Keywords
GCLP, Clinical, Capacity, Accreditation, Strengthening
This article is included in the Global Public Health gateway.
GCLP, Clinical, Capacity, Accreditation, Strengthening
HIV/AIDS is a major public health concern and cause of death globally. At end of 2018, nearly 37 million people were living with HIV, with the majority of infections occurring in sub-Saharan Africa.1 Despite the progress made in providing infected individuals with life-saving antiretroviral treatment and the scale up of preventive measures, 1.7 million people became newly infected with HIV in 2018.1 The development of efficacious and effective HIV vaccines is an essential strategy to end the HIV epidemic and during all stages of their development, candidate vaccines need to be assessed in areas with a high disease burden. It is therefore imperative that clinical trials of HIV vaccines be carried out in geographically relevant populations with the highest disease burden.2
Clinical trials of vaccine candidates in resource-limited settings can be hampered by several factors including lack of capacity of infrastructure and inadequate trained personnel, inefficient regulatory and ethical approval processes and lack of disease surveillance systems, amongst others.2 Laboratory facilities often lack quality assurance systems to ensure accurate and reliable safety and immunogenicity data that are crucial in clinical trials.3–5
For more than twenty years IAVI has been involved in HIV vaccine research and development as part of its mission to translate scientific discoveries into affordable, globally accessible public health solutions. In this regard, IAVI partnered with Clinical Research Centers (CRCs) in Eastern Africa (Kenya, Uganda and Rwanda) and Southern Africa (Zambia and South Africa). Through these partnerships IAVI has strengthened the capacity at the CRCs to conduct HIV vaccine clinical trials and epidemiological studies. One of the main components of this capacity building has been the establishment and compliance to Good Clinical Laboratory Practice (GCLP) standards,6 to ensure the results of clinical laboratory are comparable, repeatable and auditable data across the different laboratories in the network. This article describes processes implemented by IAVI to build and strengthen laboratory capacity and infrastructure in the Eastern and Southern Africa CRCs, and how GCLP compliance and accreditation was achieved and sustained in these centers.
Study locations: This was conducted in IAVI CRC laboratories.
Capacity building activities were organized in four main areas1: Infrastructure development2; Trainings3; Quality management systems; and4 Sample management. To enhance the capacity building at the CRCs, two other laboratories; Clinical Laboratory Services (CLS) in Johannesburg, South Africa and IAVI Human Immunology Laboratory (IAVI-HIL) at Imperial College in London acts as reference laboratories and provide technical support to these CRC laboratories.
To achieve infrastructure development for the selected laboratories the following were done: selection of locations for establishing fully-fledged research testing, site qualification and development of collaborating laboratories.
IAVI identified 9 CRC Laboratories in Southern and Eastern Africa (Figure 1) to form the African CRC network for purposes of conducting clinical trials and epidemiological/observational studies. The criteria for selection was based on labs performing clinical safety and immunology testing, labs located within large teaching and research institutions. These CRCs required different type of laboratory support based on their various needs. Some of the laboratories existed within a research or an academic institution and were already equipped to undertake most of the required clinical safety and immunology testing. These included Kenya Aids Vaccine Initiative-Institute of Clinical Research (KAVI-ICR) laboratory at the University of Nairobi, Kenya Medical Research Institute (KEMRI) -Kilifi and Uganda Virus Research Institute (UVRI)/Medical Research Center (MRC)-Entebbe and UVRI-Iavi) located within large teaching and research campuses at KEMRI and UVRI, respectively, and the Medical Research Laboratories (MRC – Masaka) located at an MRC research site in Uganda. Although these laboratories were well established they had no previous experience in conducting clinical trials hence had no GCLP accreditation.
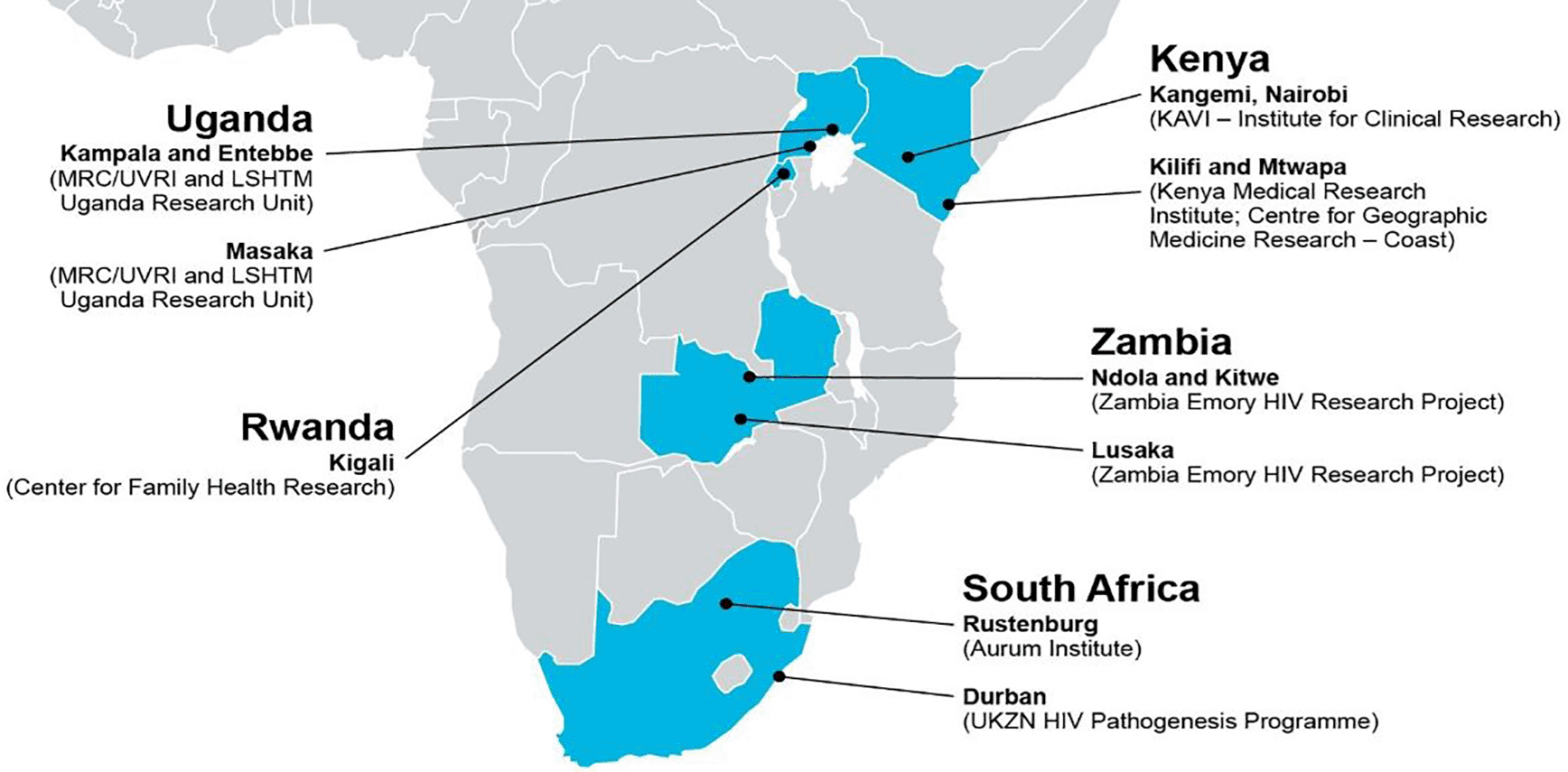
MRC – Medical Research Council, UVRI – Uganda Viral Research Institute, LSHTM – London School of Hygiene & Tropical Medicine, KAVI – Kenya AIDS Vaccine Initiative and UKZN – University of Kwazul-Natal (QGIS software version 7.4.4).
There were also CRCs with small satellite laboratories that needed to be scaled up to a level required for conducting clinical trials. This involved upgrading infrastructure and staff training to receive full GCLP Accreditation. These CRCs included the Zambia-Emory HIV Research Project (ZEHRP-Lusaka and ZEHRP-Ndola) in Zambia, and Center for Family Health Research (CFHR) in Kigali, Rwanda.
The process involved collaboration with partners in situations where there was no existing laboratory. IAVI and the partner institution agreed on the support needed and a physical space for location for the laboratory was identified. The design of the laboratory (architectural plan) was drafted, and the actual building implemented to completion. The newly built laboratory was then equipped, staff were identified and trained in both clinical safety and immunology testing. The Aurum Institute for Health – Rustenburg (South Africa) laboratory is one example of a CRC that received this kind of IAVI support.
Immunology and clinical safety testing laboratories underwent a qualification procedure prior to the initiation of any clinical testing.7 This included review of the required infrastructure (laboratory buildout, purchase and installation of equipment), assessment of compliance to GCLP and appropriate technology transfer qualifying assays. Standardized checklist was used to assess the site’s capacity to conduct the proposed clinical trial or study and the investigators were qualified by their training, education and experience, as per GCP requirement. Any gaps or other issues identified from the site qualification visits were rectified beforehand. Successful performance of qualified assays ensured that collaborating center personnel had gained adequate familiarity with equipment, SOPs and other documentation, and the correct equipment and reagents were provided.
Training of four different types were conducted to the nine CRCs within the IAVI network. The trainings were aimed at ensuring that all CRCs conduct research efficiently and to the highest ethical standards. The first type of training performed was basic GCLP. The training aimed at enhancing compliance to international standards for conducting clinical trials. The second type of training was advanced GCLP training. This training was designed to provide comprehensive guidance and practical help to ensure implementation of GCLP guidelines in all the sites conducting IAVI-sponsored clinical trials.
Biosafety was the third training type performed. This training was provided by IAVI HIL who aimed at providing a structure and set of standards upon which to develop a practical and realistic health and safety program in the IAVI-supported laboratories. The assessment was conducted as a written multiple-choice test and by observation of the trainee performing the work.
The last training type was focused on advancing the technical skills of the laboratory staff from the nine CRCs within the IAVI network. This training aimed at improving the technical knowhow on the clinical and equipment procedures. These included procedures for hematology testing, ELISPOT, flowcytometry, liquid nitrogen plant and HIV testing.
A four-approach implementation model was used to develop quality management system (QMS) in the CRCs and prepare them for accreditation. GCLP guidelines was used to achieve the journey of accreditation because it is the recommended guideline by World Health Organization (WHO) as the standard for clinical laboratories involved in the evaluation of diagnostics for infectious diseases.8 The implementation model was made up of four main components namely: a comprehensive audit program, External Quality Assessment program, equipment standardization and sample management. The audit program aimed at ensuring the CRCs adhere to GCLP compliance. The audit program was made of two external audits and one internal audit. The external audits were mainly accreditation assessment and a bi-annual audit by a research contract organization. The accreditation assessment audit was6 carried out by Qualogy, UK, an independent accrediting body which assesses GCLP Compliance against the Research Quality Assurance (RQA) GCLP Guideline and GCP Regulations.9 The bi-annual internal audits were performed by the Clinical Laboratory services (CLS), Johannesburg, South Africa.10 This audit was a follow-up of the findings of the accreditation assessments. The internal audit was performed by the trained site staff and was aimed at ensuring that the established QMS remains abreast compliance with GCLP standards.
The EQA implementation model involved registering all the CRCs on EQA schemes with an objective of providing accurate and reliable results. The intention of EQA schemes was to provide an independent external assessment of a laboratory's ability to provide an accurate results by comparing it to a peer group of laboratories or to a reference laboratory.11,12 EQA scheme providers were identified and CRCs enrolled on the clinical safety and immunology assays. The CLS coordinated the EQA enrollment and reports for all the CRCs in collaboration with IAVI. CLS reviewed all the CRCs’ EQA reports and worked with CRCs to ensure corrective actions were implemented where EQA results are not scored, out of range and if panels were not submitted To standardize the testing analyzers and methods across the CRCs, the hematology and clinical chemistry analyzers were purchased and distributed among the CRCs. This helped in coordinating the equipment service/maintenance management as well as purchase of their reagents and kits including performing method validation. The CLS in collaboration with IAVI gave guidance to the CRCs on how to develop the validation plans, carry out validations, review of data and development of reports using Psmile resources/tools.13
To strengthen sample management across the 9 CRCs within the IAVI network, site laboratory staff were trained on laboratory information management system (LIMS). The SQL*LIMS v5 data system (Applied Biosystems, San Jose, California) was used to manage the input, collection and analysis of data from the laboratory network. Documented procedures for sample reception and chain of custody were also developed to ensure sample integrity is maintained.
IAVI has established a program that provides capacity development and support for immunology and clinical safety testing at the 10 CRC laboratories in Eastern and Southern Africa region. As a result of the infrastructure development, IAVI CRC laboratories have been accredited, developed essential documents for quality management, respective staff trained, established EQA program, methods standardized, equipment maintenance/service program developed and sample management system well established.24
A total of 10 laboratories were accredited by Qualogy limited, United Kingdom between 2004 and 2016 as shown in Figure 2. The accreditation timeline was dependent on when the site was identified and their readiness for the accreditation.

KEMRI – Kenya Medical Research Institute, ZEHRP – Zambia Emory HIV Research Project, HPP – HIV Pathogenesis Project.
All the laboratories went through a comprehensive audit and implemented corrective actions to address any audit findings UVRI-IAVI Entebbe, Uganda was the first site to obtain GCLP accreditation in 2004 (and the second laboratory in Africa to achieve this status), followed by KAVI-ICR, University of Nairobi Kenya in 2005. The following laboratories received their accreditation after 2005: CFHR Kigali, Rwanda (previously known as Project San Francisco, PSF); MRC Masaka, Uganda; ZEHRP Lusaka and Ndola, Zambia; KEMRI Kilifi, Kenya; AURUM Rustenburg, South Africa; HIV Pathogenesis Programme (HPP), Durban, South Africa; MRC-Entebbe, Uganda (Figure 2).
During the period of study, a total of 82 trainings were done to 1859 laboratory staff. The highest number of trainees were in GCLP training at 61.2% (n = 1138), followed by QMS trainings at 20.3% (n = 378), technical trainings at 20.3% (n = 311) and Safety training at 1.7% (n = 32). Of the total trained for GCLP, 68% (n = 725) were trained in basic GCLP while 32% (n = 347) were trained in Advanced GCLP training (able 1). Basic GCLP training had the highest number of staff trained because all staff at each site were to be trained to understand GCLP guidelines (Table 1).
Biosafety training: All of the staff who scored above 80% were deemed competent. If the score was less than 80%, trainees were retrained and were not allowed to work in the laboratory until they obtained the required results.
The number of trainees across the years were varied per training category. Basic GCLP training was consistently done at least each year followed by Advanced GCLP. The highest number of trainees were in 2008 at 102(13.6%) and 41(10.6%) in 2021 for Basic and advanced GCLP trainings respectively (Table 2). QMS based trainings were initiated in 2007 and were done every 2 years upto 2015. Thereafter the trainings were annual up to 2019 (Table 2). Safety trainings had only been done in 2018 (Table 2).
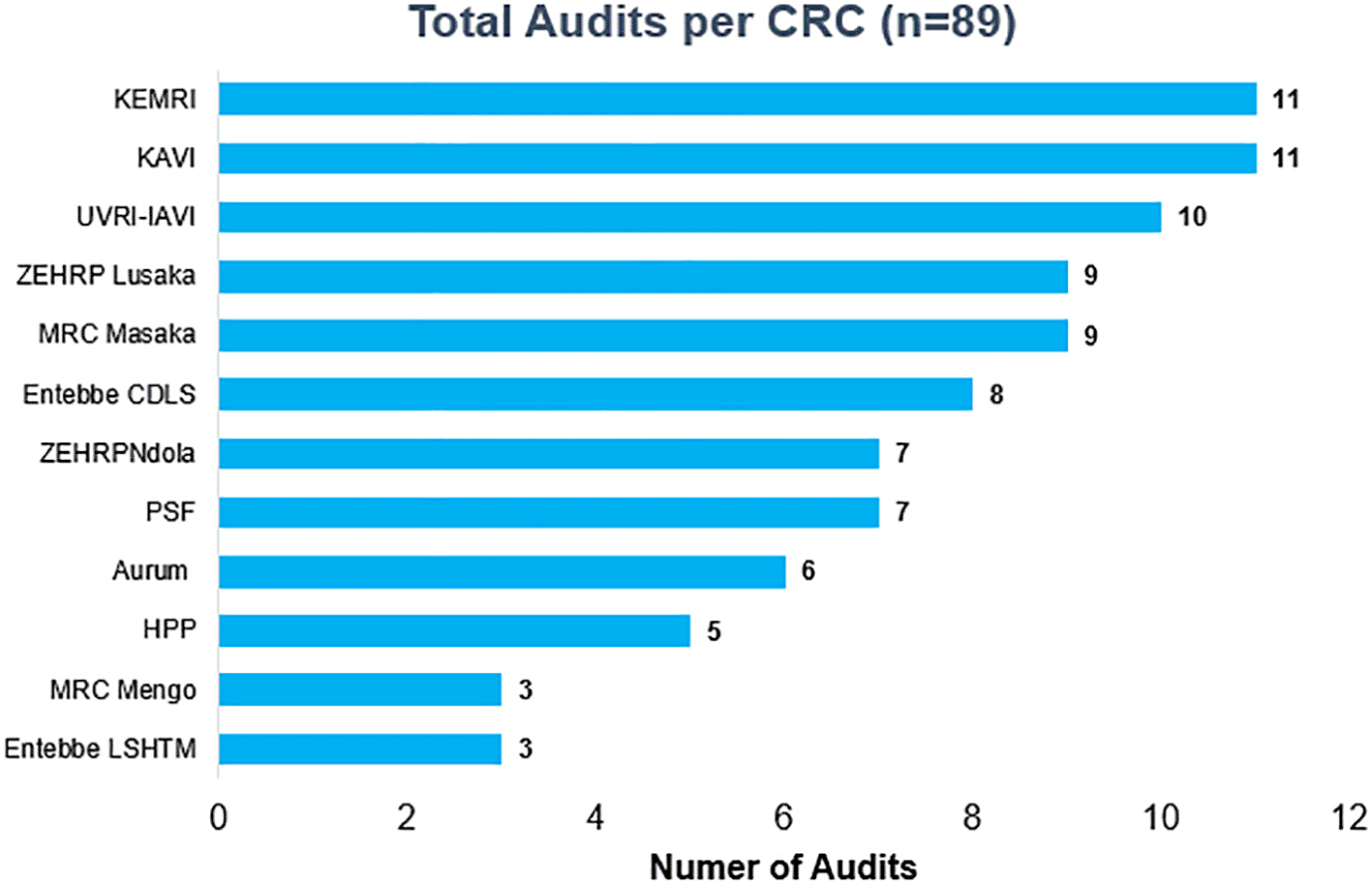
To strengthen the laboratory capacity through QMS, a total of 89 accreditation assessments audits were performed by Qualogy between 2005 and 2019. The highest number of audits (n = 10) was observed in the year 2010, 2017,2018 and 2019 as shown in Figure 3. KAVI and KEMRI had the highest number of accreditation audits (n = 11), followed by UVRI-IAVI and MRC Masaka (n = 9) and MRC Entebbe (LSHTM) had the least (n = 3) as shown in Figure 4.
In using EQA to strengthen QMS a laboratory capacity strategy, a total of three EQA scheme providers namely: CAP, RCPA and HIL were identified to provide EQA services. All the CRCs were enrolled on EQA schemes based on their testing scope covering all safety testing parameters and Elispot assay to ensure accuracy of results (Table 3). The frequency of the EQA panels is determined by the service provider and varies per assay/test.
In using standardization of testing analyzers and testing methods to implement QMS as part of laboratory capacity strengthening strategy, IAVI purchased ten chemistry analyzer, ten hematology analyzers, ten serology equipment, ten flow-cytometry equipment and ten temperature monitoring equipment which were distributed across the ten CRCs (Table 4).
Additionally, 311 number of staff were trained on the equipment, analyzers and standardized protocols to ensure accurate and reliable results are generated. HIV testing and clinical chemistry had the highest number of trainees followed by Hematology (Figure 5).
The capacity to conduct clinical trials/studies in Africa is limited in part, by a lack of adequate laboratory research infrastructure. Laboratory support of clinical trials/studies is integral to their quality; defining the analytical parameters for inclusion/exclusion criteria, monitoring the safety of the participants, determining the baseline data, and the safety and efficacy of the investigational products.
Staff training: Well trained and organized staff are essential for the successful operation and management of a clinical laboratory for quality and reliable data. In Sub-Saharan Africa, there has been lack of advanced-level training programs for laboratory staff and this has resulted to inadequacy in the laboratory sector in the region.14
Basic GCLP training had the highest number of trainings and was consistent across the years. The training is carried out for new staff or as refresher to the old staff. This implies that CRCs employ at least annually and for them to be competent in the laboratory, Basic GCLP training was to be done for them to understand the basic concepts of GCLP and the implementation process required for a clinal laboratory. As a refresher it provides staff with renewed knowledge in GCLP.
Basic GCLP training was followed by Advanced GCLP in consistency and number of trainings executed. The training is need based and offered to all CRC laboratory and QA Managers every year. Need based training has been reported elsewhere to enhance Quality improvement when emphasize is put on actionable training needs.15 The aim was to discuss challenges faced when executing GCLP and get an in-depth understanding on how to interpret and implement GCLP in their respective CRCs. The basic advanced model of training used has also been reported elsewhere.
QMS trainings evolved in 2007. This were in support of the already accredited CRCs and in preparation for the GCLP accreditations the new CRCs that were accredited around this time. At around this time adaption of QMS to local priorities had proven difficult in Sub Saharan Africa.16 Therefore, there was need to support the CRCs.
IAVI realized that one of the novel ways to obtain the most accurate and reliable clinical trial data from multiple participating CRCs in different countries was to have the least number of variables that could affect clinical trial data and processes. In view of the latter, analyzers at IAVI participating Africa CRCs for clinical trials/studies were standardized for safety testing parameters (chemistry, haematology, serology, flow-cytometry), including standardized temperature monitored cryogenic equipment for sample storage), typical of clinical trial testing set up CRC staff were also trained on standardized methods for immunology end-point testing required for the multi-centred clinical trial/epidemiological studies. Although, this was very costly, it ensured processes were in place to obtain the most accurate and reliable data. New or upgraded equipment (chemistry and haematology) were either acquired centrally or purchased locally and placed at participating IAVI CRCs to improve technology and still maintain standardization. The centrally acquired equipment allowed IAVI to negotiate for discounts and extended warranties. The standardization also enables maintenance and training of equipment to be streamlined. As a result, SOPs and related documents were developed as recommended by WHO.17
All CRC laboratories have documented SOPs which are approved by the CRC laboratory management and are periodically reviewed by CRCs staff, in accordance with GCLP guidelines. These processes are monitored during onsite audits by IAVI. A list of current SOPs is maintained and kept up to date by the CRC’s laboratory/Quality assurance manager. To standardize the immunogenicity assays, standardized SOPs were developed and distributed to the CRCs by IAVI HIL. SOPs detailing the preparation (isolation and thawing) of human peripheral blood mononuclear cells (PBMC), plasma and serum for use in assays, counting and freezing of human PBMCs and the evaluation of interferon-γ producing cells by ELISpot assay are validated at the IAVI’s HIL prior to their distribution to CRCs laboratories. In addition, the Analytical Project manager drafts an analytical plan for each of the clinical trial/study the laboratory is undertaking. This analytical plan which details the laboratory study procedures to be followed, is agreed upon with the CRC and approved by the sponsor and the laboratory management.
External audits by Qualogy limited were to ensure that all CRCs were accredited. CRC accreditation implies an implementation and monitoring of a comprehensive laboratory quality management systems that reduces variability of test results and laboratory errors.18 Before accreditation were awarded a baseline, audit was carried out. The CRCs were expected to close the findings prior to accreditation award. The accreditations were awarded at different time points depending on when the CRC was identified and how fast they were able to close out the findings. Accreditation has been a sign of compliance to the established quality and competence standards.18,19
As the CRCs developed and maintained GCLP compliance over the years, the frequency of the bi-annual internal audits by a research contract organization was changed from biannual to annual and the CRCs were enhanced to strengthened internal audit systems. As reported elsewhere internal audits were meant to stimulate the maintenance and improvement of quality.19
Enrollment to an EQA program is mandatory in established countries.18 Prior to accreditation or implementation of study protocols, IAVI enrolls the CRC networks in identified EQA programs. enrollment in EQA programs is among the first steps of implementing a quality system.19 This ensures that analytical results are reliable globally accepted.20 Most of the HIV vaccine clinical trials are multi-site trials where vaccine immunogenicity data is generated by different CRCs. An important end-point assay for HIV and other vaccines has historically been the interferon-gamma (IFN-γ) Elispot assay.21,22 Extensive efforts have been made by IAVI and others to validate and standardize the T-cell Elispot assay. To ensure reliability of the results Elispot EQA, relevant CRCs were enrolled in the program.
IAVI has established standardized protocols for validation of the assays and analyzers. All new assays are validated prior to being used and validation of new equipment is also undertaken upon installation. This has ensured that analytical results generated across the CRCs are dependable.23 This is a documented program of work intended to evaluate or demonstrate the performance of a process. Validation protocols are developed in conjunction with the CRC laboratories. Data is generated, and the results are compared against pre-defined acceptance criteria. In addition, all CRCs assess and document lot-to-lot verification of new reagent performance. This is because performance of new reagent lot can be affected by several factors including instability of reagent during transportation or storage, and defects in manufacturing. A standardized protocol has been developed to assess the lot-to-lot verification.
Availability of properly functioning laboratory equipment with appropriate maintenance is critical to the conduct of clinical research and the production of reliable and accurate results, however in developing countries it can be an enormous challenge.24 Laboratory equipment requires regular maintenance as recommended by the manufacturers. The maintenance of laboratory equipment requires a wide range of technical abilities and skills and laboratories must have a preventative maintenance contract with engineers from the manufacturers, their agents, or appropriately trained biomedical engineers.
One of IAVI’s focus has, been to strengthen and equip laboratories in its clinical research centers. IAVI has provided financial assistance to procure state of the art laboratory equipment for the safety and immunology laboratories. Among this equipment were hematology, serology & chemistry analyzers, Elispot readers, flow cytometry, deconvoluted microscopes, liquid nitrogen plants, and cryogenic equipment as shown in Table 3. IAVI has also procured generators and uninterrupted power supply (UPS) to provide continuous backup power to essential equipment in the laboratory and pharmacy.
A comprehensive equipment maintenance and service program is managed by IAVI. Independent biomedical engineering companies who have the skills, tools and the necessary experience to maintain laboratory equipment are contracted to perform such work. The service and maintenance program are undertaken twice annually at all participating collaborating CRC. The external vendors provide a written report for each CRC laboratory of the work carried out, problems encountered and the proposed resolution. They also maintain a list of small spare parts required by the CRCs and the small spare parts are replaced during the service visit. In the event a major spare part is required to be replaced, an order is generated and submitted to the manufacturer of the equipment. To ensure proper functioning of the laboratory equipment laboratory staff at each CRC was trained to perform regular preventive maintenance as recommended by the manufacturer. In addition, each CRC laboratory was provided with toolkits to make minor repairs like electric fuse replacement.
All CRC laboratories have SOPs and procedures for sample receipt, preparation and processing. Documenting chain of custody and sample integrity is a standard practice across centers. Samples collected in the clinic are given unique identifiers using automated sample labeling. A study/visit specific laboratory requisition form is sent with the samples to the laboratory. All samples received are logged into the laboratory information management system (LIMS). The SQL*LIMS v5 data system (Applied Biosystems, San Jose, California) is used to manage the input, collection and analysis of data from the laboratory network. The LIMS web-based interface allows for remote data entry, centralized technical management and near real-time access to data and sample inventory. The system is highly customizable and offers electronic signatures as well as an automatic audit trail and user security checks and data integrity. The HIL laboratory oversees quality assessment of processing, storage and shipment of PBMC samples across the IAVI CRC network. All sites have staff who have been trained on LIMS and the system has been operational at these centers for several years in support of IAVI funded studies/trials. This efficient, centralized sample management system is utilized for all the IAVI supported CRCs with support from IAVI HIL. To date, the HIL has managed the custody of over seven hundred thousand samples from a global network of CRCs and collaborative partners. On average 8,000 to 12,000 (15-20 shipments per year) samples per year were shipped to HIL from IAVI CRCs. Only one failed shipment (Dec 2017) has occurred within the past 15 years. The sample repository, which is based at the HIL has a storage capacity of over 900,000 specimens in both liquid nitrogen vapor tanks and ultra-low temperature (−80°C) freezers, managed by a customized GCLP compliant LIMS.
High quality standards in international shipping practices have led to a standardised procedure of 7-day calibrations of dry shippers for the shipment of PBMCs. PBMCs are the main samples obtained for cell based immunological assays, which are typically integral to measuring the immunogenicity of vaccine clinical trials. The cold chain from PBMC preparation, storage, shipping, through to sample use is controlled and closely monitored, with alarm systems and back up corrective procedures in place at all laboratories. The relevant staff at each site is trained to meet the minimum requirements for dangerous goods according to 49 CFR 172.700 and IATA 1.5 and to ensure that the shipment is compliant with these regulations. This is achieved by completing the training in the SAF-T-PAK that is shipped to CRCs laboratories every 2 years.
The IAVI Africa laboratory program with the support of the IAVI HIL has enabled real-time safety testing and state-of-the-art immunology assays at all their affiliated facilities. Infrastructure development, training, site support visits, and ongoing quality assurance programs were essential parts of setting up a network of African laboratories conducting clinical trials. The Quality Assurance (QA) programs have shown that the labs at the research centers in Africa can perform clinical assays consistently in line with counterparts in industrialized countries
With IAVI’s support, laboratories at CRCs have received GCLP accreditation and standardized testing procedures to ensure reliable and accurate data, especially for multi-center studies. The establishment of GCLP accredited laboratories in Eastern and Southern Africa with well-trained personnel has created centers of excellence and allowed these CRCs to attract independent research funding.
Standardization of laboratory equipment at IAVI sponsored sites has allowed efficient training, maintenance, and easier purchase of spare parts. The centralized vendor is based in Kenya oversees this process for all CRCs which are part of the IAVI network. Standardized temperature monitoring systems are included, and these can be evaluated across CRCs which ensures that equipment downtime and service interruptions are minimized.
The IAVI HIL coordinates and supports the flow of samples across the network of clinical trial laboratories ensuring full chain of custody of all specimens collected. The HIL Unit, which includes IAVI QA Africa and HIL-London have consistently maintained and driven the expansion of capacity of the CRCs within the IAVI network to support high quality sample management infrastructure.
IAVI’s continued capacity building across the CRCs in Africa has led to the following: 1) Transfer of clinical research skills to African researchers to support clinical trial programs and a broad range of epidemiological studies at the research sites2; Implementation of quality systems and standardized testing procedures leading to generation of reliable and attributable data, especially for multi-center studies across various countries3; Participation in EQA programs, which enables comparison of performance between laboratories globally and provides an early warning for systematic errors; and4 Ability to conduct and participate in clinical trials and epidemiological studies.
BF contributed towards conceptualization, data collection, drafting and final revisions of the manuscript. MMaraka contributed to the technical content, Analyzed data,created the figures and tables and revision of the manuscript. MMshai contributed to the technical content of the information and conducted the technical and GCLP trainings, PC contributed towards revising the manuscript and oversaw the capacity building of the laboratories at the CRCs. LM contributed towards the intellectual content and the final revisions of the manuscript. DO is the Director of IAVI Africa laboratory program and oversaw the capacity building projects and conceptualization of the manuscript. JG is the Executive Director for HIL at IAVI and contributed towards conceptualization of the laboratory capacity building program and contributed to GCLP accreditation program in all the CRCs. All authors read and approved the final manuscript.
Figshare: GCLP Audits and Training Raw Data, https://doi.org/10.6084/m9.figshare.16817482.v1.25
This project contains the following underlying data:
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4).
Special thanks to laboratory staff and management of the ten clinical research centers in Eastern and Southern Africa. This manuscript was submitted for publication with the permission of all the authors.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Viral (HIV) immunology
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: I have worked one in one of the laboratories described in the article; this does not impact my ability to give an impartial review.
Reviewer Expertise: Infectious Diseases, global Health, One Health
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 21 Jan 22 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)