Keywords
Pediatric malaria, incidence, mapping, Sussundenga, Mozambique
This article is included in the Pathogens gateway.
This article is included in the Emerging Diseases and Outbreaks gateway.
Pediatric malaria, incidence, mapping, Sussundenga, Mozambique
Malaria is a parasitic-borne disease that affects red blood cells. The World Health Organization (WHO) recorded 229 million malaria cases in 2019 and, of these, there were 409,000 deaths in 87 endemic countries and 67% of these occurred in children under five years. Worldwide, 95% of malaria cases occurred in 29 countries.1
Mozambique is among the six countries that account for half of all malaria cases and deaths globally and has the fifth highest number of cases. Due to the control programs, malaria prevalence and incidence decreased by 50 and 40%, respectively. In Southern Africa, the malaria decrease was between 50 to 91%. In Mozambique, the reduction is slow, and between 2015 and 2018, cases reduced only by 14%.2,3
The disease is preventable, detectable, treatable, and more common in poor regions. It has socioeconomic impacts, representing a significant burden on countries' revenue where it is endemic.
In Mozambique, malaria is undoubtedly one of the main public health concerns impacting families and the economy. In 2020 the country reported 8,921,081 cases and 1,114 deaths.1 Although the entire population of Mozambique is at risk, children and pregnant women are at higher risk owing to lower immunity. In the Chimoio region/province, children under five years old are three times more prone to malaria than adults.4
Malaria transmission in Mozambique occurs all year round, and peaks between January and March. Malaria transmission strongly correlates with rainfall temperature, with relative humidity accounting for 72 to 80% of the cases.5–7
Despite those weather conditions, malaria occurrence is also influenced by socio-demographic determinants such as age, gender, level of education, residence type (rural and urban).8–10 Age can affect the course and progression of the diseases and correct treatment.11 Very few studies in pediatric malaria exist in Mozambique, and the existing ones use a simplistic and coarse grouping of 0 to 4 and over 5 years, not reflecting stages of development and the current standard age categories.
The malaria environmental dependency results in a complex pattern of geographical variation in transmission at almost every scale. Malaria risk is rarely uniform, whether between households in a village, villages in a district, or districts in a country. The knowledge of the spatial distribution of malaria and the evaluation of local heterogeneity by generating malaria risk maps can improve the understanding of pediatric malaria, especially in children.
Hence, this study intends to determine the malaria determinants and map the risk of malaria in children with and without clinical data. The knowledge generated by the present research can help formulate malaria control programs and policy strategies for malaria control in under 14 age categories in Mozambique.
This is a pilot study to determine malaria prevalence, risk factors, and health seeking behaviors in Sussundenga to map the risk of malaria in children. Retrospective data were collected from Sussundenga Health Center from January to May 2020.
Sussundenga village is a rural municipality in the center of Manica Province, Mozambique (33°29′52″ and -19°41′30″), administratively divided into 17 residential areas within 156.9 km2 (Figure 1).
It has a population of 41,354 inhabitants, 52% female and 48% male, with a 2.5% annual growth rate. The pediatric population (0 to 14 years) comprises 45.6% of the total population, being 4.4% less than 1 year, 11% from 1 to 4 years, and 30.2% from 5 to 14 years old.12 The livelihood of the inhabitants is based on subsistence agriculture.
The hydrographic network comprises six main rivers with a permanent flow. Most of the population has deficient access to health services in the municipality since there is only one public hospital.
The climate is warm-temperate with dry winters from April to July, hot and dry summers from August to October, and hot, humid summers from November to March. The average rainfall is 1,067.6 mm, varying significantly in the amount and distribution within and between October and November are the hottest months recording averages of 30°C. July is the coldest month with averages of 12°C. The predominant vegetation is associated with open, deciduous forests, evergreen forests, prairie, shrubs, and savanna.13
In public hospitals, all patients are registered in a record book upon arrival. A malaria test at Sussundenga District Hospital (SDH) uses a rapid diagnostic test (RDT) and a few microscopic tests for confirmation. Patient daily data were collected from 24 book records of the Pediatric Department of SDH from November 2018 to October 2020. The dataset can be found under Underlying data.49 The following variable data were recorded for the positive malaria cases: date, sex, origin, and age category. The age was grouped into five pediatric age categories: 0 to 5, 6 to 11, 12 to 23, 24 to 59 months, and 5 to 14 years old. Missing data comprised only 1.2% and were removed from the study.
The disease's weight was calculated by dividing the positive malaria cases by the total patient visits. The malaria incidence per 100 persons in each neighborhood was calculated by dividing the total number of cases occurring in each Bairro by the total population of the Bairro and then multiplied by 100.14 Chi-Square and G test tests for the proportion of gender and age category were used to test statistical significance.
For malaria risk mapping. Bioclimatic (WorldClim),15 Diva GIS 7.4.0,16 Landsat 8 image,17 accessed on September 29, 2019 with a resolution of 30 m × 30 m and, Digital Elevation Model (DEM)18 of 30 m × 30 m were used.
A three-step strategy was applied to develop the map: Step 1. Malaria risk factors identification, Step 2. Determining risk factor weights (Analytical Hierarchical Process and consistency check), Step 3, Mapping risk of malaria and accuracy assessment of the produced map.
Step 1. Malaria risk factors identification
Ten risk factors used in the weights, classes, and rank are in Table 1.
i) Population density (Inhabitants/km2)
To calculate population density, Mozambique national statistics projections for 2020 was used and, data were related to the neighborhood administrative units.19 Density lower than 2,000 inhabitants per km2 was considered low risk; from 2,001 to 4,000 was considered moderate risk while over 4001 was considered high malaria risk.
ii) Malaria Incidence per 100 persons per residential area
The malaria incidence per 100 persons in each neighborhood was calculated. Incidence from 0 to 40 per 100 persons was considered low risk, 41 to 80 was considered moderate risk, and over 80 was considered high risk of malaria occurrence.
iii) Altitude (m)
To estimate altitude, a digital elevation model (DEM) at 30-meter × 30-meter resolution was used. Altitude lower than 200 meters was considered to have high malaria risk, from 201 to 1000 meters was considered to have moderate malaria risk, and over 1000 meters was considered to have a low malaria risk.20
iv) Average temperature (°C)
The surface temperature in degrees Celsius was extracted in the terminal infrared range of the TIRIS/Landsat-8 sensor in Band 10.21 The formula for temperature calculation was:
Temperatures ranging 22 and 32°C were classified as high risk, while less than 22°C were considered low malaria risk and, above 32°C was considered moderate malaria risk.20
v) Rainfall (mm)
Rainfall data were extracted from worldclim 2-5m.cli data and processed in Diva GIS.
Areas that received an amount of rain less than 450 mm were considered to have low malaria risk, those that received an amount of rain ranging 450 to 700 mm were considered moderate malaria risk and above 700 mm of rain were considered high malaria risk.20
vi) Slope (degrees)
The slope was derived from the 30 m × 30 m DEM obtained from the ArcGIS spacial analysis tool. Areas ranging 0 to 5 degrees were considered to have high malaria risk, from 5 to 15 degrees were considered to have moderate malaria risk and, above 15 degrees were considered to have lower malaria risk.20
vii) Normalized Difference Vegetation Index (NDVI)
The Landsat Normalized Difference Vegetation Index is used to quantify vegetation greenness, and greater amounts of green vegetation in the soil indicate greater NDVI. The NDVI was extracted from a Landsat image and calculated by the following formula:
Where:
NIR = Reflectance in the near infrared band.
RED = Reflectance in the red range of the spectrum.
The NDVI from -0.288 to 0 was considered as low malaria risk, from 0 to 0.25 was considered as moderate malaria risk and from 0.255 to 0.986 was considered as high malaria risk.20
viii) Distance from the road (km)
The Euclidian distance to the nearest road determines accessibility and malaria interventions capability and, was calculated using the measuring distance function in ArcGIS 10.2.2 software from a Landsat image 30 m × 30 m from September 2019. Distances less than 2.5 Km from the nearest road were considered as low malaria risk, from 2.5 to 5 Km considered as moderate malaria risk and more than 5 km considered high malaria risk.20
ix) Distance to water bodies (km)
Stagnant water bodies constitute mosquito breeding sites. ArcGIS was used to calculate the nearest waterbody by classifying a 30 m × 30 m Landsat 8 image from 2019 for water and in defined areas. The measurement distance function of ArcGIS software was employed. Areas above 1.5 km were considered as low malaria risk, from 0.5 to 1.5 km considered moderate malaria risk and lower than 0.5 km low malaria risk.20
x) Land use and cover (LULC)
Landsat 8 satellite image (September 2019) was used to retrieve LULC data. The reclassification of the image into different classes was carried out using the manual training sampling technique and the maximum likelihood algorithm. Areas with crops, grass and waterbodies were considered as high malaria risk, Shrub and mosaic cover areas were considered moderate malaria risk while forest, bare and urban settlements were considered low malaria risk.20
LULC Classes
1. Agricultural crop area, grass and water body.
2. Shrub area and mosaic cover vegetation
3. Forest, nude and urban settlement areas21
Step 2. Determining risk factor weights (Analytical Hierarchical Process) and consistency check.
The analytical hierarchic process was determined as previously described.20 To check for consistency, an actual consistency ratio for the corresponding matrix was calculated by dividing the consistency index for the set of judgments by the index for the corresponding random matrix. Saaty suggests that if that ratio exceeds 0.1, the judgments may be too inconsistent to be reliable.22
Step 3. Mapping risk of malaria risk and accuracy check
Two risk maps were produced using spatial, anthropic, and environmental variables; one risk map used ten risk factors, while the other excluded the incidence data. Figure 2 presents the spatial analyses carried out.
A raster dataset was created using the variables. The reclassification was done, with all the measures given equal numeric scale, assigning them the same level of importance. For model suitability, reclassified outputs of the variables were combined assigning them weights. For the comparison of class values among layers and, numeric values of classes within each map layer, the variables were assigned values from 1 to 3, representing low, moderate and high malaria risk.
Applying the overlay method, where each of the input maps is allocated a weight and every class and spatial unity existed in each variable map, different classes on a single map presented different weights and each variable map also had its own weight.
By summing all of the input variable maps after each had been multiplied by its overall weight, two final maps of risk were produced, one using the incidence variable and other without as presented in the equation:
Where:
Wi = weight of i-th variable map,
Sij = the i-th spatial class weight of j-th
S = is the spatial unit value in the output map.
As a result, a new raster surface was generated representing different levels of risk of malaria based on the variables.20
Tests were performed using SPSS IBM version 2023 and for mapping ArcGIS 10.7.3.24
From November 2018 to October 2020, 42,248 patients visited the pediatric department of SDH, and 21,663 (51.2%) were positive for malaria. Of the malaria-positive patients, 89.9% were from the Sussundenga municipality. The incidence per 100 persons was 45.7 for the year 2019. As per gender, the positive malaria patients were equally distributed, 48.4% male and 51.6% female.
The average age of pediatric malaria patients was 5.7 SD (Standard deviation) = 1.38 years. Figure 3 presents age group distribution, and the age group 0 to 5 months showed the most negligible percentage of cases (3.3%), while the age group 5 to 14 years old presented the highest rate of patients (45.6%). No difference was found between sex among the age categories. The full dataset can be found in the Underlying data.49
Malaria incidence by residential areas
The incidence per residential area varied from 6.6 per 100 persons to 118 per 100 persons. There is a difference between residential areas in malaria incidence G = 377.38, P = 0.0001, DF = 16 and, Nhamarenza present the highest incidence, 118 per 100 persons, and the most negligible incidence was given in residential areas Chizizira and Tave with 6.6 and 6.7 per 100 persons respectively (Figure 4).
Malaria cases by month
The percentage of pediatric malaria cases for 2019. January showed the highest rate of patients (19.7%), followed by May (19.6%), and the most negligible percentage of cases occurred in September (0.1%) (Figure 5).
i Analytical Hierarchical Process and consistency check
The comparison matrix of 1 × 10 and 9 × 9 risk factors was used in this study. A value of 1 means that the malaria risk factors being compared have the same weight. A value of one means that variables being compared have the same weight in terms of malaria risk. A value of four for example, means that the variable in the column is four times more important in malaria risk than the comparison in the row (Tables 2 & 3). The consistency ratio was 0.081 and 0.096 for 10 × 10 and 9 × 9 matrix, respectively, which was considered good enough.
Mapping risk of malaria and accuracy check
The risk factor maps before reclassification are presented in Figure 6: the average temperature in the area ranges from 20.14 to 21.17 °C, and rainfall ranges from 1028 to 1,082.24 millimeters, altitude ranges from 459 to 791 meters, slope ranges from 0 to 37.5°, distance to water bodies ranges from 0 to 15,134 meters, NDVI ranges from -0.14 to 0.5, population density ranges from 41 to 59,091 people per km2, malaria incidence ranges from 2.9 to 118.
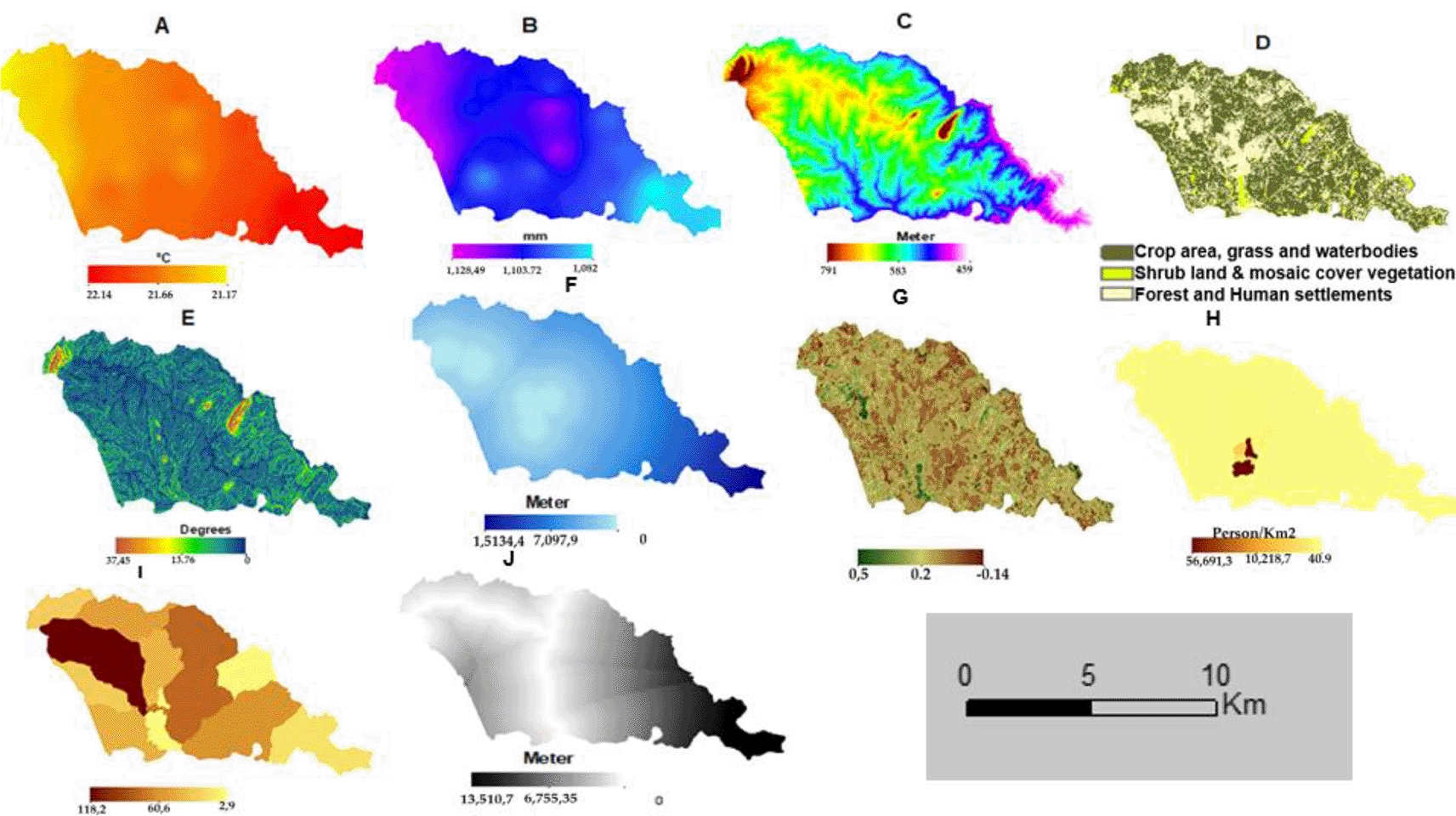
(A). Temperature. (B) Rainfall (C). Altitude. (D). LULC, (E) Slope (F). Distance to water bodies. (G) NDVI for Sussundenga Village (H). Pop. Density. (I). Malaria incidence. (J) Distance to roads.
Figure 7 presents the maps for the reclassified risk factors and the malaria risk in percentage. Rainfall (100%) and slope (73%) have the highest chance, altitude (100%), and NDVI (92%) have moderate risk in the municipality.
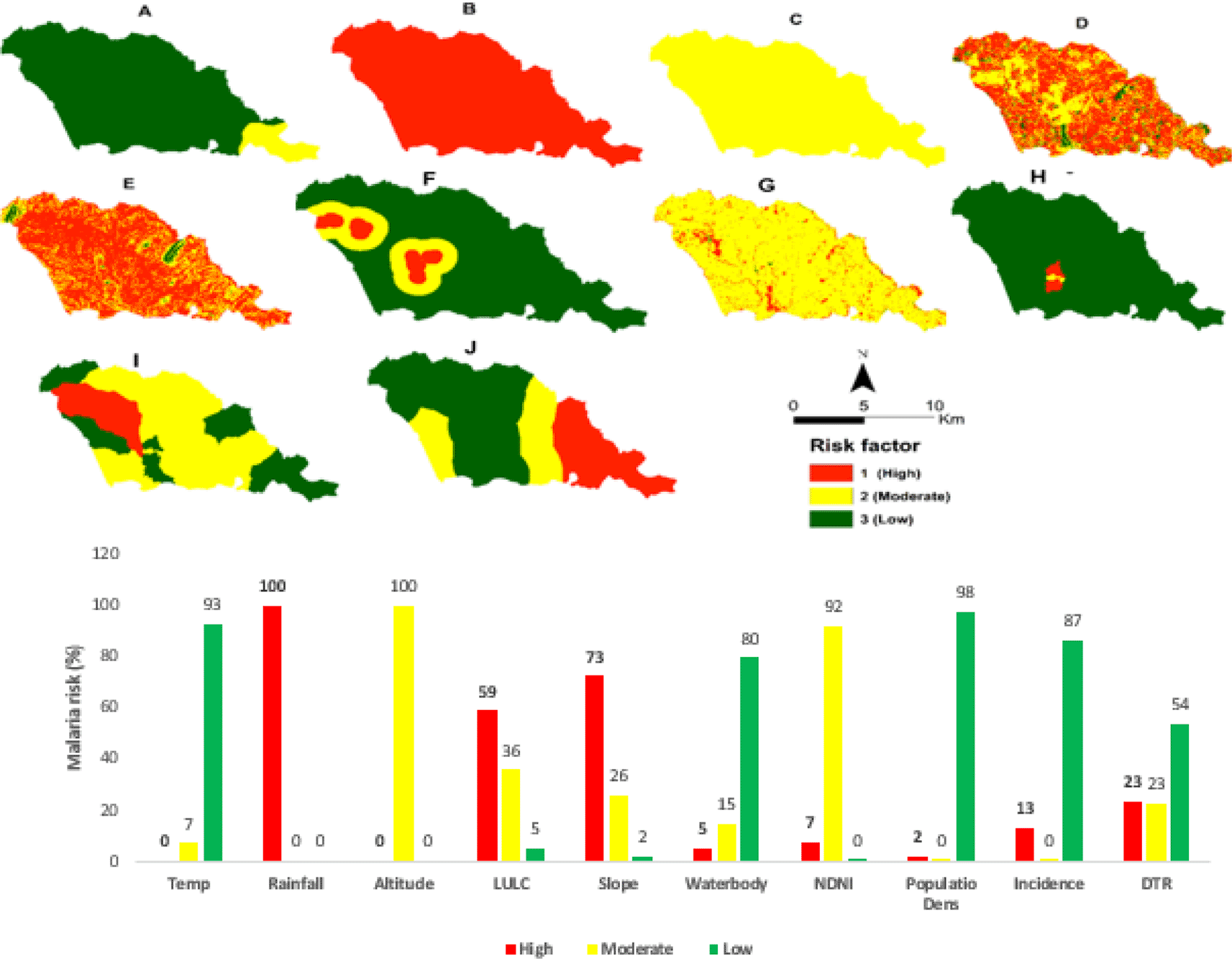
The spatial model derived to produce the two malaria risk maps from the risk factors are presented in formulas 4 and 5.
Figure 8A and Figure 8B present the final malaria risk maps for Sussundenga municipality after the consolidation and the weighting using the incidence data and excluding the incidence data. The entire Sussundenga municipality is at risk of malaria, varying from moderate to high risk. Malaria's high risk coincides with the highly populated area and surrounding water bodies.
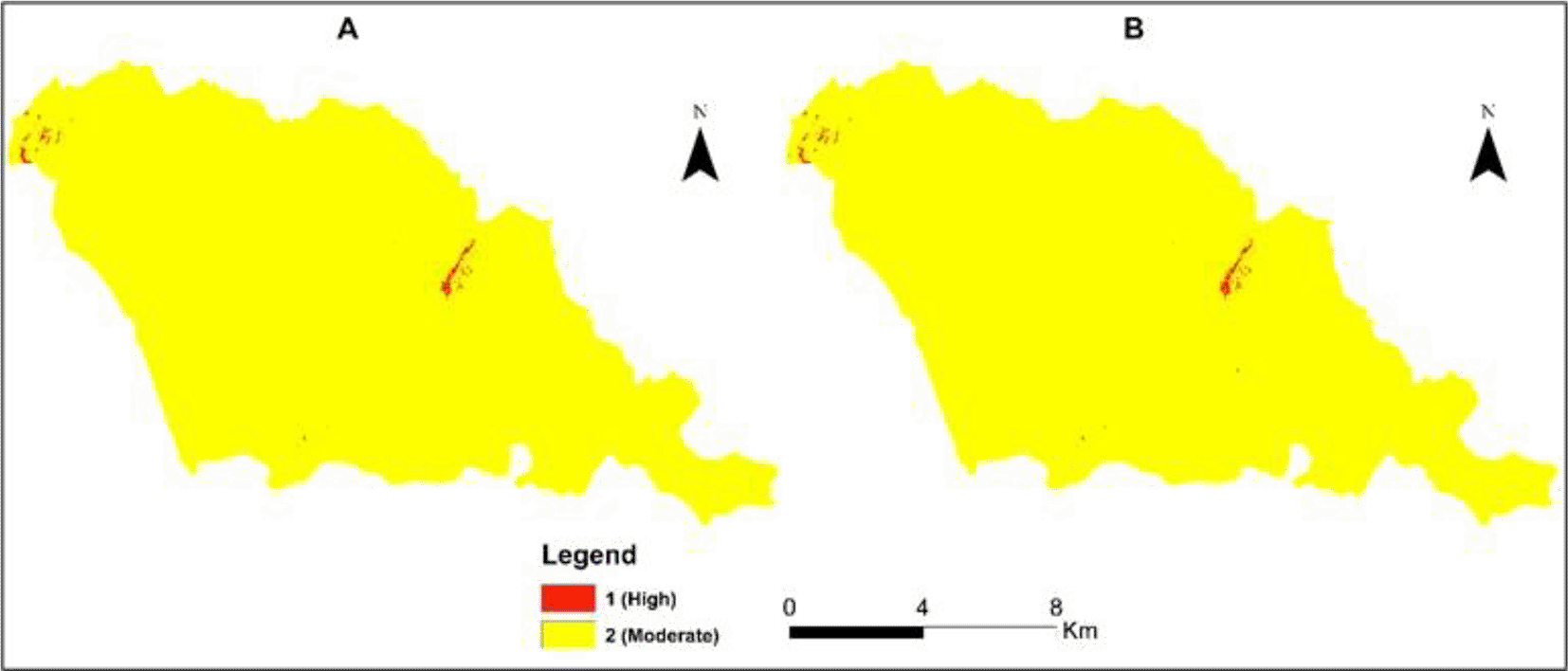
A. Including incidence. B. Excluding incidence.
Table 4 presents the percentage of malaria risk in Sussundenga, and there is no difference in the risk areas using and excluding the clinical data (incidence data).
| Risk of Malaria | Area (hectares) | % |
|---|---|---|
| A | ||
| 2 (Moderate) | 16,333,11 | 99,72 |
| 1 (high) | 46,44 | 0.26 |
| B | ||
| 2 (Moderate) | 16,332,55 | 99,7 |
| 1 (high) | 46,89 | 0.3 |
Table 4A and B present the area of the high and moderate malaria risks.
In this study, the pediatric weight of malaria was 51.2%. In 2015 malaria was responsible for 45% of outpatient visits and 56% of pediatric admissions in Mozambique,2 consistent with these results. A study in Manhiça, a rural area in the south of Mozambique in 200825 indicated 30.5% had malaria, lower than the results of the present study, and this can be related to environmental conditions since the south of Mozambique is drier. In Malawi, a neighboring country, the weight of the disease ranged from 26% in Salima to 64% in Mwanza.26
In this study, the malaria incidence in pediatric malaria was 45.7 per 100 persons. A 2018 study reported an incidence of 39 per 100 in children under 10 years in the Central region of Mozambique.27 A study in Malawi indicated 35 to 37 per 100-person incidence in children under 15 in 2017.9 In Zimbabwe, significant progress was made in malaria cases reduction, and the incidence is 2.5 per 100 persons, while in Zambia is 20 per 100 persons.28,29
The mean age of malaria patients in this study was 5.6 SD 1.3 years. In Southern Africa, 29 studies in 2010 reported a median age of clinical malaria of 32 months highly intense and not markedly seasonal transmission areas and 72 months in low-intensity and markedly seasonal transmission areas consistent with this study.30 In China, the mean average of most childhood diseases was reported to range from 20 to 80 months and, for high incidence pediatric disease approximately 3 years.31
In this study, the age group 5 to 14 years presented the highest percentage of cases, 45.6%, while this category comprises 30.2% of the municipality inhabitants. A community study in the same municipality in 2021 reported 50% of positive malaria cases in children aged 5 to 14 years,32 consistent with these results. A study in Manhiça Mozambique22 reported 36% of cases among age group 5 to 14 in 2008 less than in this study, while, in Inhambane, Mozambique33 in 2015, reported a higher figure of 67.7%. In Malawi and Kenya, results consistent with the present findings were reported.34,35
A shift in the peak age of cases from 1 to 4 years old to 5 to 9 years old was reported in 2009 in a study in Inhambane, Mozambique.36 In many malaria-endemic areas, successful control programs have substantially reduced transmission levels. Consequently, in such communities, the peak age of clinical attacks of malaria is shifting from very young to older children.37–39
The most minor malaria cases in this study occurred in children aged 0 to 5 months. For approximately six months after birth, antibodies acquired from the mother during pregnancy protect the child. This maternal immunity is gradually lost as the child develops their immunity to malaria. In areas where malaria is endemic, children are believed to achieve a high level of immunity up to 5 years of age.40,41 Higher usage of bed nets was also reported in pregnant mothers and children less than one year than in children 5 to 14 years.30,42
This study showed a difference in malaria incidence between residential areas varying from 6.6 to 118 per 100 persons. The results are consistent with Chimoio, Sussundenga, and Mozambique.4–6 In Ethiopia and Kenya, spatial variation of malaria incidence in a geographically homogeneous area was also reported,41,43 and this can be a result of high endemicity. Heterogeneous rates from 2.5 to 10.5 episodes per 100 children per year were also reported in Senegal.44 In Malawi, geographical groups of households where children experienced repeated malaria infections overlapped with high mosquito density areas.45 In Brazil, the high incidence of malaria at a low scale was due to the heavily modified landscape.46
Most malaria cases occur from January to March in Mozambique.5,6 For 2019, the high number of malaria cases continued from January to May. This is a result of the Cyclone Idai that occurred in March 2019 and resulted in heavy rain and floods in the region. The malaria temporality was also reported in other countries in Africa.47,48
The age of pediatric malaria is shifting from 0 to 4 to the age category 5 to 14 years, and targeting this to combat malaria should be addressed. Mapping malaria risk at a low scale is feasible without using clinical data and can provide tools to improve the strategy of malaria combat in children. Malaria eradication needs to involve medical disciplines and other fields such as economics, geography and ecology, and social sciences. The use of GIS and mapping can contribute to the design and implementation of control malaria strategies by defining precisely the pattern of malaria occurrence.
This study used retrospective data to calculate pediatric malaria incidence. With climate change and extreme events such as Cyclone Idai, different results may occur in the future due to different circumstances. Previous mapping of Sussundenga was carried out using clinical data. This study aimed to prove that, even without clinical data, malaria risk can be forecasted.
The retrospective data were collected at Centro de Saude de Sussundenga, EN216, 2207 Sussundenga, Mozambique. Coordinates: -1940954, 33.29355. Permission was granted by the District Director of Health of Sussundenga for the use of this data.
Harvard Dataverse: Replication data for: Pediatric malaria incidence and risk mapping in Sussundenga Municipality, Mozambique, https://doi.org/10.7910/DVN/UL1CW7.49
This project contains the following underlying data:
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
This study is part of the Malaria Risk, Prevention, and Health Seeking Behaviors in Sussundenga, Mozambique Project. Ethical review and approval for the study was completed by the Institutional Review Board (IRB) at the University of Minnesota [STUDY00007184] and from A Comissão Nacional de Bioética em Saúde (CNBS) at the Ministry of Health of Mozambique [IRB00002657].
We would like to thank the Provincial and district directorates of health for granting permission to carry out this study, especially Dr. Firmino Jaqueta, Dr. Serafina Benesse, Dr. Filipe Murgorgo and Mrs. Elsa Trabuco. We thank Mrs Jesca Mutowo for the English revision. An earlier version of this article can be found on Research Square (https://orcid.org/0000-0002-3543-6459).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Micronutrients, malaria, anaemia and food fortification or supplementation are my areas of research. I am also involved in clinical trials, especially among infants and young children
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Infectious Disease,MDR , Congenital Malaria , Nanotechnology & Microbes, Candidemia ,Bacteriology , ESS & section systems , Covid -19
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
References
1. Ibrahim OR, Alao MA, Suleiman BM, Mokuolu OA: Outcomes of childhood severe malaria: a comparative of study pre-COVID-19 and COVID-19 periods.BMC Pediatr. 2023; 23 (1): 177 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Pediatric Infectious disesase: Malaria, Tuberculosis
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 1 07 Jul 22 |
read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)