Keywords
Obesity, executive function, cognitive flexibility, working memory, verbal fluency, diet, meta-analysis
This article is included in the Agriculture, Food and Nutrition gateway.
Obesity, executive function, cognitive flexibility, working memory, verbal fluency, diet, meta-analysis
According to the 2017 global disease burden report, more than four million people die yearly from being overweight or obese. The prevalence of obesity continues to rise in adult individuals, with a global trend of 4% to 18% between 1975 and 2016 (World Health Organization, 2018). Of note, the rise of obesity prevalence is irrespective of sex, social, and economic status (Blüher, 2019). Indeed, a recent review pointed out that the economic growth and urbanization experienced by many Low Middle-Income Countries (LMICs) were significant drivers of obesity trends as they promoted unhealthy dietary patterns and sedentary physical activity (Malik et al., 2020). In general, obesity status can be defined using body mass index (BMI), although this measure is not sensitive to determine the amount of body fat (Nuttall, 2015; World Health Organization, 2018).
The effects of obesity are usually deleterious on several organ systems. Furthermore, metabolic syndrome, an obesity disorder, is often characterized by several comorbid conditions, such as large waist size, high triglyceride levels, glucose intolerance, and hypertension, has contributed to metabolic disorders. This condition is also a risk factor for developing type 2 diabetes (T2D), systemic hypertension, coronary arterial disease, and heart failure. Obesity further increases the incidence of gastrointestinal and musculoskeletal disorders, thromboembolism, stroke, cancer, and respiratory diseases, such as obstructive sleep apnea (Blüher, 2019). This disease affects the central nervous system, namely the subregions essential to learning, memory, and executive functions (EFs) (Nguyen et al., 2014; Nuttall, 2015).
Notably, a recent meta-analysis has suggested that deficits in EF domains have already been observed in adult individuals with obesity. It has been indicated that adult individuals with obesity performed inhibition tasks worse as compared to normal-weight individuals. According to pieces of literature on EF (Miyake et al., 2000; Miyake & Friedman, 2012), inhibition is defined as the ability to suppress impulsive responses. Next to that, obese individuals had impairment on tasks that require cognitive flexibility, which refers to the ability to shift attention when situationally appropriate (Yang et al., 2018). Furthermore, obese individuals lacked working memory performance, known as the ability to monitor the incoming stimuli and update information in memory as required (Yang et al., 2018). In addition to that, other EF domains have also been investigated in that meta-analysis (Collins & Koechlin, 2012; Lezak et al., 2012; Suchy, 2009; Testa et al., 2012), including i) decision making (Rangel et al., 2008), ii) verbal fluency (Troyer et al., 1997), and iii) planning (Lezak et al., 2012) suggesting that healthy individuals performed tasks related to EF domains better than obese individuals (Yang et al., 2018).
Recently, it has been proposed that weight loss may be associated with improvement in EF in obesity (Eichen et al., 2021). Various lifestyle modification approaches could be used for individuals to lose weight or overcome obesity problems, including calorie reduction and macronutrient composition balancing. The Mediterranean diet (MD) is an example of one of these methods used to treat obesity. In addition, Rodrigues et al. (2020) stated that this regimen was associated with higher structural connectivity between the left hemisphere brain regions, specifically the amygdala, lingual, middle occipital gyrus, and calcarine area (Rodrigues et al., 2020). The benefits of this diet are also positively associated with brain health, namely EFs, and white matter integrity, which are associated with taste processing and integration, reward, and decision making (Devere, 2016; Martin & Davidson, 2014). However, the effect of dietary intervention (modification) on EF domains in adult individuals with obesity remains unknown. Therefore, this systematic review and meta-analysis aimed to determine the effect of dietary modification on several EF domains in adult individuals with overweight/obesity.
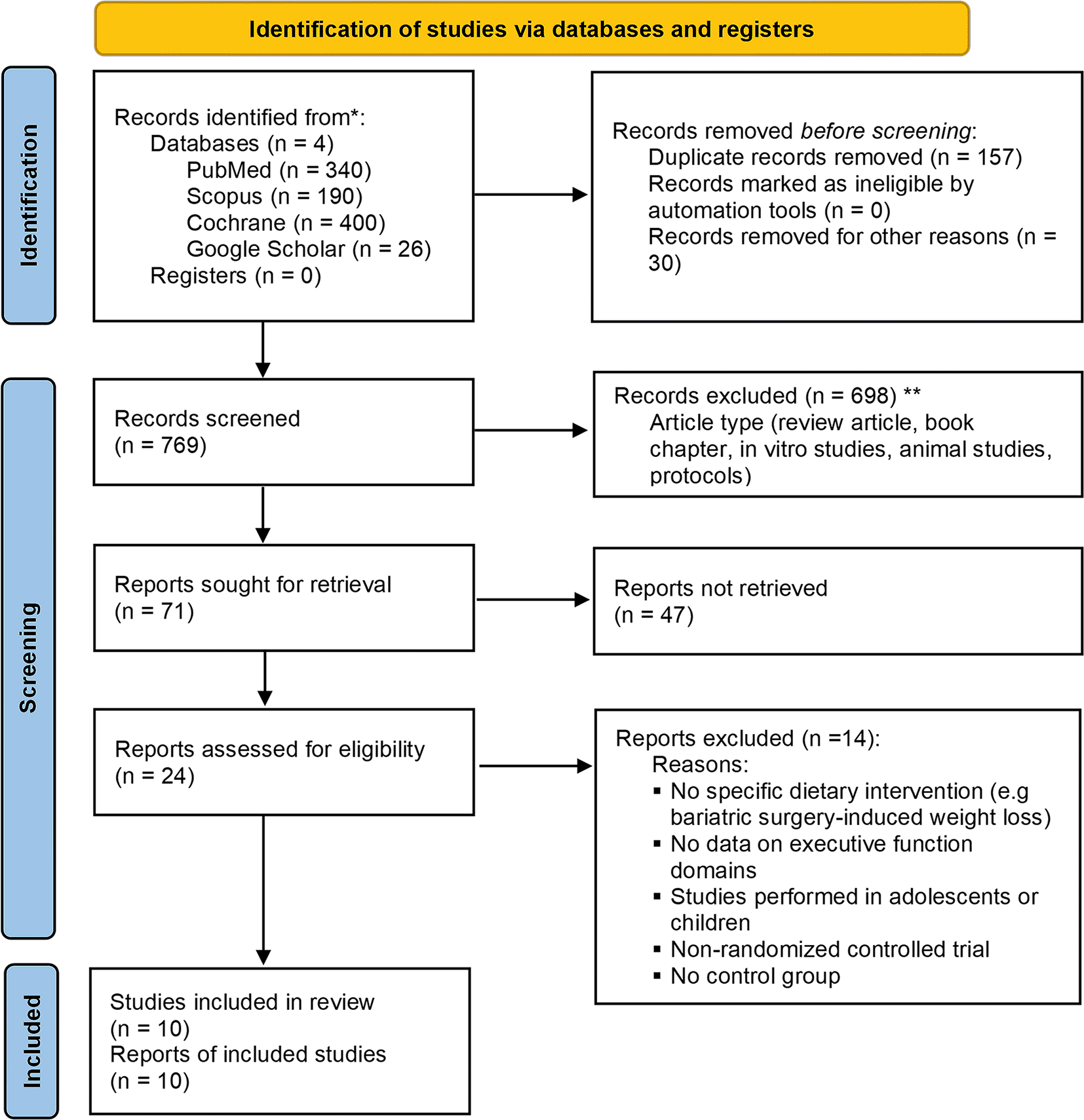
This systematic review and meta-analysis are reported in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) updated guidelines (Page et al., 2021) (completed PRISMA checklist (Gunawan, 2022) and flow chart (Gunawan, 2022)). This systematic review is registered in the PROSPERO database (CRD42022332572; 24 May 2022).
The main outcome of interest was the change in EF domains based on a previous study (Yang et al., 2018), including i) inhibition measured by several tests such as the Stroop task, the Stop Signal Task or Reaction Time Tasks, ii) cognitive flexibility as determined by Trail Making Test or Wisconsin Card Sorting Test (WCST) or Modified WCST, iii) working memory as assessed by the Digit span tasks, or the Letter–number sequencing task or memory performance test, iv) decision making as determined by Tower of London Task or Iowa Gambling Task or Delay Discontinuing Task, v) verbal fluency as measured by verbal learning test or verbal fluency test or verbal paired associates or word list fluency or verbal free recalls, and vi) planning as determined by The Wechsler Memory Scale – Fourth Edition (WMS-IV) or Tower of London Task following dietary intervention (dietary modification).
Comprehensive literature searches on Pubmed (RRID: SCR_004846)/Medical Literature Analyses and Retrieval System Online (MEDLINE) (RRID: SCR_002185), Cochrane Library (RRID: SCR_013000), Science Direct (SCOPUS), and Google Scholar (RRID: SCR_008878) were performed to identify articles from 1980 until 30 April 2022. The main keywords used were as follows: ‘overweight’, ‘obesity’, ‘EF’, ‘cognitive flexibility’, ‘inhibition’, ‘working memory’, ‘decision making’, ‘verbal fluency’, ‘planning’, ‘dietary intervention’, ‘dietary modification’, ‘Caloric restriction’, ‘Mediterranean diet’, ‘Diet’. These keywords were combined with Boolean operators (e.g., ‘OR’, ‘AND’, ‘NOT’), and all fields or Medical Subject Heading (MeSH) terms or Title/Abstract (TIAB). This set of search terms was slightly modified when we searched in every database due to a different system and technical limitations.
Eligible studies met the Patients/participants, Intervention, Comparison/control group, outcome, and Study design (PICOS) criteria: i) study was a randomized controlled trial (RCT); ii) study population consisted of individuals with overweight/obesity (without complications); iii) interventions were dietary modifications (any modes of dietary interventions) versus the appropriate control; iv) no limit of the duration for intervention (weekly, or monthly); v) measured outcomes of EF domains (inhibition and/or cognitive flexibility and/or working memory and/or decision making and/or verbal fluency and/or planning); vi) RCT; and vii) study was published in English.
Exclusion criteria were as follows: i) non-clinical trial studies; ii) studies without the outcome of EF domains (inhibition and/or cognitive flexibility and/or working memory and/or decision making and/or verbal fluency and/or planning); iii) study populations with end-stage renal disease (kidney disease), cancers, gestational diabetes mellitus (GDM), nonalcoholic steatohepatitis, cardiovascular diseases complications, and infectious diseases; iv) intervention periods of < 4 weeks; and v) study performed in children or adolescents (<18 years old).
Following the search, duplicates were removed. Titles and abstracts were screened by two authors (A.P. and W.B.G.). Based on the inclusion criteria, the final study selection was done by two authors (A.P. and W.B.G.) and approved by another author (J.M.P.). Any disagreements between the authors were resolved through discussion with other authors.
Data were extracted by two authors (A.P. and W.B.G.). Extracted data were approved by the other authors. Data extracted from each study included the following items: first author, reference, year of publication, country of study, study design, sample size, age, BMI, dietary interventions, duration, participants’ characteristics [n, sex (% male)], outcome measures of EF domains [as described i) inhibition measured by several tests such as the Stroop task, the Stop Signal Task or Reaction Time Tasks, ii) cognitive flexibility as determined by Trail Making Test or WCST or Modified WCST, iii) working memory as assessed by the Digit span tasks, the Letter–number sequencing task, memory performance test, iv) decision making as determined by Tower of London Task, Iowa Gambling Task, Delay Discontinuing Task, v) verbal fluency as measured by verbal learning tests or verbal fluency tests, verbal paired associates, word list fluency, verbal free recalls, and vi) planning as determined by WMS IV-VWMIS, Tower of London Task] (Table 1).
| No | Author, Year | Country | Subject characteristics | Intervention characteristics | Intervention outcomes | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age range | Mean age, years | Sex (M/F) | % M | Mean body mass index (BMI) | Type of diet intervention | Diet rules | Duration of study | Executive function domain tests | |||
| 1. | Kretsch et al., 1997 | USA | 25–42 years old | Treatment (T) = 35.0±5.2; Control (C) = 30.1±7.5 | F | 0 | T = 31.5±4.1; C = 34.2±1.9 | Caloric restriction (T) | Caloric restriction (63% or 43% energy from carbohydrate 18–20% protein); an American-type menu consisting of conventional food was fed throughout the study. | 18 weeks (without 3-week weight stabilization period) | |
| 2. | Bryan & Tiggemann, 2001 | Australia | Not available (NA) | T = 48.9±8.2; C = 50.9±7.3 | F | 0 | T = 34.1±4.3; C = 35.2±4.8 | Caloric restriction-induced weight loss | 15% fat, weight reducing diet. Guidelines for the diet were presented in written form and during regular twice-weekly consultations with a dietician. Participants were presented with lists of foods to be included in their diet and the frequency with which they should be eaten, foods that should be avoided and a sample menu. The diet was designed to produce a 20% energy deficit. | 12 weeks | |
| 3. | Cheatham et al., 2009 | USA | 20–42 years old | 34.9±4.3; 34.6±5.5 | NA | NA | 27.7±1.7; 27.9±1.4 | High glycemic; Low glycemic | Energy from carbohydrate 60, protein 20, fats 20 (%); Energy from carbohydrate 40, protein 30, fats 30 (%) | 12 months | |
| 4. | Smith et al., 2010 | USA | NA | 51.7±9.0 | 15/27 | 35 | 32.7±3.9 | 4 months | |||
| 52.3±9.5 | 15/23 | 39 | 32.8±3.4 | Dietary Approaches to Stop Hypertension (DASH) | Follow DASH Rules | ||||||
| 5. | Green and Elliman, 2013 | UK | 20–45 years old | NA | F | 0 | Baseline: 27.17±0.62; Week-8: 27.05±0.67 | Diet-receiving group (D) | D: Commercially available organised weight-loss group for this period. This commercial weight-loss plan comprised an energy-restricted, nutritionally balanced diet in conjunction with weekly weighing and group support sessions. No foods were provided to the participants. | 8 weeks | |
| Baseline: 29.27±0.62; Week-8: 29.30±0.67 | C: No diet | ||||||||||
| 6. | Napoli et al., 2014 | USA | ≥65 years old | 69±4 | 9/18 | 33.33 | 37.3±4.7 | C | No diet rules | 12 months | |
| 70±4 | 9/17 | 34.62 | 37.2±4.5 | D | Prescribed a diet that provided an energy deficit of 500–750 kcal/d from daily requirements | ||||||
| 70±4 | 10/16 | 38.46 | 36.9±5.4 | Exercise | Structured exercise | ||||||
| 70±4 | 12/16 | 42.86 | 37.2±5.4 | Exercise + diet | Structured exercise + prescribed a diet that provided an energy deficit of 500–750 kcal/d from daily requirements | ||||||
| 7. | Boraxbekk et al., 2015 | Sweden | 52–69 years old | 61.1±1.6 | F | 0 | 31.9±1.7 | Paleolithic diet | 30% protein, 40% fat, 30% carbohydrate, high monounsaturated fatty acid (MUFA) & polyunsaturated fatty acid (PUFA) | 6 months | |
| 61.6±1.7 | 32.4±2.4 | Standard (The Nordic Nutrition Recommendations) | |||||||||
| 8. | Prehn et al., 2017 | UK | 40–80 years old | 61±6 | F | 0 | 34.7±4.3 | Caloric restriction | 8–12 weeks: low caloric formula diet (800 kcal/day), all meal replaced by very low energy drink. After 12 weeks: isocaloric diet | 16 weeks | |
| 61±4 | 35.0±3.7 | C | |||||||||
| 9. | Soldevila-Domenech et al., 2021 | Spain | 55–75 years old (M) 60–75 years old (F) | 65.2±4.7 | 241/246 | 50 | Overweight/Obesity | Mediterranean diet | 30% calorie reduction | 6 years intervention 2 years follow-up | |
| C | |||||||||||
| 10. | Arjmand et al., 2020 | Iran | 40–60 years old | C = 48.95±1.07 | F | 0 | C = 32.55±3.51 | C | Plant-based food | 3 months | |
| D = 48.86±1.56 | D = 32.32±4.35 | Mediterranean – DASH Diet (MIND) | |||||||||
The quality of selected RCTs was assessed independently by two authors (A.P. and W.B.G.) using the risk of bias checklist (RoB 2.0) from the Cochrane Collaboration (Sterne et al., 2019). The quality assessments of the checklist included: i) bias from the randomization process, ii) bias due to deviations from intended interventions, iii) bias due to missing outcome data, iv) bias from measurements of the outcome, v) bias from the selection of reported result. Each criterion could be answered in three ways: yes (adequate information), no (inadequate information), or unclear (some concerns). Study quality and the risk of bias in the eligible RCTs were systematically assessed using RoB 2.0 tools (MS Excel format).
To calculate the effect size of each study, we used the mean change and standard deviation (SD) change of the outcome measures from baseline to the end of the intervention in the control and intervention groups. When the outcome measure was reported as median and range or 95% confidence interval (CI), mean and SD values were estimated using the method described previously (Wan et al., 2014). If the standard error of the mean (SEM) was reported, the SD was estimated using the following formula: SD = SEM x square root (n), where n is the number of subjects (Wan et al., 2014). SDs of the mean difference were estimated using the following formula: SD = square root [(SD pre-treatment)2 + (SD post-treatment)2) - (2R * SD pre-treatment * SD post-treatment)]. Because the pretest-posttest correlation coefficients (r) were not reported in studies, an r-value of 0.5 was assumed throughout this meta-analysis.
A meta-analysis was conducted to combine the individual study results using Stata version 16 software (RRID:SCR_012763; StataCorp LLC). Alternatively, RStudio (RRID:SCR_000432) can be used as a free alternative. If a study included more than two intervention groups (e.g., diet modification and exercise), groups were included compared to the placebo (control) group is exercise. If the outcome measurement was performed multiple times after the intervention period, we only used the endpoint after the intervention.
Random-effects models (DerSimonian-Laird method) were used to estimate outcomes. Heterogeneity was assessed using the I2 index, with values above 60% indicating substantial heterogeneity. The Egger test’s funnel plot followed by a meta-bias analysis was performed to detect publication bias. The effect size is reported as the standardized mean difference with its 95% confidence interval (Higgins et al., 2022). A p-value < 0.05 is considered statistically significant. The forest plots and funnel plots will be produced using Stata version 16 software.
In the initial search, a total of 956 publications have been reported in PubMed (340); Cochrane library (400); SCOPUS (190); and Google Scholar (26). After removing duplicate and unrelated titles and screening the titles and abstracts, about 71 articles were selected. The authors read the full text of these selected articles and selected 10 eligible articles (Arjmand et al., 2022; Boraxbekk et al., 2015; Bryan & Tiggemann, 2001; Cheatham et al., 2009; Green & Elliman, 2013; Kretsch et al., 1997; Napoli et al., 2014; Prehn et al., 2017; Smith et al., 2010; Soldevila-Domenech et al., 2021) to be qualitatively and quantitatively synthesized. The detail of the study selection process can be seen in Figure 1.
Characteristics of these studies (10 studies) are shown in Table 1. The age and mean BMI of participants were comparable between studies. The types of dietary intervention (diet modification) varied between studies. The duration of studies ranged between eight weeks and six years (Arjmand et al., 2022; Boraxbekk et al., 2015; Bryan & Tiggemann, 2001; Cheatham et al., 2009; Green & Elliman, 2013; Kretsch et al., 1997; Napoli et al., 2014; Prehn et al., 2017; Smith et al., 2010; Soldevila-Domenech et al., 2021). The outcomes of EF domains varied between studies. There are limited data on the ethnicity of subjects within the study intervention. However, the study’s country of origin was described as The United States of America (three studies), Australia (two studies), The United Kingdom (two studies), Spain, Sweden, Japan, and Iran.
All 10 studies (Arjmand et al., 2022; Boraxbekk et al., 2015; Bryan & Tiggemann, 2001; Cheatham et al., 2009; Green & Elliman, 2013; Kretsch et al., 1997; Napoli et al., 2014; Prehn et al., 2017; Smith et al., 2010; Soldevila-Domenech et al., 2021) reported the use of several types of dietary modifications such as caloric restriction (Bryan & Tiggemann, 2001; Kretsch et al., 1997; Prehn et al., 2017), The Paleolithic Diet (Boraxbekk et al., 2015), The MD (Soldevila-Domenech et al., 2021), the Dietary Approaches to Stop Hypertension (DASH) Diet (Smith et al., 2010) as well as modified Mediterranean – DASH Diet (Arjmand et al., 2022). Dietary modification (dietary intervention) was designed for weight loss in all studies (Arjmand et al., 2022; Boraxbekk et al., 2015; Bryan & Tiggemann, 2001; Cheatham et al., 2009; Green & Elliman, 2013; Kretsch et al., 1997; Napoli et al., 2014; Prehn et al., 2017; Smith et al., 2010; Soldevila-Domenech et al., 2021). The duration of intervention ranged from eight weeks to six years. Most of the studies (except Arjmand et al., 2022) have reported a higher dropout rate, whereas those who left the study either did not comply with the dietary protocol during dietary modification (dietary intervention) or left for personal reasons. More importantly, no major adverse effect of the dietary modification (intervention) program was reported. This suggests that all dietary modifications are considered safe for adult individuals with overweight/obesity.
Furthermore, changes in EF domains were determined by varied tasks of inhibition domain, cognitive flexibility, working memory, decision making, verbal fluency, and planning (Arjmand et al., 2022; Boraxbekk et al., 2015; Bryan & Tiggemann, 2001; Cheatham et al., 2009; Green & Elliman, 2013; Kretsch et al., 1997; Napoli et al., 2014; Prehn et al., 2017; Smith et al., 2010; Soldevila-Domenech et al., 2021). Among these outcomes, the decision-making and planning domains were not quantitatively synthesized because the data from included studies could not be extracted. Based on qualitative analysis, the decision-making domain tested by Iowa Gambling Task (IGT) by (Soldevila-Domenech et al., 2021) suggests an improvement following three years of the MD trial. Of interest, the planning domain assessed by The Tower of London task (Green & Elliman, 2013) showed no differences between a supervised-diet group and the control group.
The effect of dietary modification (diet interventions) on EF domain inhibition
In this meta-analysis, the domain inhibition of EF was determined based on the Stroop test and reaction time test. About eight RCTs described the outcome of the Stroop test (five studies) (N dietary intervention 361 vs. N control 279) and reaction time test (three studies) (N dietary intervention 44 vs. N control 37). Based on a random-effect model in this meta-analysis, dietary modification (dietary intervention) did not improve the inhibition domain of EF as measured by the Stroop test and Reaction Time Test (the Stroop test SMD: −0.055; 95% CI: −0.356 to 0.246, P=0.721; Reaction Time Test SMD −0.527; 95% CI: −1.423 to 0.369, P=0.249) (Figure 2a and 2b, respectively).
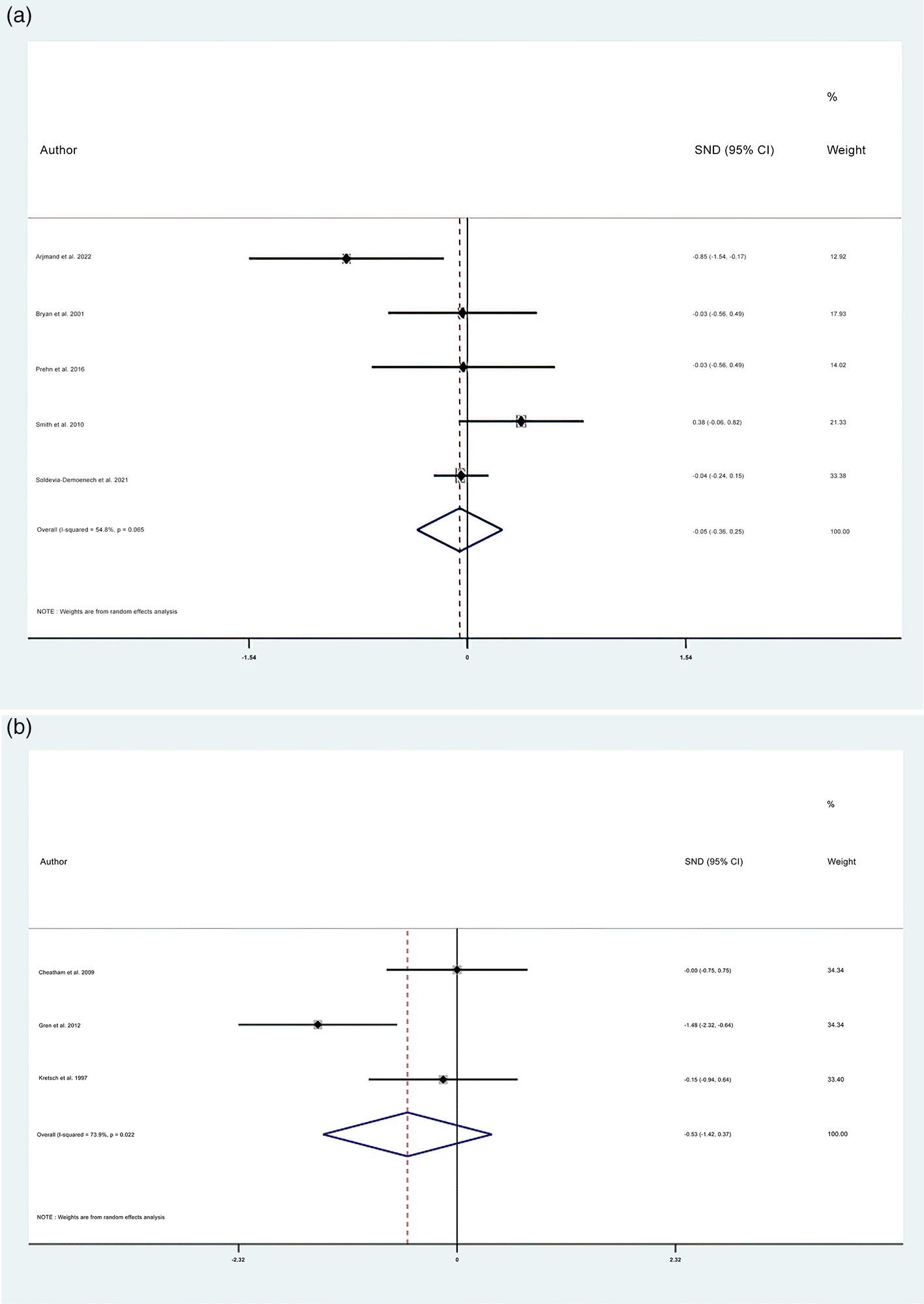
(a) Forest plots of SMD of inhibition domain measured by Stroop test between intervention and control groups (represented as Diamond). Horizontal lines span individual study 95% confidence intervals. (b) Forest plots of SMD of inhibition domain measured by reaction time tests between intervention and control groups (represented as Diamond). Horizontal lines span individual study 95% confidence intervals.
The effect of dietary modification (diet interventions) on EF domain cognitive flexibility
About six RCTs described the outcome of the cognitive flexibility domain of EF (N dietary intervention 161 vs. N control 154) was determined based on the Trail Making Test. Based on a random-effect model in this meta-analysis, dietary modification (dietary intervention) decreases the score of the Trail Making Test (SMD: −0.303; 95% CI: −0.527 to −0.079, P=0.008), with no heterogeneity observed (I2=0%, P=0.513) (Figure 3). This result implies that dietary modification (dietary intervention) resulted in an improvement of the cognitive flexibility domain of EF.
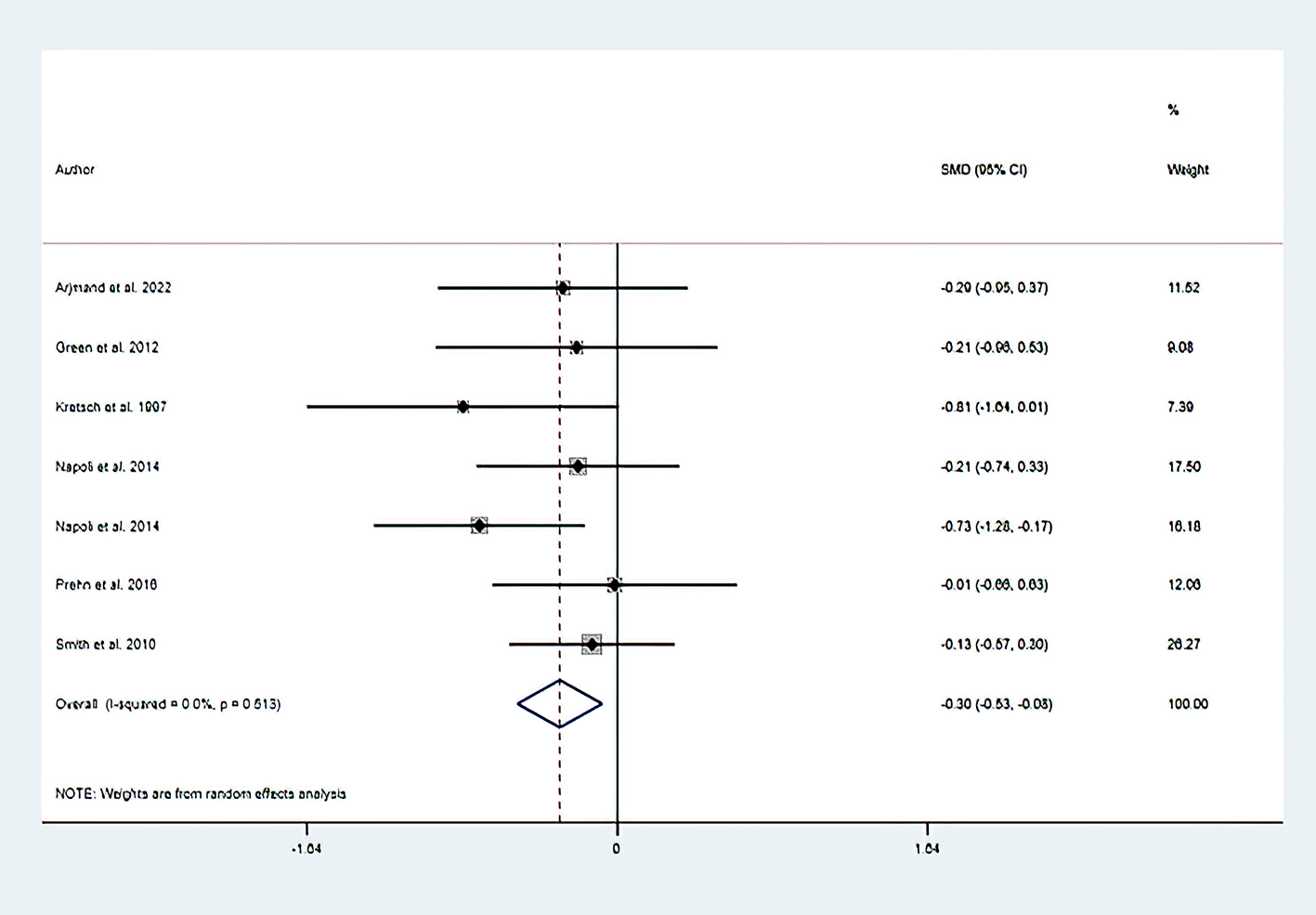
Horizontal lines span individual study 95% confidence intervals.
The effect of dietary modification (diet interventions) on EF domain working memory
In this meta-analysis, about seven RCTs described the outcome of EF’s working memory domain, which was determined based on the Digit Span test, the Letter Number Sequencing Task. Based on a random-effect model in this meta-analysis, dietary modification (dietary intervention) (N dietary intervention 260 vs. N control 193) improves the working memory domain of EF (SMD: 0.601; 95% CI: 0.076 to 1.125, P=0.025), with substantial heterogeneity observed (I2=83.6%, P=0.000) (Figure 4).
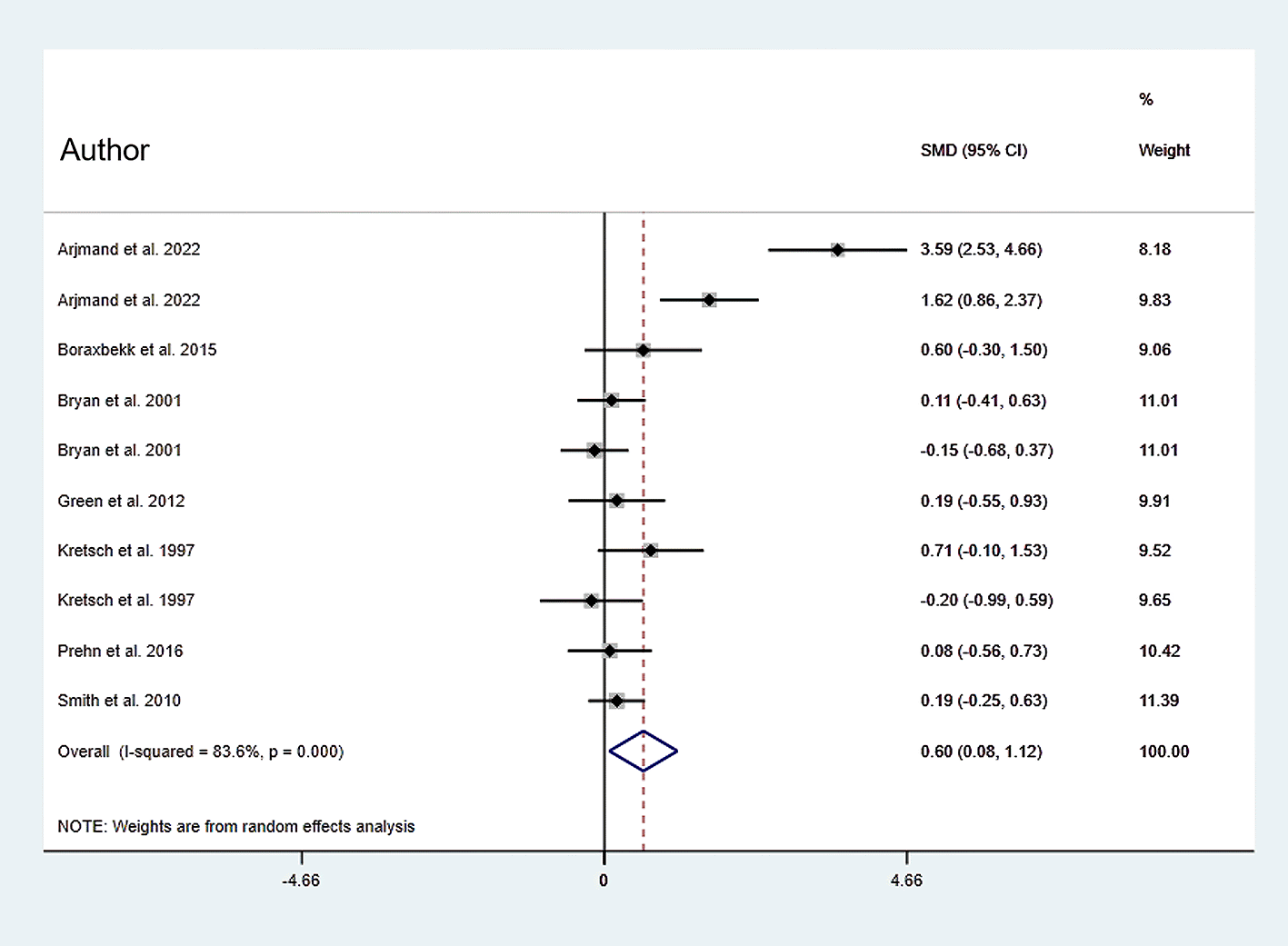
Horizontal lines span individual study 95% confidence intervals.
The effect of dietary modification (diet interventions) on EF domain verbal fluency
About six RCTs described the outcome of the verbal fluency domain of EF that was measured based on verbal learning test (Auditory Verbal Learning Test, Verbal initial and excluded letter fluency, Verbal Fluency test, Animal naming, Verbal paired associates, and control oral word association). Based on a random-effect model in this meta-analysis, dietary modification (dietary intervention) (N dietary intervention 274 vs. N control 234) enhances the verbal fluency domain of EF (SMD: 0.223; 95% CI: 0.002 to 0.428, P=0.033), with low heterogeneity observed (I2=16.3%, P=0.297) (Figure 5).
The output of the improved EF domains from most included studies suggests that the effect of all dietary modification (dietary intervention) may be similar and not superior to other dietary modification regimes. Due to the limited number of selected articles, we could not perform a subgroup analysis to determine that question. However, perhaps a varied improvement of EF domains may also suggest that more specific approaches might be taken into account when implementing a dietary modification to improve specific domains of EF in adult patients with overweight/obesity.
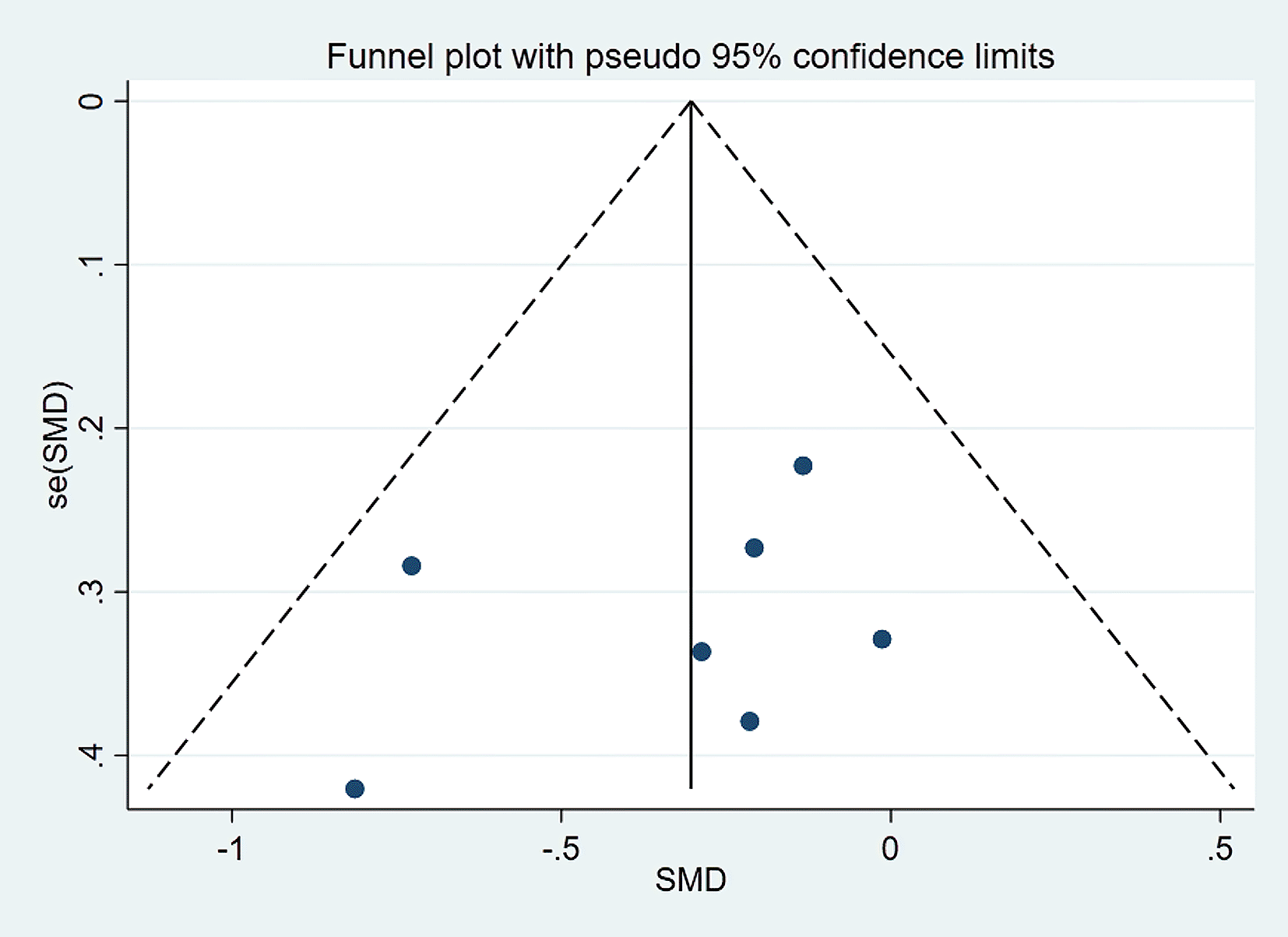
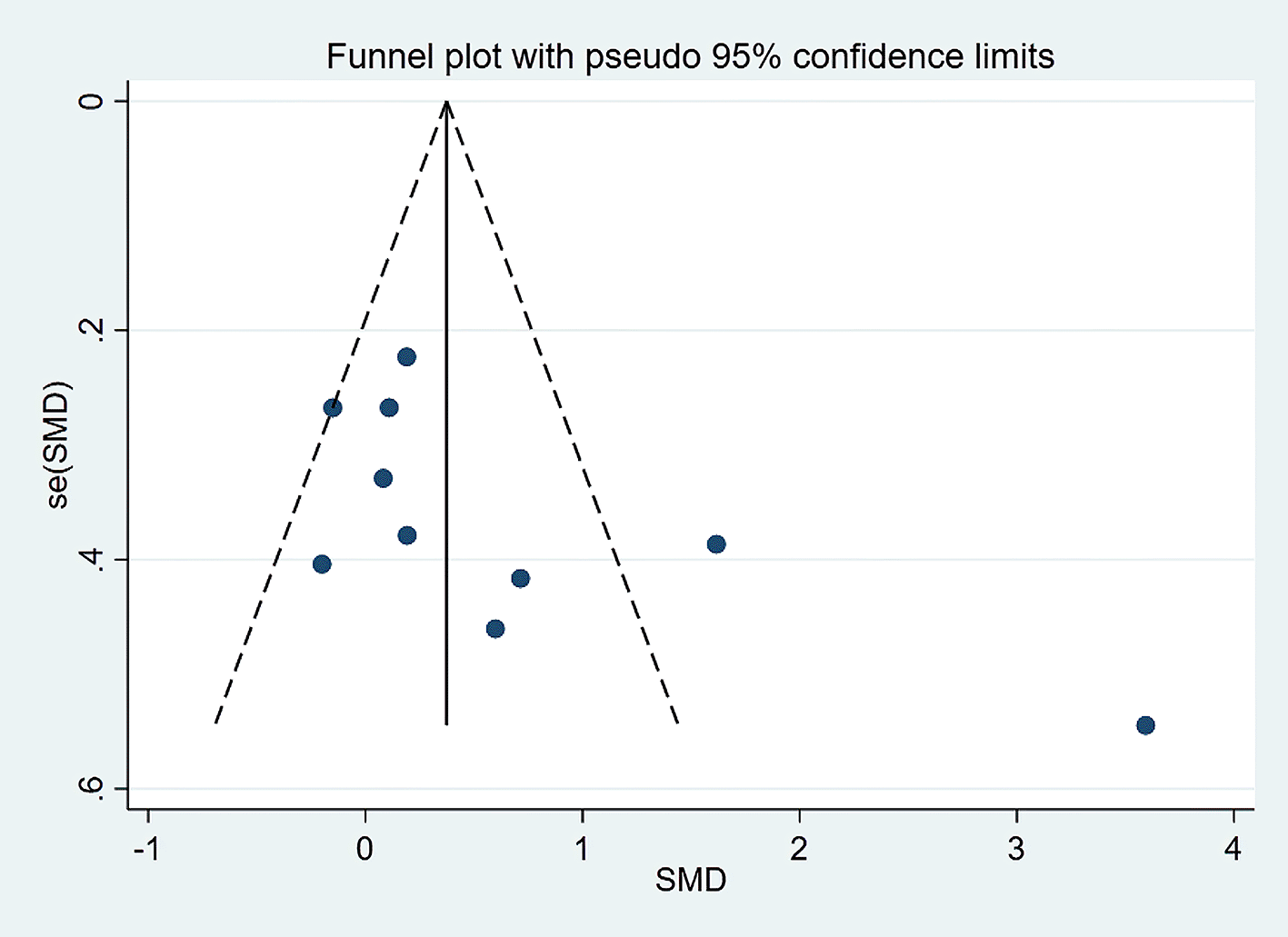
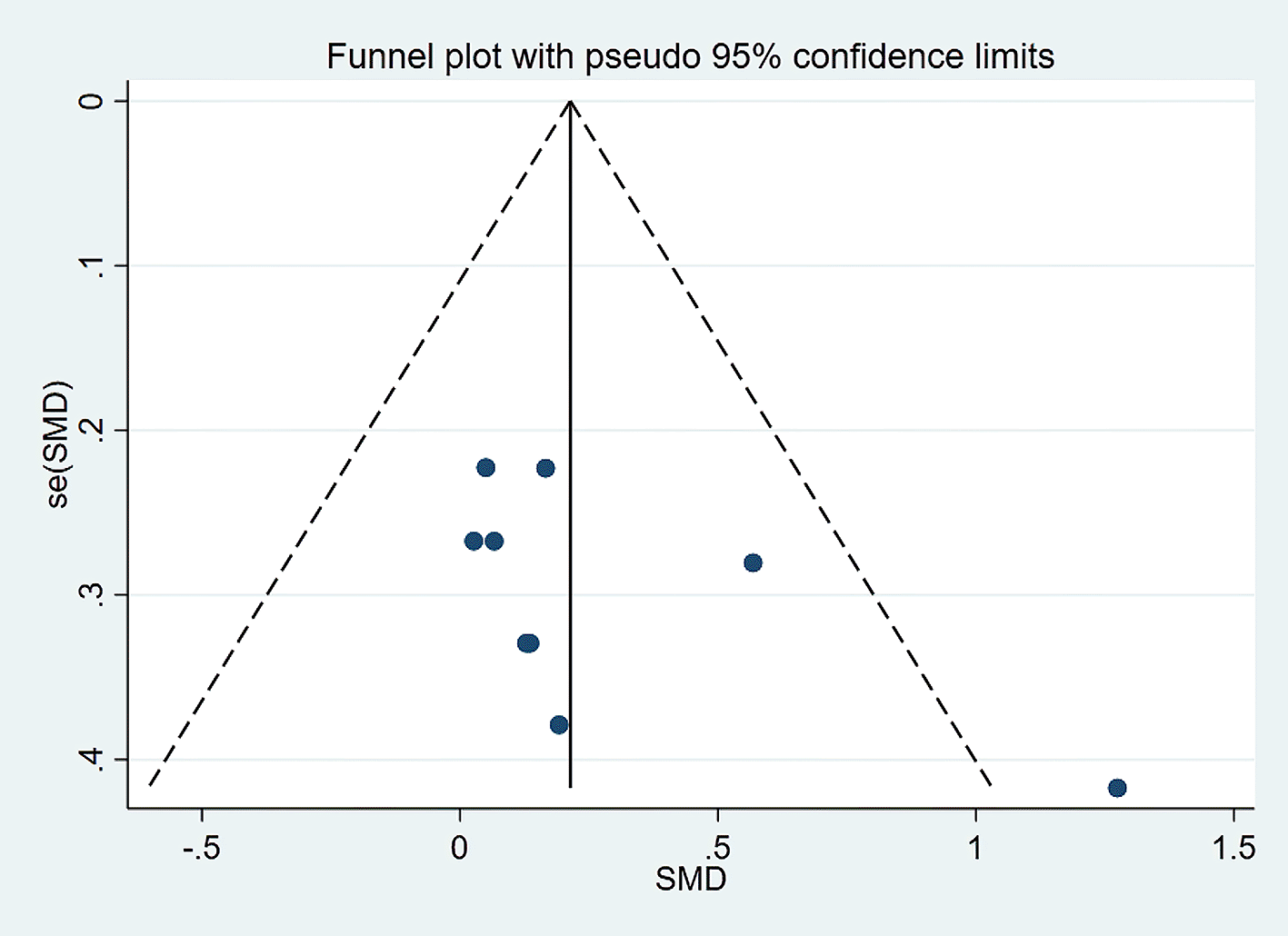
Visually inspected funnel plot symmetry did not indicate any potential publication bias for the comparison of EF domains, inhibition domain, cognitive flexibility, and verbal fluency, between the dietary modification (intervention) groups and control groups (Figures 6-9). Regarding the outcome of working memory, the further inspection of the funnel plot, including a meta-bias analysis using the Egger test, illustrates publication bias from included studies (Egger test Root MSE=2.021 P=0.049).
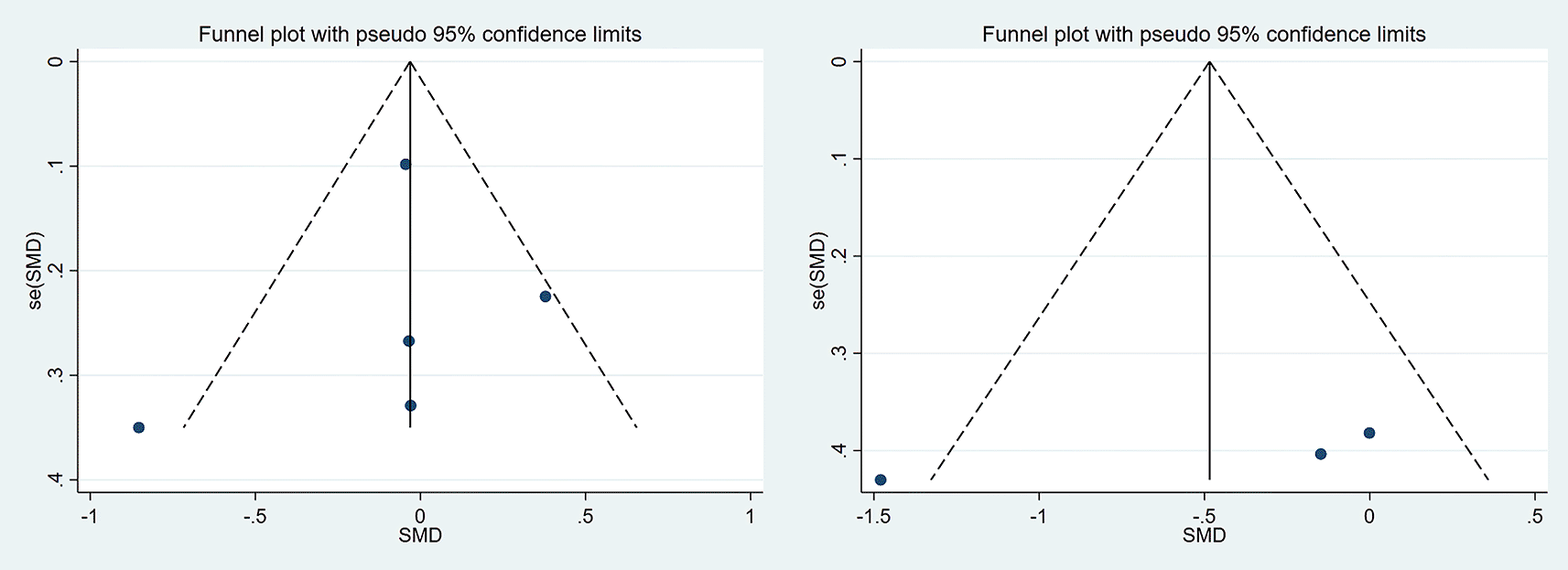
SMD, standardized mean difference.
SMD, standardized mean difference.
SMD, standardized mean difference.
SMD, standardized mean difference.
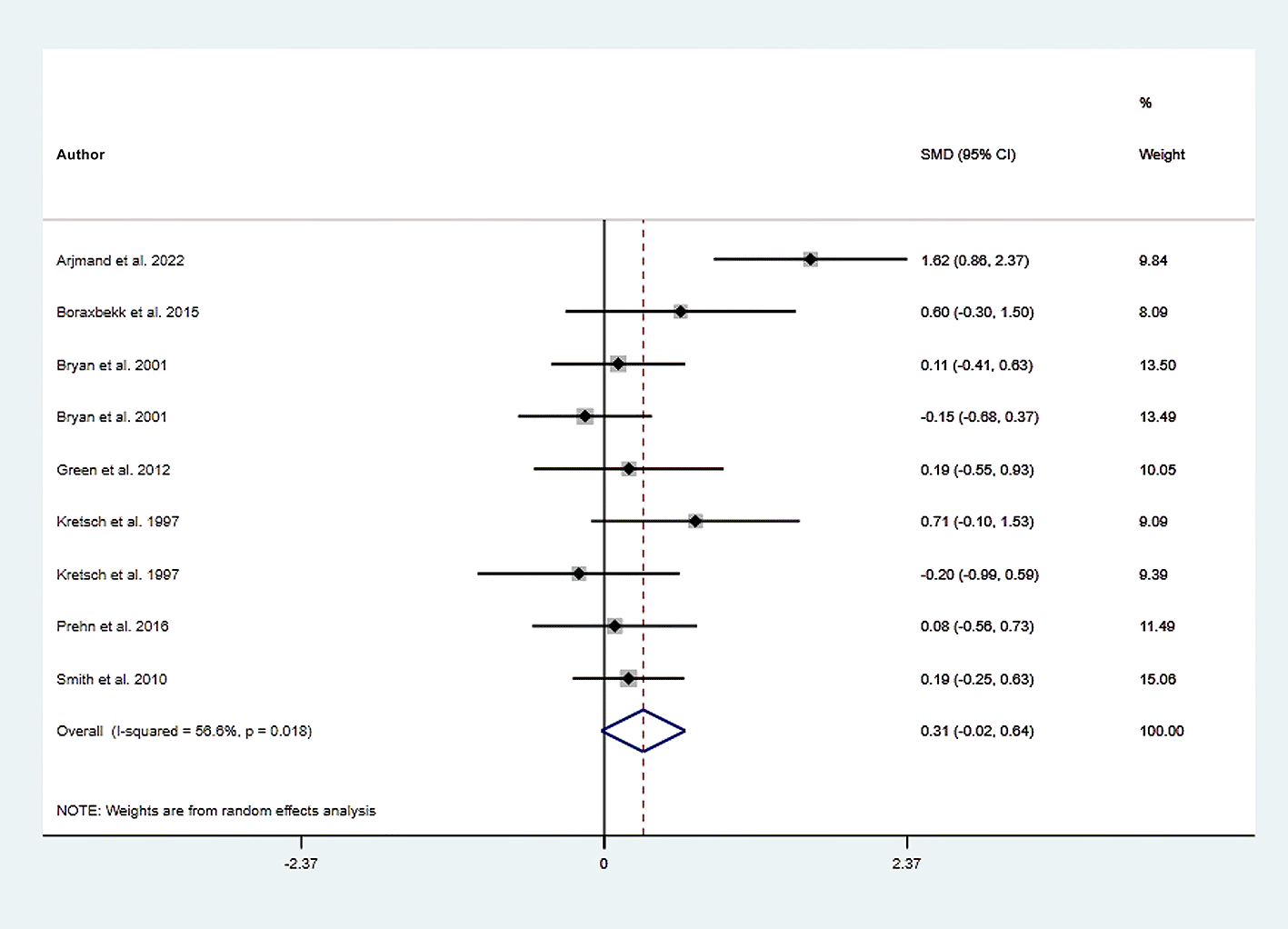
Horizontal lines span individual study 95% confidence intervals.
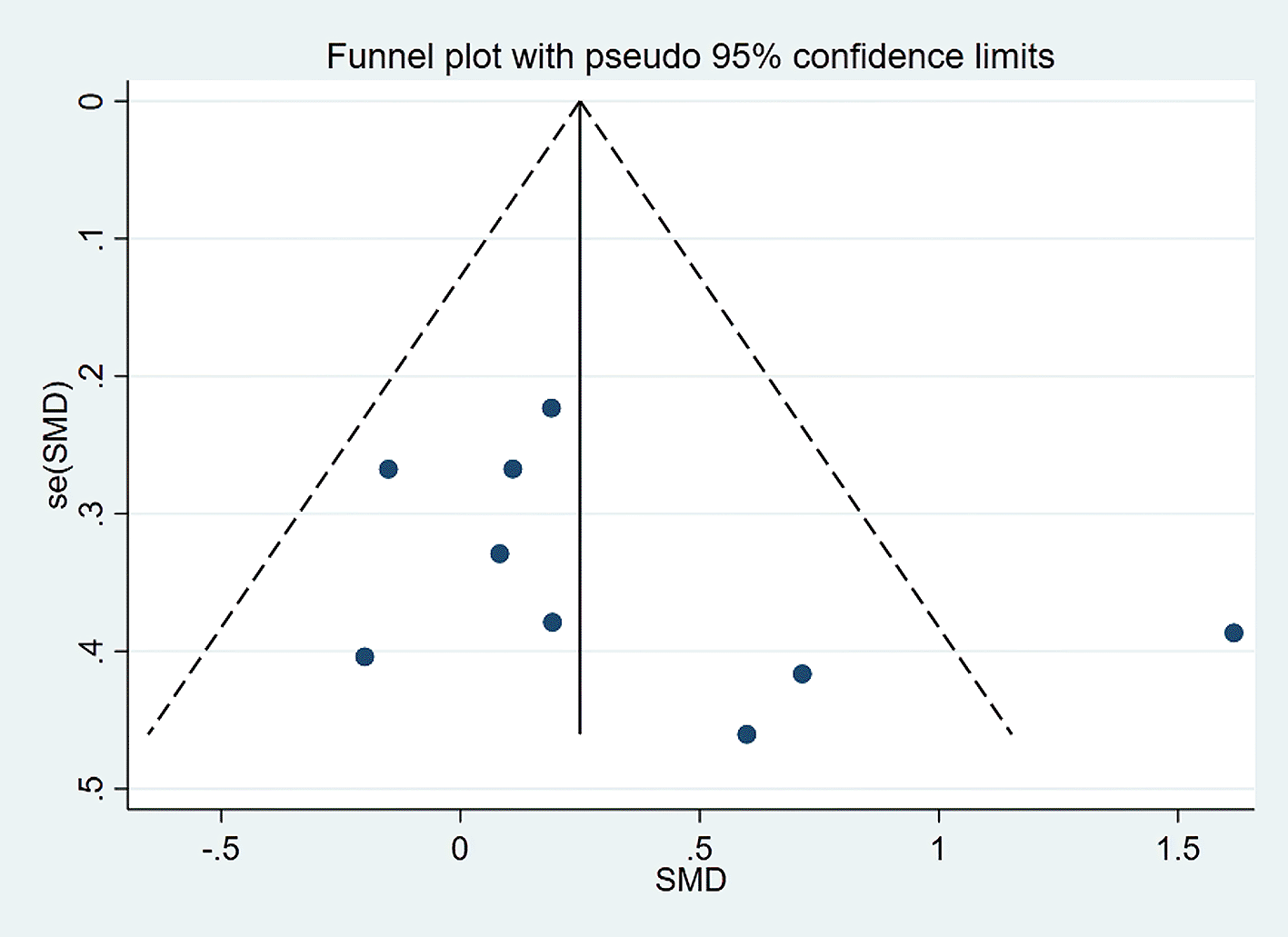
We excluded the study in which the outcome had the highest effect size, and the interpretation was slightly changed to address that issue. We found that working memory was slightly improved in the dietary intervention (dietary modification) group as compared to the control group (SMD=0.306; 95% CI −0.024 to 0.635, P=0.069) with moderate heterogeneity (I2=56.6%, P=0.018). Further funnel plot inspection and the Egger-test analysis revealed no publication bias was observed (Root MSE=1.457, P=0.235) (Figures 10 and 11).
Risk of bias assessment (RoB 2.0)
Overall, the RoB assessment using RoB 2.0 tools of the included studies using the Cochrane risk of bias tool 2020 showed some concerns in six out of 10 studies (Figure 12). This assessment illustrated only four out of 10 studies with a low risk of bias. Several domains such as “randomization processes”, “allocation concealment” and “deviations from intended intervention” were recognized as sources of concern. In addition, this type of study (i.e., dietary intervention/dietary modification) trials, the domains that mostly contributed to concern were the “blinding of participants and personal (performance bias)” as well as “blinding of outcome assessment (detection bias)”. These can be attributed due to the nature of this type of intervention (dietary modification/dietary intervention).
Quality of evidence assessment
The study’s quality assessment was evaluated using the GRADE assessment based on the Cochrane Handbook 2022 (Higgins et al., 2022) in a narrative format with overall analysis results on all selected articles. This software assessed the article results, which are following the initially set research outputs according to PICOS, such as changes in several domains (inhibition, cognitive flexibility, working memory, verbal fluency, decision-making, and planning) of the EF after dietary intervention (dietary modification). All outputs were measured for quality in each selected article by assessing the risk components of bias, inconsistency, indirectness, and impressions. Subsequently, the outputs were grouped based on the outcome priorities. We also determined whether each output had a very low, low, medium, or high certainty level (Schünemann et al., 2013) (Table 2). Table 2 describes the quality of evidence assessment based on GRADE. We evaluated the assessment of each effect of dietary modification on several domains of the EF. No serious issues were observed concerning the components of inconsistency and indirectness since the results were relatively consistent between studies and all participants were adults with overweight/obesity. Regarding impression, four (inhibition, cognitive flexibility, working memory, and verbal fluency) out of six domains in the EF domains can be determined with meta-analyses. We could not perform meta-analyses for outcome decision-making and planning. Furthermore, the majority of included studies had some concerns, as depicted in Figure 12. Therefore, the intervention treatment (dietary modification) might be implemented and with careful attention on some concerns such as randomization, allocation concealment and high rate of dropout. Table 2 shows that all outcomes had distinct certainty values of the selected articles.
Based on the risk of bias assessment, the two outputs did not pose a serious risk because a randomization technique was conducted in the study design. The output assessment was also evaluated with an objective tool to minimize the risk of bias. No serious issue was observed concerning the components of inconsistency and indirectness since the results were relatively consistent between studies and all participants were adults with overweight/obesity. Regarding impression, four (inhibition, cognitive flexibility, working memory, and verbal fluency) out of six domains in the EF domains could be determined with meta-analyses. We could not perform meta-analyses for the outcome of decision-making and planning. Furthermore, the majority of included studies had some concerns, as depicted in Figure 6. Therefore, the intervention treatment (dietary modification) may be implemented with low-risk precision. Table 1 shows that all outcomes had distinct certainty values in the selected articles.
This systematic review and meta-analysis reported findings regarding the effect of dietary modification (dietary intervention) on the EF domains in adult individuals with overweight/obesity. This meta-analysis determined the EF domains as described in literature such as inhibition, cognitive flexibility, working memory, verbal fluency, decision-making, and planning. Overall, this meta-analysis showed that the dietary intervention (dietary modification) was safe to implement in adult individuals with overweight/obesity. Furthermore, we observed improvements in cognitive flexibility, working memory, and verbal fluency after dietary intervention in the treatment groups as compared to control groups. By contrast, we did not see any dietary intervention effects on the EF inhibition domain. We could not draw results from the decision-making and planning domains due to a limited number of studies to perform a meta-analysis.
A growing body of evidence suggests that being overweight and obese are linked to cognitive decline and an increased risk of vascular dementia and Alzheimer’s disease, particularly in middle-aged people (Pedditizi et al., 2016). Notably, the incidence of dementia in those under the age of 65 years was associated with obesity according to a previous meta-analysis (Pedditizi et al., 2016). Furthermore, in another systematic review and meta-analysis, there was a clear association between overweight/obesity and the EF domains (Favieri et al., 2019). Supporting this evidence, a recent meta-analysis also demonstrated that overweight/obesity had the worse response regarding several domains of the EF (Yang et al., 2018).
A recent review has indicated that the EF domains may be associated with lower weight loss (Eichen et al., 2021). Losing bodyweight is still recognized as the main target of treatments for overweight/obesity. There are several weight loss treatment programs; bariatric surgery, pharmacological treatment, and behavioral weight loss (BWL), including dietary modification (National Institute for Health and Care Excellence, 2020; Tchang et al., 2021). Currently, behavioral weight loss is still the recommended behavioral treatment for obesity. One strategy to establish behavioral weight loss is by implementing dietary modifications. The majority of human trials on dietary modification-induced weight loss are by implementing caloric restriction. Indeed, it has been shown that caloric restriction may reduce visceral adipose tissue (VAT) (Ard et al., 2018). The optimum weight reduction as a result of dietary modification strategy is varied for each individual. Several reports determine that a 5 to 10% weight loss is adequate enough to influence the clinical outcomes (Haase et al., 2021).
In this meta-analysis, we found that dietary modification rules varied between studies. Notably, several included studies employed a combination of specific diets such as MD or DASH diet and caloric restriction (Arjmand et al., 2022; Soldevila-Domenech et al., 2021). A meta-analysis determined that adherence to the MD increased working memory and verbal cognition compared to the control group (Loughrey et al., 2017). Of note, populations in that meta-analysis were individuals with relatively healthy/normal BMI.
In addition, several studies have reported that the direct effect of weight reduction on cognition is biologically plausible (Horie et al., 2016; Siervo et al., 2011). Accordingly, BMI reduction during the intervention period might be associated with improvements in working memory. It may also be that cognitive improvements accumulate based on time course. Our results also suggest that weight loss is often accompanied by the dietary intervention (dietary modification) and was associated with the EF domains.
The EF consists of several domains (Miyake et al., 2000; Miyake & Friedman, 2012), inhibition is defined as the ability to suppress impulsive responses. Next to that, obese individuals had impairment on tasks that require cognitive flexibility, which refers to the ability to shift attention when situationally appropriate (Yang et al., 2018). Furthermore, obese individuals lacked working memory performance, known as the inability to monitor the incoming stimuli and update information in memory as required (Yang et al., 2018). In addition to that, other EF domains have also been investigated in that meta-analysis (Collins & Koechlin, 2012; Lezak et al., 2012; Suchy, 2009; Testa et al., 2012), including i) decision making (Rangel et al., 2008), ii) verbal fluency (Troyer et al., 1997), and iii) planning (Lezak et al., 2012), suggesting that healthy individuals performed tasks related to EF domains better than obese individuals (Yang et al., 2018).
Several tools were used in the assessment of cognitive performance, such as Rey’s auditory verbal learning test (RAVLT) for evaluating short-term and long-term auditory memory. The Rey-Osterrieth complex figure test (RCFT) was used to assess visuospatial function, the symbol digit modalities test (SDMT) to measure thinking speed, and the Stroop color-word interference test to evaluate inhibitory and attentional functions. Furthermore, the IGT was another tool used to assess decision-making abilities, the conner’s continuous auditory test of attention (CPT) evaluated attention, impulsivity, and alertness, while the screening assessment of general cognitive function was examined with a mini-mental state examination (MMSE) (Soldevila-Domenech et al., 2021).
The EF outputs in the selected articles showed improvements in the attention, decision-making, and speed of thinking performances through the CPT – commission errors, IGT, and SDMT examinations, respectively. Meanwhile, the inhibition function through the Stroop test did not show any statistically significant changes. This finding is in accordance with several hypotheses, which showed that cognitive function and obesity have a two-way relationship, where this condition results in mental decline and vice versa. According to Kamijo et al. (2014), an impaired inhibitory control function in the prefrontal cortex can lead to excessive caloric consumption, which significantly results in weight gain (Kamijo et al., 2014). Volkow et al., 2011 further revealed that imaging studies in obese patients indicate hypoactivation of dopamine D2 receptors and decreases neural metabolism in areas involved in EF. Dopamine also plays a role in the “reward system”, therefore, disruption of its production can impact excessive calorie consumption (Schiff et al., 2015; Volkow et al., 2011). Another hypothesis by Gonzales et al. (2010) depicted that obesity can affect insulin resistance, inflammatory processes, changes in cerebral areas related to analytical functions, and cerebrovascular blood flow, resulting in structural modifications (Gonzales et al., 2010).
Stinson et al. (2018) mentioned that prolonged incorrect food consumption is connected with excessive body weight maintenance, which leads to decreased EF performance and impaired behaviors. Furthermore, these factors are linked to an increased risk of cognitive impairment in old age (Sanderlin et al., 2017) and trouble reacting correctly to external stimuli. These traits are indicative of impaired EFs, which will have a detrimental impact on the lives of overweight people (Favieri et al., 2019). Dohle et al. (2018) also revealed that dietary behavior influences brain performance, and that healthy eating habits can increase cognitive function maintenance throughout life (Morris et al., 2005; Smith & Blumenthal, 2015). Other research, however, tends to disagree with this viewpoint, claiming that cognitive functions are regarded as separate entities. On the other hand, several disagree with this viewpoint, claiming that cognitive abilities predict eating behavior connected to weight change. According to this viewpoint, decreased brain function is the underlying of disordered eating behavior, causing alterations in adults with overweight/obesity (Dassen et al., 2018; Dohle et al., 2018; Favieri et al., 2019).
This study had several strengths; first, we performed a systematic review and meta-analyses of RCTs, which is considered high-quality evidence. Second, we determined detailed domain analysis regarding the EF e.g., inhibition, cognitive flexibility, working memory, verbal fluency, decision-making, and planning. There were several limitations in this meta-analysis. First, even though the dietary intervention (dietary modification) is considered safe, the majority of included studies had low adherence to the dietary intervention, and other personal circumstances exist during dietary interventions. Second, whether the dietary modification directly affects the EF domains or due to the mediator effect of weight loss remains unclear. Third, while measuring the six domains of the EF (inhibition, cognitive flexibility, working memory, verbal fluency, decision-making, and planning), included studies employed varied tasks. Nevertheless, these meta-analyses showed that dietary modification might influence the changes in some of the EF domains.
This meta-analysis highlights the effects of dietary modification on several domains of the EF such as cognitive flexibility, working memory, and verbal fluency. However, we did not find any effects of dietary intervention on the inhibition domain of the EFs. In addition, the effects of dietary modification on decision-making and planning domains remain unclear. Nevertheless, dietary modification may have beneficial effects on improving the EF domains. More large, well-controlled studies are needed to confirm these findings.
Figshare: PRISMA checklist for ‘The effect of dietary modification on executive function domains in adult with obesity: A systematic review and meta-analysis of randomized controlled trials’. https://doi.org/10.6084/m9.figshare.19843111 (Gunawan, 2022).
Figshare: PRISMA flow diagram for ‘The effect of dietary modification on executive function domains in adult with obesity: A systematic review and meta-analysis of randomized controlled trials’. https://doi.org/10.6084/m9.figshare.19843873 (Gunawan, 2022).
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
Junita Maja Pertiwi, Adriyan Pramono, Martha Marie Kaseke, David Susanto, Andisty Ate, Ahmad Syauqy, and William Ben Gunawan: Conceptualization, Formal Analysis, Writing – Original Draft Preparation and Writing – Review & Editing. Adriyan Pramono, Nelly Mayulu, Mochammad Rizal, and Fahrul Nurkolis: Supervision, Validation, Writing – Review & Editing.
J.M.J., M.M.K., D.S., and A.A. thank the Faculty of Medicine, Sam Ratulangi University, Manado for supporting this research. A.P., A.S., and W.B.G. also thank the Faculty of Medicine, Diponegoro University, Semarang.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Partly
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
Yes
Are the conclusions drawn adequately supported by the results presented in the review?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: nutrition and health
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are the conclusions drawn adequately supported by the results presented in the review?
Yes
References
1. Veronese N, Facchini S, Stubbs B, Luchini C, et al.: Weight loss is associated with improvements in cognitive function among overweight and obese people: A systematic review and meta-analysis.Neurosci Biobehav Rev. 2017; 72: 87-94 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Diet, cognition, neuroscience, psychology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 16 Jun 22 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)