Keywords
Circulating Tumor Cell (CTC), Colorectal Cancer, Primary Culture, Spheroid Culture
This article is included in the Oncology gateway.
Circulating Tumor Cell (CTC), Colorectal Cancer, Primary Culture, Spheroid Culture
Circulating Tumour Cells (CTCs) are commonly defined as tumour cells that enter circulating systems such as the blood or lymphatic vessels Grover et al., 2014). CTCs are cells that shed from either primary tumors or from metastatic sites (Wang et al., 2018). Recently, CTCs have been found to initiate tumorigenesis in Patient-Derived Xenograft models (Rodrigues and Vanharanta, 2019). Therefore, CTC might play a key role in understanding such metastatic processes (Castro-Giner and Aceto, 2020).
The tumorigenic capacity of CTC may be demonstrated using xenograft models as previously described (Rodrigues and Vanharanta, 2019) or by tumor spheroid formations in in vitro assay (Hu et al., 2021). However, xenograft models and 3D culture using Matrigel cell culture matrix is expensive, in particular for developing countries such as Indonesia. In this study, we report the interesting findings from our pilot study that show CTCs were able to be cultured and that they can form spheroid aggregations spontaneously. Therefore, such methods may be promising and should be further investigated, in particular in developing an economical assay to test the tumorigenesis ability of CTCs. This pilot study is a part of a larger study that aims to assess CTC as a diagnostic tool for colorectal cancer (CRC).
This pilot study is a part of a larger study that was approved by the Ethical Committee of Faculty of Medicine, Universitas Indonesia (Protocol ID: 20-04-0643, version 02, June 29th, 2020). Recruited subjects provided their written informed consent for blood donation (biobanking) and for the use and publication of their data for research purposes. All participants signed an informed consent form before participating in the study. In addition, the two patients involved in this pilot study provided written informed consent for participation and publication of their data.
Patient blood samples were taken in the endoscopy room of the Cipto Mangunkusumo Hospital, Jakarta, Indonesia. Isolation and culture were carried out at the Indonesian Institute of Medical Research and Education, Faculty of Medicine, University of Indonesia. Subjects were recruited (as part of the overarching study) from June 2020 until the culture process was carried out in August/September 2020. Primary culture images were taken separately in August 2021.
A total of 73 patients with colorectal cancer, who were older than 18 years old and who were scheduled for colonoscopy were recruited for the overarching study. After patient consent was obtained, demographic and clinical data were collected from each patient. The demographic data included gender and age, and the clinical data included cancer location, stage, and metastasis. Histological data (cancer type, differentiation) were also noted. Then, 2 ml of patient blood were drawn from a peripheral vein (cubiti vein) and transferred in BD vacutainer (Becton, Dickinson and Company, USA, www.bd.com) heparinized tubes. During the recruitment process for the overarching study, we observed two patients with similar metastatic features and included these patients in the CTC pilot study.
To obtain the CTCs for this pilot study, two colorectal cancer patients who had similar clinical profiles, namely liver metastases (see Table 1) were chosen. Approximately 1 ml of blood was taken from each patient and placed in separate heparin tubes. The blood was then transferred from the heparin tubes to separate polypropylene tubes to undergo CTC isolation using Easysep™ Direct Human CTC Enrichment kit (Catalog #19657, StemCell Technologies, Canada), according to the manufacturer's protocol. The enriched cells were then pooled in 15 ml centrifuge tubes and centrifuged 300 × g for five minutes. Supernatant was removed afterward. Cell pellets were resuspended in 1 ml complete Dulbeco’s Modified Eagle medium (Gibco, USA) supplemented with 10% Fetal Bovine Serum (Gibco, USA) and 1% of Antibiotic-antimycotic solution (Gibco, USA).
The process of spheroid formation was carried out by implanting cells directly on the nuncsphera u-plates and allowing the cells to immediately aggregate at the bottom of the plate. Approximately 10,000 isolated cells of colorectal CTC’s primary cells were seeded directly in Nunclon™ Sphera™ 96-Well, Nunclon Sphera-Treated, U-Shaped-Bottom Microplate (Catalog number: 174929) (Thermoscientific, USA) and incubated under standard culture conditions (37 °C, 5% CO2) for three days. For controls, we seeded 5,000 cells of primary breast cancer culture, obtained from the Human Cancer Research Center, Indonesia Medical Education, and Research Institute. This culture was originally collected for optimizations of primary cultures and was obtained from discarded tissues. Written informed consent was provided by the patient for this purpose. All spheroids were examined using Carl Zeiss inverted microscope using 10× magnification in Zen blue 2.1 embedded software (Carl Zeiss, Germany).
In this study, we identified two patients with similar clinical profiles which rectal adenocarcinoma and metastatic cancer in the liver. The demographic information of the two patients is shown in Table 1. At first glance, we wanted to optimize the culture conditions for CTC of the metastatic cancer patients in the short term for approximately 3-7 days using standard culture media. The first question we wished to address was how the CTC culture had grown in vitro under standard culture conditions. Our suggestion was to increase the chance of cell-to-cell contact using 3D cultures.
In this study, we were able to identify two cultures from two patients, namely ID 001 and ID 002 after three days of incubation. Surprisingly, we found that ID 001 was able to form aggregation of cells (Figure 1A) while the CTC 002 did not have aggregation formation (Figure 1B). The ID 002 displayed clustered grape-like morphology. Therefore, we suspected that the aggregation formation may vary between samples and may depend on intrinsic factors between samples such as homophilic interactions of Cluster of Differentiation (CD)-44/CD44 antigen/CD44 that induced multicellular aggregations.
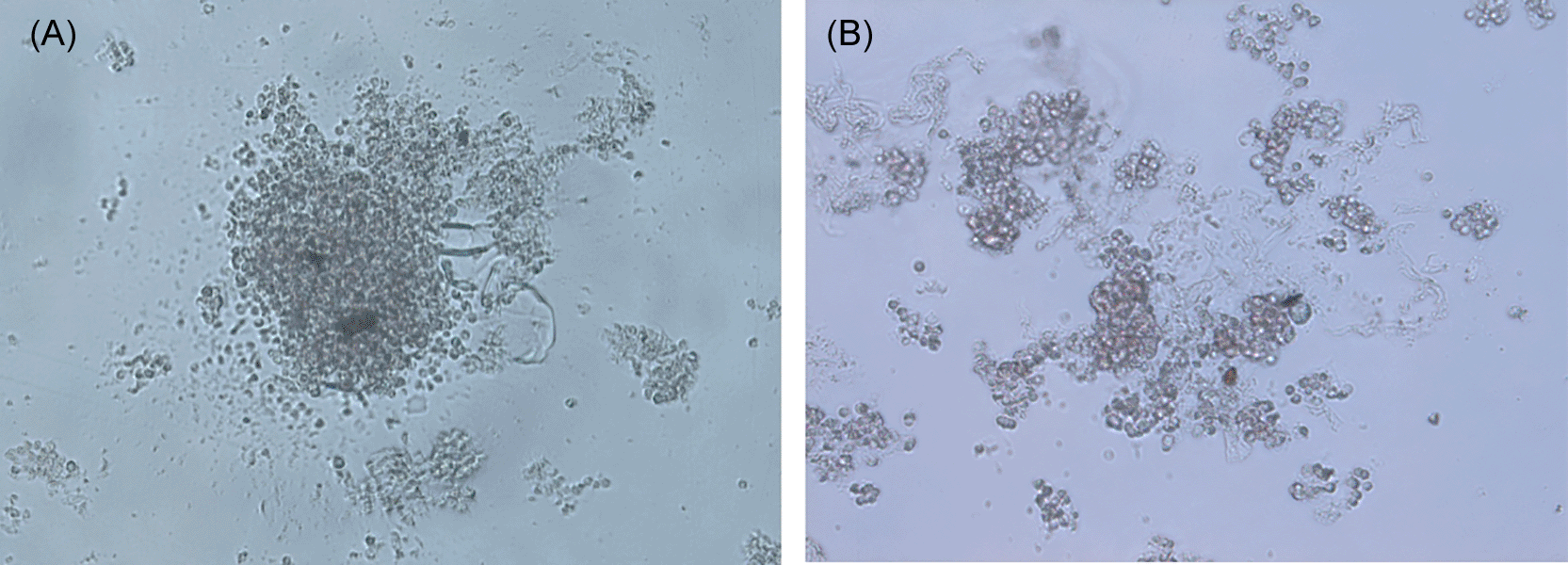
(A) The CTC with ID001 was able to spontaneously aggregate at the bottom of the well, forming a spheroid aggregation. (B) The CTC with ID 002 was unable to form uniform aggregation.
Since tumors are known for their ability to form aggregations, we chose an aggressive solid type of tumor, namely breast cancer as a control for the study. Breast cancer was a type of solid tumor with a known lobular structure and estimated behold potency to form aggregation in the 3D culture platform. We tried to seed these primary cells directly to the same type of plates used for the CTCs, for the duration of three days. We found that this produced a more rigid structure of spheroid formation (as shown in Figure 2).
In this study, we wanted to know the characteristics of isolated CTC cells by observing the ability of isolates to form spheroids. Based on this study, it was shown that CTC can form spheroids spontaneously. We recommend using an u-plate for the test to allow the aggregation process at the bottom of the plate. After that, it is recommended that the sample be left for three days. Our results showed that, surprisingly, CTC ID 001 had the ability to spontaneously form spheroids within three days, whereas CTC ID 002 did not spontaneously form spheroids (Figure 1).
The spheroid formation has been found as in vitro assay to evaluate the tumorigenic capacity of the cells (Hu et al., 2021) In this study, we found facts that CTC was able to form spheroids and aggregations. Therefore we suspected that CTCs might have some tumorigenic capacity, which may be displayed by the aggregations. We confirmed these aggregation processes in primary breast cancer cells using the same methods and observed a more dense and darker spheroid formation (Figure 2). Thus, the aggregations may also be related to tumorigenesis of the CTCs or at least some similar features, as those primary cells were able to form spheroids.
Previously, the tumorigenicity of CTCs has been reported using Patient-Derived Xenograft (PDX) models (Rodrigues and Vanharanta, 2019). From our perspective, such conditions of spheroid formation may be related to the tumorigenic potential of CTC. At least, these CTC cultures were isolated from two metastatic colorectal cancer patients (shown in Table 1). Our results suggested that the CTCs have the ability to mimic primary tumors in their ability to spontaneously aggregate in vitro. The capacity of the CTC to form aggregation may vary depending on intrinsic factors such as expressions of CD44 in the CTC’s that could not be identified in this study. A previous study by Liu et al. showed that homophilic interactions between CD44 increase the chance of cell to cell contact, leading to multicellular aggregations of the patient-derived breast cancer models (Liu et al., 2019). In the future, we suggest that our findings regarding spheroid aggregation assay may be used as a potential alternative to predict either patient metastasis or tumorigenic capacity of CTC in a larger study.
Open Science Framework: Circulating Tumor Cells from CRC CIptoMangunkusumo. https://doi.org/10.17605/OSF.IO/7CGYA (Abdullah, 2021).
This project contains the following underlying data:
- Figure 1A. tiff (CTC ID 001 ability to spontaneously form spheroid aggregation in vitro)
- Figure 1B. tiff (CTC ID 002 3D culture formations, which could not spontaneously form aggregation)
- Figure 2. tiff (Breast cancer primary cells’ ability to form spheroid aggregation in vitro)
- Table 1. Patient demographic.xlsx
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
We thank Dr. Nadhira Nizam and Dr. Asiyah Nurul Fadilah for their valuable contribution in gathering patients and samples resources. We also thank Dr Erwin Danil from Department of Surgery Oncology CiptoMangunkusumo Hospital and Human Cancer Research Center for supporting the development of breast cancer primary culture.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Liquid Biopsy, CTCs, Cancer Molecular Pathology
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Liquid biopsy, CTCs, tumour microenvironment
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 21 Jan 22 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)