Keywords
mortality, biomarkers, pandemics, hospitalization, COVID-19, Infection, Cardiovascular disease, Respiratory disease
This article is included in the Emerging Diseases and Outbreaks gateway.
This article is included in the Pathogens gateway.
This article is included in the Coronavirus (COVID-19) collection.
mortality, biomarkers, pandemics, hospitalization, COVID-19, Infection, Cardiovascular disease, Respiratory disease
The past years have been marked by the emergence of a novel coronavirus (called SARS-CoV-2) and its global spread (Yi et al., 2020). The coronavirus disease 2019 (COVID-19) has a broad spectrum of clinical manifestations, ranging from no or mild symptoms to severe pulmonary involvement requiring hospitalization for respiratory support (Hazra et al., 2020; Mao et al., 2020). Older adults, immunocompromised subjects, and those with high comorbidity burden are more prone to severe illness, although the reasons are not yet fully understood (Pott Junior and Cominetti, 2021; Maquet et al., 2020; Richardson et al., 2020).
Several studies have shown an association between severe illness and elevated serum levels of inflammatory markers such as erythrocyte sedimentation rate, C-reactive protein (CRP), ferritin, procalcitonin, interleukin-6 (IL-6), and interleukin-10 (IL-10), among others (Shu et al., 2020; Samprathi and Jayashree, 2021). This evidence has led to the assumption that disease severity is somehow related to an immune response shifts towards a systemic inflammatory state caused by unknown driving factors (Foo et al., 2021). Recently other markers have also been associated with disease severity, including N-type natriuretic peptide (NT-proBNP), myoglobin (MYO), D-dimer, myocardial creatine kinase band (CK-MB), and cardiac troponin I (cTnI) (Qin et al., 2020). In particular, there seems to exist an association between COVID-19 severity and cardiovascular injury, especially among those experiencing longer lengths of hospitalization (Wang et al., 2020).
Severe cases of COVID-19 often present elevated serum levels of D-dimer and cTnI, which have been associated with respiratory failure, thrombotic events, hospitalization, ICU admission, and mortality (Rajendran et al., 2021; Wibowo et al., 2021). In addition to the systemic inflammatory process, studies have shown that SARS-CoV-2 infects endothelial cells, cardiomyocytes, and cardiac endothelial cells (Liu et al., 2021; Lei et al., 2021). Thus, there are two possible concurrent mechanisms for cardiovascular involvement, including a direct effect of the virus on target cells and cell injury secondary to the systemic inflammatory process. These mechanisms are not mutually exclusive and probably act on each other (Balse and Hatem, 2021; Ho et al., 2021).
The objectives of this study were to examine (i) whether admission cardiovascular diagnostic testing with D-dimer and troponin measurement predicted early mortality among adults with COVID-19 and (ii), if so, how the disease severity influenced the association between these laboratory parameters and mortality.
This study is a prospective cohort of adults admitted to the COVID-19 Unit of University Hospital at the Federal University of São Carlos (HU-UFSCar). This study was carried out according to the recommendations of the STROBE statement.
The study population included individuals aged ≥18 years diagnosed with COVID-19 when admitted to the COVID-19 Unit between June and January 2021, before the period of vaccination against the disease. All individuals must have had their diagnosis of COVID-19 confirmed by RT-PCR technique in at least 72h of the beginning of the symptoms. Exclusion criteria included a previous diagnosis of heart failure with known left ventricular (LV) reduced ejection fraction (LVEF<0.5); diagnosis of interstitial pulmonary fibrosis or known severe pulmonary disease that courses with fibrosis on chest tomography; and a previously known diagnosis of supraventricular or ventricular arrhythmias. Individuals' follow-up status was determined through hospital records; time-to-outcome was recorded in days.
The study followed the guidelines laid down in the Declaration of Helsinki, and all procedures involving research study participants were approved by the UFSCar's Research Ethics Committee (Number: CAAE: 34344520.8.0000.5504).
Data collection occurred through face-to-face interviews at hospital admission and included sociodemographic and clinical data, chronic comorbidities, and disease severity. Within the first 12 hours of admission, venous blood was sampled to analyze laboratory parameters. After admission, a professional involved in this study accompanied the clinical course of each participant until their discharge or death.
Measurements
Clinical evaluation. The following data were obtained: sex (male, female), age (years), systolic and diastolic blood pressure (mmHg), heart rate (bpm), respiratory rate (bpm), peripheral oxygen saturation (%), smoking habit (never, current), history of hypertension (yes, no), diabetes mellitus (yes, no), stroke (yes, no), myocardial infarction or known coronary artery disease (yes, no), and chronic obstructive pulmonary disease (yes, no). Medications in use and respectively diary dosage were also registered.
Laboratory tests. The following tests were performed, in accordance with institutional protocol: complete blood count, PT/INR, D-dimer (mg/L), cTnI (ng/mL), c-reactive protein (mg/dL), urea (mg/dL), creatinine (mg/dL), sodium (mEq/L) and potassium mEq/L).
Quantitative variables are presented as mean ± standard deviation or median (interquartile range) according to the Shapiro-Wilks normality test. The Mann-Whitney Wilcoxon test compared quantitative variables between groups. Categorical variables are presented as frequencies (percentages), and the Pearson's Chi-square test compared categorical variables between groups.
Kaplan-Meier analysis estimated overall survival probability and compared it between groups using the log-rank test. Cox proportional hazards regression models estimated each outcome's hazard risk (HR) and the 95% confidence interval (CI). Youden Index determined optimal cutoffs, while sensitivity, specificity, accuracy, and ROC curves assessed each model's diagnosis performances.
Statistical significance was assessed at a two-sided p-value < 0.05. R version 4.0.3 (The R Foundation for Statistical Computing, Vienna, Austria) in R-Studio 1.3.1093 (RStudio Inc., Boston, USA) was used for all analyses.
The total of 105 patients met the inclusion criteria, but the analysis of the biomarkers was not possible in five patients, due to the temporary lack of kits. The final sample consisted of 100 hospitalized patients with COVID-19, as illustrated in the Figure 1.
The basal and clinical characteristics of the included patients are presented in the Table 1. Half of these patients needed admission in ICU and 19% died during the hospitalization. It is noteworthy that about 46% of hospitalized patients SAH, 23% had DM-2 and 16% known CAD.
The comparison of clinical and laboratory characteristics of survivors (N = 81) or non-survivors (N = 19) for COVID-19 during the hospitalization are shown in the Table 2. The patients who died were older (p > 0.001), predominantly of male gender (p = 0.02) and had lower platelet count (p < 0.001) and higher levels of cTnI (p = 0.003) and D-dimer (p = 0.002) dosed in the first 24 hours of admission compared with the survivors. There were no differences between the oxygen saturation in the admission and prevalence of comorbidities in the groups.
Regarding other signals and symptoms in the admission, patients who died during hospitalization had more dyspnea sensation in the admission (p = 0.03). There was no difference between survivors and non-survivors in the following signals and symptoms: cough (p = 0.36) temperature (p = 0.90), systolic arterial pressure (p = 0.45) and heart rate (p = 0.35). In addition, there were no differences in the other laboratory markers between the groups.
In a multiple logistic regression model adjusted for age and oxygen saturation in the admission, including the biomarkers, platelets count was independently associated with death during the hospitalization (p = 0.019). The ROC curve showed area under the curve (AUC) of 0.74 [95% CI: 0.62-0.86; p = 0.001] and cutoff point < 185000/μL, sensitivity of 63% and specificity of 80% for mortality during hospitalization.
Regarding other biomarkers, D-dimer had AUC of 0.74 [95% CI: 0.62-0.86; p = 0.001] and cutoff point ≥ 1.1 mg/L, sensitivity of 83% and a specificity of 65% for the prediction of mortality during hospitalization for COVID-19. These results are presented in Figure 2. The cTnI had not a good accuracy for mortality in this analysis, with AUC = 0.6 [95% CI: 0.44-0.77; p = 0.18].
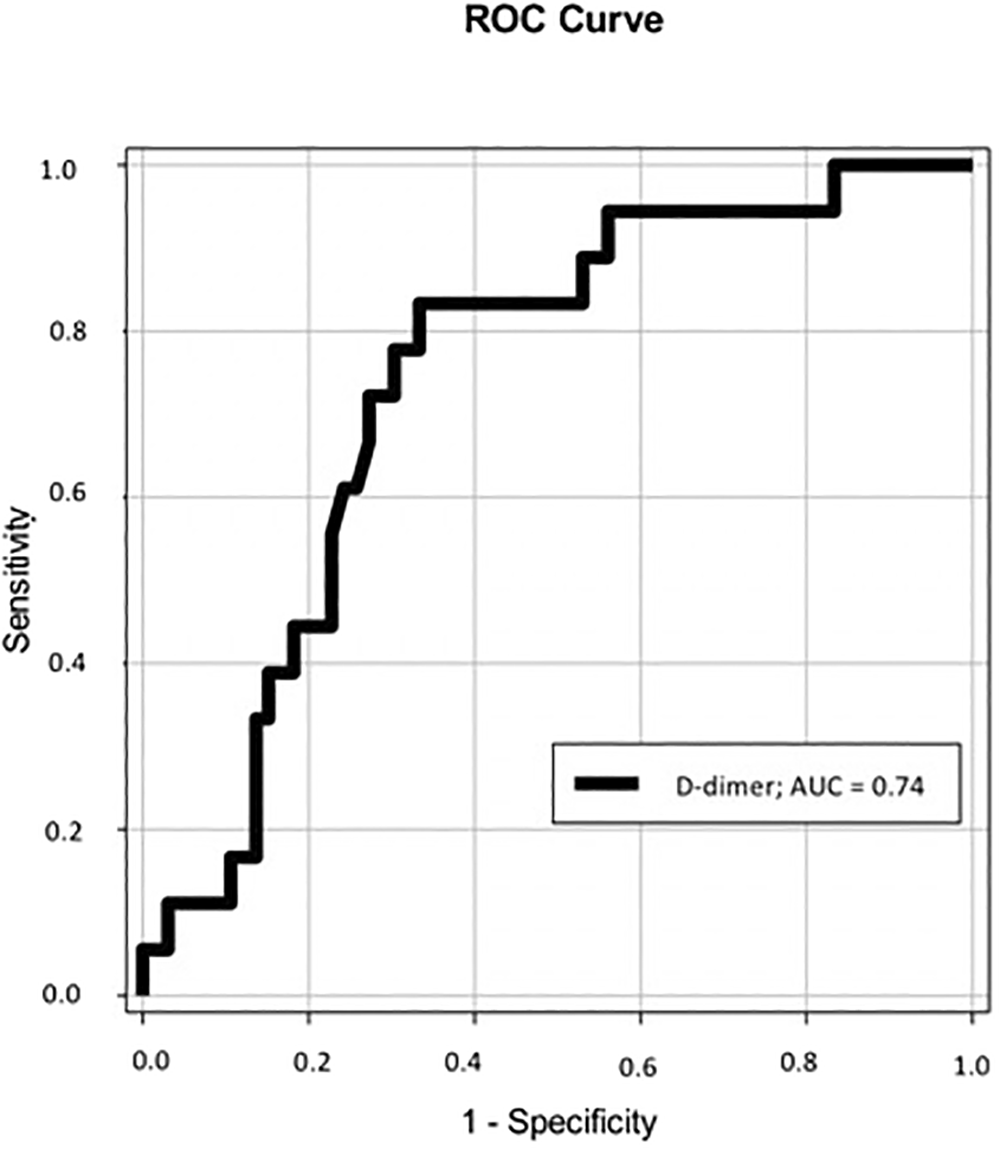
Legend. ROC: Standard operating curve.
Considering the Kaplan-Meier curves, as shown in Figure 3, hospitalized patients for COVID-19 and D-dimer ≥ 1.1 mg/L have a higher mortality when compared to those with D-dimer < 1.1 mg/L, dosed in the first day of the hospital admission. The curves of survival differed significantly in the log-rank test (p = 0.04).
The great contribution of this manuscript is the possibility to identify hospitalized patients with infection by COVID-19 with higher risk of death during hospital stay. Lower platelets counts and higher levels of D-dimer showed good accuracy to detect unfavorable outcomes in these patients, in the pre-vaccination period. Values of D-dimer above or equal to 1.1 mg/L were predictors of in-hospital mortality in these patients.
Platelets are small cells without a nucleus, with a disc shape and are considered the smallest cell units synthesized by bone marrow megakaryocytes. The release of platelets are mediated by cytokines and chemokines (Ghoshal and Bhattacharyya, 2014). These cells play a role in blood clotting, wound healing, inflammation, tumor homeostatic balance, with an average life span between seven to ten days (Jurk et al., 2005; ((Lyn) Greenberg and (Sue) Kaled, 2013). Platelet count is also used as a biomarker in the identification of several diseases, especially those with a large acute inflammatory response, due to their ability to modulate the inflammatory response (Wu et al., 2020). Currently, one of the phenomena identified in patients hospitalized with COVID-19 is thrombocytopenia (Stasi, 2012). Our results corroborate with the literature and add new information: platelets count in the first hours of admission, in a model adjusted for age and risk factors, were independently associated with death in patients infected by COVID-19. Therefore, patients with thrombocytopenia need to be closely medically monitored, because of their increased risk of death during the hospitalization.
Although the exact mechanism leading to thrombocytopenia related to patients hospitalized with COVID-19 is not known, there are studies that already showed a relationship between low platelet count and death for severe acute respiratory syndrome (SARS-CoV). The mechanism of SARS-COV-2 is very similar to SARS-COV: the inhibition of hematopoiesis in the bone marrow occurs through the interaction with certain receptors that cause a decrease in platelet formation and, consequently, thrombocytopenia (Xu, Zhou and Xu, 2020; Maquet et al., 2020). It is also described more aggregation of megakaryocytes and platelets and increased expression of P-selectin and CD40 that alter transcriptomes, changing the size, maturity and number of platelets that become more reactive to the organism itself. This leads to increased mortality in hospitalized patients with COVID-19 (Barrett et al., 2021). Furthermore, there is the release of mature megakaryocytes that cause a decrease or morphological alternation in the pulmonary capillary bed, leading to morphological destruction of platelet cells (Lei et al., 2021).
Lymphocytopenia was recently identified in association with SARS-COV-2, being considered an indicator of severity and hospitalized patients with COVID-19, in addition to having a direct association with the mortality rate (Tan et al., 2020; Zhao et al., 2020). In the present study, there was no significant association with low lymphocytes count and higher mortality. This probably occurred because of the limitation of the small sample.
D-dimer levels are related to COVID-19 infection, mainly with disease severity and mortality during hospitalization (Zhang et al., 2020). Values of D-dimer above 1 mg/L are associated with changes in coagulation and inflammatory response (Yu et al., 2020). Patients with mild symptoms had lower levels than those with more severe symptoms (Logothetis et al., 2021). Thus, due to the cytokine storm caused by the acute infection of COVID-19, together with hypoxia secondary to lung injury and diffuse intravascular coagulation, it causes venous thromboembolism due to coagulopathy (Rostami and Mansouritorghabeh, 2020). Interestingly, in our study, mild elevation of D-dimer in the first hospital day (cutoff point 1.1 mg/L), even with no direct association with thromboembolic phenomenon, had a good accuracy for prediction of death during the days of hospitalization (Nadeem et al., 2021). Recent studies directly have described high levels of D-dimer during hospitalization as a predictor of mortality, especially in patients with DM and the elderly (Soni et al., 2020; Mouhat et al., 2020). Elevation of the D-dimer is usually due to a cytokine storm, and the increase in pro-inflammatory cytokines causes an imbalance in the coagulation system. Consequently, this leads to hypoxia through activation of the hypoxia-inducible transcription factor-dependent signaling pathway, favoring the onset of thrombosis (Poudel et al., 2021). Hypercoagulability is a direct factor related to increase D-dimer, being present in higher levels in hospitalized patients who died for lung tissue damage (Chocron et al., 2021). In addition, individuals with increased D-dimer often have myocardial injury and disseminated intravascular coagulation (DIC) on radiological examinations (Varikasuvu et al., 2021).
Currently, cTnI has been used as a biomarker related to COVID-19 infection. It is released mainly because of the myocardial injury caused by excessive inflammatory reaction through cytokine storms. Thus, these cardiac injuries are also related to increased mortality and hospitalizations, including respiratory complications (Liao et al., 2020). In our study, higher cTnI levels were more prevalent in non-survival patients. Nevertheless, cTnI had no good accuracy to identify patients with adverse outcomes. Despite this, one study pointed out that cTnI is a great predictor of 30-day mortality (Tersalvi et al., 2020).
The limitation of the study was the small sample size and limited kits for biomarkers analysis. The great relevance of this study was to identify biomarkers associated with more in-hospital mortality in the natural history of COVID-19, in the pre-vaccination period and without specifically aimed at the effective cure of this disease.
Future clinical perspectives implicate the comparison of the behavior of these markers in completely vaccinated hospitalized patients and how new advanced treatments may influence the inflammatory response.
D-dimer high levels and platelet count at the first 24 h of admission have good accuracy in the detection of increased risk of death in hospitalized patients with COVID-19. These findings are important to identify in the first hours of hospitalization which patients have more risk of death and need a more intensive medical approach.
The role of biomarkers in the prediction of mortality in hospitalized patients for COVID-19. DOI: https://doi.org/10.17605/OSF.IO/ND9QS (Cruz et al., 2022).
This project contains the following underlying data:
The authors thank the entire medical, nursing and multidisciplinary team of the university hospital that dedicated themselves to the care of patients with COVID-19 included in this study.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Systematic review and meta-analysis for COVID-19-related treatment.
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
No
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: My specialty is respiratory medicine, especially infectious diseases such as pneumonia and tuberculosis.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 06 Jul 22 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)