risk factor. We considered all articles in English which analysed cohorts, reported OR/HR/RR, and associated 95% confidence interval (CI) or standard error. If multiple meta-analyses were eligible for inclusion per given risk factor, we selected the study with the highest total number of participants.
A search of three databases, OVID, Scopus and Web of Science, found 5211 unique articles. After applying inclusion and exclusion criteria to the abstracts, 29 articles were retained ( Figure 1). Seventeen articles were not included for meta-analysis after full text review because either the results were not reported per unit increase in risk factor or there was an overlap of between outcome cohorts. Of those seventeen articles, ten studies were reserved for secondary outcome analyses to give a total of 22 articles included in this study (Supplementary Material 2, S1).
There were three primary MR studies each for type 2 diabetes16–18 and LDL cholesterol19–21; two studies each for HDL cholesterol,19,22 total cholesterol,19,23 triglycerides,19,21 and systolic blood pressure20,24; and one study each for diastolic blood pressure,25 BMI26 and circulating glucose27 ( Figure 1). The MR studies for diastolic blood pressure, BMI, and circulating glucose reported an overall estimate produced through the meta-analysis of two or more outcome cohorts. As such, the estimates from these studies were included in the present study.
Quality assessment was performed on the 12 selected studies ( Table 2). All studies addressed the three assumptions of MR. The MR results for each of the six vascular risk factors with more than one MR study were meta-analysed ( Figure 2). BMI was significantly associated with dementia as stated in the original study by Li et al. (2021), with higher BMI increasing the odds of developing dementia (1.03 [1.01, 1.05] per 5 kg/m2 increase, p = 0.00285), and met the corrected significance threshold.
Yes = Reported, No = Not reported. Each question corresponds to the questions outlined in Table 1.
| 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Are the samples used to identify the genetic IVs for the risk factor and outcome drawn from the same ethnic population? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 2. Are the outcome and exposure samples independent (2SMR)? | Y | Y | N* | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 3. Assumption 1 - Is there sufficient evidence that the variants are robustly associated with the risk factor? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 4. Assumption 2 - Do authors consider confounders of association between genetic variants and outcome, and take measures to minimize confounders? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 5. Assumption 3 - Do authors consider other biological mechanism of genetic variants other than risk factor of interest, and take measures to mitigate alternative biological mechanisms? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 6. Are variants manually picked only with known mechanism? | N | N | N | N | N | N | N | N | N | N | N | N |
| 7. Are variants pruned to only include independent instruments (i.e. account for linkage disequilibrium)? | N | Y | N | Y | Y | N | Y | Y | Y | Y | Y | Y |
| 8. Are the effect and other alleles coded in the same direction for the exposure and outcome (variable harmonization)? | Y | Y | N | N | N | N | N | N | Y | Y | N | N |
| 9. Is sensitivity analysis performed? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 10. Are data available in a supplement or by request to allow researchers to reproduce their findings? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |

UKB = UK Biobank, IGAP = International Genomics of Alzheimer's Project, HRS = Health and Retirement Study, MRC-WTCCC2 = Medical Research Council (MRC)-Wellcome Trust Case Control Consortium, IOP+ = Institute of Psychiatry Plus, ADNI = Alzheimer's Disease Neuroimaging Initiative. Copenhagen Studies = Copenhagen General Population Study and the Copenhagen City Heart Study.
Comparison of different MR studies using the same cohort (IGAP) had similar estimates (I2 = 0%) for all risk factors, apart from for LDL-c (I2 = 65.2%) and systolic blood pressure (I2 = 25.2%) ( Figure 3). The MR studies included in this analysis all fulfilled the three core assumptions of MR. Sensitivity analysis to replace MR studies using the IGAP (2013) outcome GWAS with another MR study with the most extreme values rendered the meta-analysed effect estimate for all risk factors to remain non-significant (Supplementary Material 1, Figure 1). Diastolic blood pressure, BMI and circulating glucose were excluded from sensitivity analysis because only a single study was reported for each risk factor in this study.
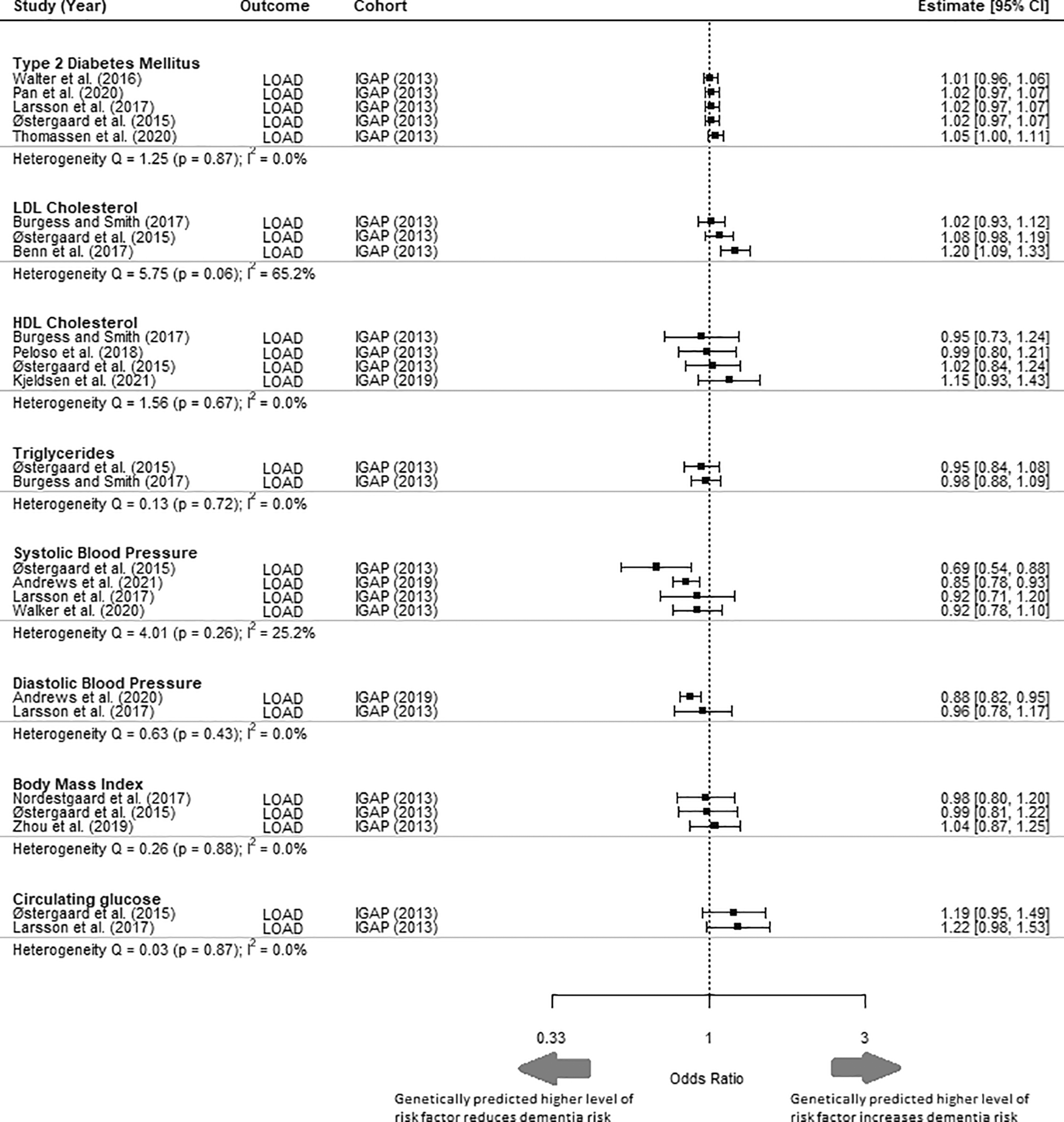
This figure includes studies that have not been selected in the meta-analysis and is shown to highlight the heterogeneity of results despite using the same outcome cohort. LOAD = Late onset Alzheimer’s disease; IGAP = International Genomics of Alzheimer's Project; CI = Confidence Interval.
For eight of the risk factors, we compared the effect estimate from MR studies with the effect estimate from the largest available meta-analysis of observational studies ( Figure 4).28–33 One risk factor, circulating glucose, did not have an eligible meta-analysis; therefore, the primary study with largest study cohort was included as the comparator.34 The units of estimates from most meta-analyses of observational studies were not explicitly stated, so it was not possible to compare the magnitude of effect with that obtained from the meta-analysis of MR studies. There were significant associations between LDL cholesterol, diastolic blood pressure diabetes and circulating glucose with a higher risk of later dementia in observational studies, but neutral associations from studies using MR.
We performed a meta-analysis of MR studies assessing the effects of nine modifiable risk factors for vascular diseases on the odds of dementia. With the exception of for BMI, we did not obtain evidence for an association between genetically predicted levels of any vascular risk factor and the odds of developing dementia.
Fewer vascular risk factors were associated with dementia based on meta-analysed estimates from MR studies than from meta-analyses of observational studies. This may be because the estimates from cohort studies were limited by residual confounding, or by a selection bias that identified populations with particularly high risk of dementia due to vascular risk factors, or by publication bias towards reporting positive results. The MR studies may have been limited by weak genetic instruments that explained only a small percentage of the variation in the risk factors of interest. For example, in Andrews et al. (2021) and Malik et al. (2021) less than 6% of the variation in blood pressure was explained by all the SNPs identified through GWASs. Moreover, these MR studies are unable to distinguish age-dependent mechanisms. For instance, high systolic blood pressure has been linked with harmful effects during mid-life but protective effects during late-life (>75).35,36 A similar case has been made for cholesterol levels.37 There is additional uncertainty regarding the reliability of GWASs themselves in which genetic instruments were derived from. GWASs may have been underpowered, resulting in unreliable identification of IV SNPs.38 The populations included in the GWASs for exposure and outcome may differ, limiting their comparability. An attempt to mitigate this issue was, however, made by using GWAS studies that focused on populations of European descent. A further consideration is that differences in model formulation between the GWASs from which IVs are identified and observational studies has the potential to limit the comparability of the phenotype under consideration.
An exploration of the consistency between the findings of MR studies that used different exposure GWASs but the same outcome GWAS (citation) found consistency between the MR estimates for all risk factors, except for LDL cholesterol and systolic blood pressure. This may be because of differences in the SNPs used as IVs for these risk factors. Sometimes differences in the SNPs used as IVs are attributable to a decision to focus on specific pathways: for example, Benn et al. (2017) focused on PCSK9 and HMGCR variants for LDL cholesterol to tackle pathways that are therapeutically relevant based on PCSK9 inhibitors and statins.39 They reported a statistically significant causative effect of LDL-c, unlike other studies that included SNPs from other genomic regions ( Figure 3). However, the choice of SNPs may also be simply due to differences in available data. For instance, Østergaard et al. (2015) obtained 24 SNPs to act as IVs for systolic blood pressure from an up-to-date GWAS at that time, while Larsson et al. (2021) obtained 93 IV SNPs from a study published in 2017. Østergaard et al. (2015) concluded that higher systolic blood pressure lowered the odds of dementia, while Larsson et al. (2021) found no significant association ( Figure 3). Therefore, recognizing these differences is important when interpreting the estimates from MR studies.
We identified relatively few eligible studies. Although many potentially relevant primary MR studies were identified, many studies used data from the same source; for example, many studies of Alzheimer’s disease use IGAP (2013).40 Therefore, many otherwise eligible studies had to be excluded to ensure independence of estimates in our meta-analysis (Supplementary Material 2, S2). The limited number of eligible studies led us to pool our two outcomes of interest (Alzheimer’s disease and dementia). Although Alzheimer’s disease constitutes 60-80% of dementia cases, a significant proportion of dementia cases are associated with different diseases with distinct pathophysiology such as vascular dementia.41 Discrepancies in pathophysiology may potentially be reflected in the heterogeneity of estimates as seen in systolic blood pressure where outcomes Alzheimer’s disease and dementia were both present (I2 = 92.6%) ( Figure 2).
Secondly, although we meta-analysed studies with unique outcome cohorts, we did not have access to individual participant data. We were therefore unable to control any potential overlap between the cohorts. Potential overlap could result in overestimation or underestimation of effect sizes, depending on the extent of shared participants and their characteristics. This could lead to inflated precision (narrower confidence intervals) without a corresponding improvement in accuracy. However, our sensitivity analyses, which replaced overlapping studies with alternative estimates where possible, showed that the overall pooled estimates remained consistent, suggesting minimal impact on the main findings.
Thirdly, we found heterogeneity between the MR estimates obtained using the same outcome cohort for two risk factors, systolic blood pressure and LDL cholesterol. This was in spite of key similarities in the study, such as only including population of European ancestry and using the same GWAS to derive the SNPs. The heterogeneity observed is likely to stem from differences in methods, such as discrepancy in rationale for selection of SNPs, p-value cutoff for the use of SNPs as IVs, and discrepancy in covariates when analysing exposure-outcome relationship. The exact effect these differences have on the estimate and its clinical implications remains yet to be characterized. There is a need to assess the effect of such discrepancies and the robustness of MR estimates through sensitivity analyses.
Out of the nine vascular risk factors assessed in this study, only genetically predicted BMI showed evidence of being causally associated with dementia. Estimates from observational studies for many risk factors were significantly associated with dementia.
Open Science Framework: Assessing the role of vascular risk factors in dementia: Mendelian randomization meta-analysis and comparison with observational estimates, https://doi.org/10.17605/OSF.IO/UA7Z6.42
This project contains the following underlying data:
Open Science Framework: PRISMA checklist for ‘Assessing the role of vascular risk factors in dementia: Mendelian randomization meta-analysis and comparison with observational estimates’, https://doi.org/10.17605/OSF.IO/UA7Z6.42
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Cognitive Impairement in older adults, NPH, Dysautonomia in DLB
Are the rationale for, and objectives of, the Systematic Review clearly stated?
No
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are the conclusions drawn adequately supported by the results presented in the review?
Yes
If this is a Living Systematic Review, is the ‘living’ method appropriate and is the search schedule clearly defined and justified? (‘Living Systematic Review’ or a variation of this term should be included in the title.)
Not applicable
Competing Interests: No competing interests were disclosed
Reviewer Expertise: Cognitive Impairement in older adults, NPH, Dysautonomia in DLB
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are the conclusions drawn adequately supported by the results presented in the review?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Vascular factors contributing to dementia
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 07 Feb 25 |
read | |
|
Version 1 24 May 22 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)