Keywords
Acinetobacter baumannii, antibiotic combinations, time-kill, meropenem, ampicillin-sulbactam, amikacin
Acinetobacter baumannii, antibiotic combinations, time-kill, meropenem, ampicillin-sulbactam, amikacin
In accordance with the reviewers' recommendations, we made several adjustments. The Figure and Table have been altered to convey the content better. On the underlying data in FigShare, tables have been newly constructed. The writing of the manuscript has undergone a few minor adjustments.
See the authors' detailed response to the review by Sarunyou Chusri
See the authors' detailed response to the review by Ardiana Kusumaningrum
Acinetobacter baumannii is a Gram-negative rod that garners attention due to its role as a primary pathogen in healthcare-associated infections with a broad spectrum of antibiotic resistance1,2. Carbapenems are the preferred treatment for multidrug-resistant (MDR) A. baumannii infections. However, treatment options have dwindled due to high isolation rates of extensively drug-resistant (XDR) A. baumannii with concurrent carbapenem resistance3,4.
The discovery of new antibiotics is critical for treating MDR and XDR A. baumannii infections. Nevertheless, antibiotic studies take a long time to complete and are difficult to implement in developing countries with limited access to the latest antibiotics. The alternative strategy that has gathered the most interest is antibiotic combination therapy, which is theoretically supposed to boost antibiotic effectiveness compared to single antibiotics5–7.
In studies evaluating antibiotic combinations, isolates that are susceptible to at least one of the regimens are frequently used, whereas many A. baumannii clinical isolates frequently lack susceptibility to any antibiotic5,8. Additionally, because the antibiotic concentrations used in studies are typically multiple times of minimum inhibitory concentration (MIC) and are difficult to achieve during the administration of therapeutic antibiotic doses, the clinical application of study results is complicated5,9–11.
Meropenem is one of the few remaining low-toxicity treatment options for MDR and XDR A. baumannii infections12,13. Sulbactam is a beta-lactamase inhibitor with intrinsic activity against A. baumannii, whilst amikacin is an aminoglycoside with relatively maintained efficacy against multidrug-resistant Gram-negative bacteria, including A. baumannii14–17. Ampicillin-sulbactam and amikacin are two antibiotics that are available and easy to obtain in Indonesia. A sole sulbactam regimen is not available; it is marketed in conjunction with ampicillin or cefoperazone. Ampicillin-sulbactam formulations were chosen because of the availability of breakpoints in CLSI M100 2022 and technical considerations such as affordability and convenience of access to the antibiotics.
Numerous in vitro studies have demonstrated synergy between meropenem and ampicillin-sulbactam as well as meropenem and amikacin; thus, this study aimed to compare the growth kinetics of various A. baumannii strains exposed to these two antibiotic combinations at clinically relevant concentrations18–23.
Experiments were conducted on two MDR, one XDR clinical isolates from Clinical Microbiology Laboratorium Dr. Soetomo General Academic Hospital, and one standard reference isolate (ATCC A. baumannii 19606 KWIK-STIKTM Microbiologics). All clinical isolates are meropenem resistant, conforming to the Clinical and Laboratory Standard Institute (CLSI) 2022 breakpoint for A. baumannii (MIC >8 μg/ml as determined by an automatic susceptibility test using BD Phoenix® ID/AST instrument). MDR-1 is resistant to meropenem and amikacin (MIC >32 μg/ml) but is intermediate to ampicillin-sulbactam (MIC 16/8 μg/ml); MDR-2 is resistant to meropenem and ampicillin-sulbactam (MIC >16/8 μg/ml) but is intermediate to amikacin (MIC 32 μg/ml). XDR exhibited resistance to all antibiotics tested.
This study was reviewed by the Ethics Committee of the Faculty of Medicine, Airlangga University (0758/LOE/301.4.2/I/2022).
Drug concentrations were selected based on the CLSI breakpoint value for the susceptible category of tested antibiotics as it represents clinically achievable concentrations of drugs in human plasma following standard dosing. Fresh stocks of each antibacterial were prepared on the day of the experiment to achieve 0.5 MIC + 0.5 MIC, 1 MIC + 1 MIC, 2 MIC + 2 MIC, and 2 MIC + 0.5 MIC of meropenem + ampicillin-sulbactam and meropenem + amikacin (Sigma). Prior to the time-kill assay experiment, strains were subcultured onto blood agar (Oxoid CM0055 Blood Agar Base supplemented with 5% sheep blood) and incubated for 24 hours at 35°C. Mid-log phase growth suspension was obtained by inoculating isolated colony into cation-adjusted Mueller-Hinton broth (Oxoid CM0405 Mueller-Hinton Broth base) followed by 4 hours of incubation at 35°C. Static time-kill experiments were performed in sextuplicates on separate days at an initial inoculum of 6×105 CFU/ml with the combined antibiotic concentrations in the glass tube, incubated at 35°C. Samples were collected at 0, 1, 2, 4, 6, 8, and 24 h, measured for turbidity by nephelometer (BD PhoenixSpecTM Nephelometer), serially diluted in saline, plated on Mueller-Hinton agar (Oxoid CM 0337 Muelle-Hinton Agar base), and counted after 24 h of incubation for viable-cell counting. Enumeration was performed manually after 24 hours of incubation at 35°C. The limit of detection (LOD) was 102 CFU/ml. In the meantime, a control experiment was carried out simultaneously with the same procedure without antibiotic addition. Bactericidal activity was assessed as a ≥ 3 log10 reduction in a colony-forming unit (CFU)/mL over the period measured. Regrowth was defined as an initial decrease of turbidity or colony count followed by an escalation in the subsequent measurement hour.
The turbidity and colony count data did not follow a normal distribution (Shapiro-Wilk value 0.000). There were significant differences in mean turbidity between isolates of ATCC 19606, MDR-1, MDR-2, and XDR at 2, 4, 6, 8, and 24 hours following antibiotic exposure (p<0.05; Wilcoxon; CI 95%). There were significant differences in the mean colony count between isolates of ATCC 19606, MDR-1, MDR-2, and XDR at 6, 8, and 24 hours following exposure, (p = 0.001, p = 0.01, and p = 0.000; Wilcoxon; CI 95%). The full turbidity and colony count data can be found under Underlying Data24.
Exposures to meropenem and ampicillin-sulbactam yield encouraging results. In the MDR-1 isolate, which was resistant to carbapenem and intermediate to ampicillin-sulbactam, the bactericidal effect of meropenem and ampicillin-sulbactam was achieved at a 2 MIC + 2 MIC concentration, respectively (Figure 1). During 0–24 hours, concentrations of 0.5 MIC + 0.5 MIC, 1 MIC + 1 MIC, and 2 MIC + 0.5 MIC were able to sustain growth under the rate of growth control, as demonstrated by turbidity measurements. However, the turbidity was approximately indistinguishable at 48 hours (Figure 2). Changes in the number of colonies could not be observed at 0.5 MIC + 0.5 MIC and 1 MIC + 1 MIC concentration due to high colony count results. Exposure to a 2 MIC + 0.5 MIC concentration caused a transient inhibitory effect for up to 4 hours, but regrowth occurred at the hour of measurement thenceforth.
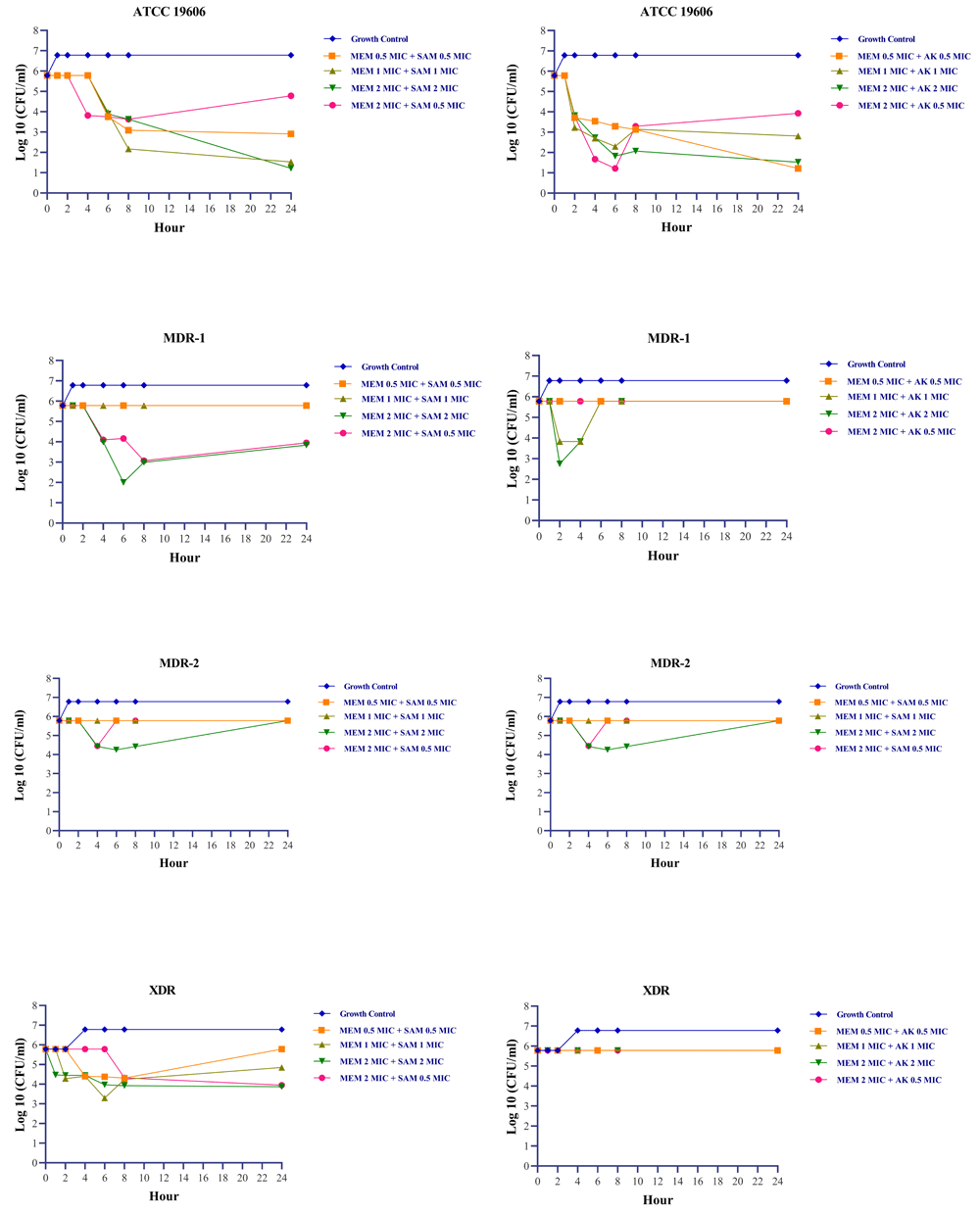
MEM: meropenem, SAM: ampicillin-sulbactam, AK: amikacin, MIC: minimum inhibitory concentration. Acinetobacter baumannii’s MIC based on CLSI 2022 susceptible breakpoint: Meropenem 2 µg/ml, Ampicillin-Sulbactam 8/4 µg/ml, Amikacin 16 µg/ml.

MEM: meropenem, SAM: ampicillin-sulbactam, AK: amikacin, MIC: minimum inhibitory concentration. Acinetobacter baumannii’s MIC based on CLSI 2022 susceptible breakpoint: Meropenem 2 µg/ml, Ampicillin-Sulbactam 8/4 µg/ml, Amikacin 16 µg/ml.
MDR-2 isolate (isolate resistant to meropenem and ampicillin-sulbactam) treated with meropenem and ampicillin-sulbactam combination at concentration equal to or less than the MIC demonstrated higher turbidity compared to positive growth control after 24 and 48 hours. At a concentration twice the MIC, there is a reduction in colony count after four hours, followed by regrowth. During post-exposure monitoring, XDR isolate exposed to meropenem and ampicillin-sulbactam did not show any signs of regrowth, except at a concentration of 1 MIC + 1 MIC, where regrowth occurred at 8 and 24 hours (Table 1).
| Isolate | Antibiotic | Concentrationa | Activityb | Δ Log 10c | Regrowthd | Turbidity measurement higher than growth controle |
|---|---|---|---|---|---|---|
| ATCC 19606 | MEM + SAM | ½ MIC + ½ MIC | Bacteriostatic | 2.87 | No | No |
| 1 MIC + 1 MIC | Bactericidal | 4.25 | No | No | ||
| 2 MIC + 2 MIC | Bactericidal | 4.56 | No | No | ||
| 2 MIC + ½ MIC | Bacteriostatic | 2.15 | Yes | No | ||
| MEM + AK | ½ MIC + ½ MIC | Bactericidal | 4.56 | Yes | No | |
| 1 MIC + 1 MIC | Bactericidal | 3.48 | Yes | No | ||
| 2 MIC + 2 MIC | Bactericidal | 4.26 | Yes | No | ||
| 2 MIC + ½ MIC | Bactericidal | 4.56 | Yes | No | ||
| MDR 1 | MEM + SAM | ½ MIC + ½ MIC | Bacteriostatic | 0 | Yes | No |
| 1 MIC + 1 MIC | Bacteriostatic | 0 | Yes | No | ||
| 2 MIC + 2 MIC | Bactericidal | 3.78 | Yes | No | ||
| 2 MIC + ½ MIC | Bacteriostatic | 2.71 | Yes | No | ||
| MEM + AK | ½ MIC + ½ MIC | Bacteriostatic | 0 | Yes | Yes, since hour 6 after exposure | |
| 1 MIC + 1 MIC | Bacteriostatic | 1.95 | Yes | Yes, since hour 6 after exposure | ||
| 2 MIC + 2 MIC | Bacteriostatic | 2.83 | Yes | No | ||
| 2 MIC + ½ MIC | Bacteriostatic | 0 | Yes | No | ||
| MDR 2 | MEM + SAM | ½ MIC + ½ MIC | Bacteriostatic | 0 | Yes | Yes, at hour 24 after exposure |
| 1 MIC + 1 MIC | Bacteriostatic | 0 | Yes | Yes, at hour 24 after exposure | ||
| 2 MIC + 2 MIC | Bacteriostatic | 1.53 | Yes | No | ||
| 2 MIC + ½ MIC | Bacteriostatic | 1.33 | Yes | No | ||
| MEM + AK | ½ MIC + ½ MIC | Bacteriostatic | 0 | Yes | Yes, since hour 8 after exposure | |
| 1 MIC + 1 MIC | Bacteriostatic | 0 | Yes | Yes, at hour 24 after exposure | ||
| 2 MIC + 2 MIC | Bacteriostatic | 1.79 | Yes | Yes, at hour 24 after exposure | ||
| 2 MIC + ½ MIC | Bacteriostatic | 0 | Yes | No | ||
| XDR | MEM + SAM | ½ MIC + ½ MIC | Bacteriostatic | 1.47 | No | Yes, at hour 24 after exposure |
| 1 MIC + 1 MIC | Bacteriostatic | 2.48 | Yes | No | ||
| 2 MIC + 2 MIC | Bacteriostatic | 1.91 | No | No | ||
| 2 MIC + ½ MIC | Bacteriostatic | 1.83 | No | No | ||
| MEM + AK | ½ MIC + ½ MIC | Bacteriostatic | 0 | Yes | Yes, since hour 6 after exposure | |
| 1 MIC + 1 MIC | Bacteriostatic | 0 | Yes | Yes, since hour 6 after exposure | ||
| 2 MIC + 2 MIC | Bacteriostatic | 0 | Yes | Yes, since hour 6 after exposure | ||
| 2 MIC + ½ MIC | Bacteriostatic | 0 | Yes | Yes, since hour 6 after exposure |
ATCC: American Type Culture Collection, MDR: multidrug-resistant, XDR: extensively drug-resistant, MEM: meropenem, SAM: ampicillin-sulbactam, AK: amikacin, MIC: minimum inhibitory concentration
a: Meropenem MIC = 2 μg/ml; Ampicillin-Sulbactam MIC: 8/4 μg/ml; Amikacin MIC: 16 μg/ml
b: Bactericidal: ≥ 3 log10 reduction in a colony-forming unit (CFU)/ml over the period measured. Bacteriostatic: < 3 log10 reduction in a colony-forming unit (CFU)/mL over the period measured (compared to initial measurement of tested isolate)
c: Δ Log 10: Log 10 of the total colony-forming unit (CFU/ml) reduction over the measurement time (compared to initial measurement of tested isolate)
d: Regrowth: initial decrease of turbidity or colony count followed by an escalation in the subsequent measurement hour
e: Comparison of the colony count between the treatment group and growth control group of isolate. Growth control: isolate without antibiotic combination exposure
Meropenem and amikacin had no bactericidal impact on intermediate and drug-resistant isolates; hence on all clinical isolates of A. baumannii in this study. The most significant reduction in the number of bacteria was observed following exposure to 2 MIC and 2 MIC; however, these concentrations had no effect on the number of colonies in XDR isolates when compared to the number of colonies at 0 hours measurement.
This investigation discovered regrowth in clinical isolates from nearly all exposure groups. Regrowth is influenced by various factors related to the concentration of antibiotics and bacterial inoculum, as well as the susceptibility of bacteria25. Regrowth may occur when bacterial growth is not fully inhibited by exposure to antibiotics (due to insufficient antibiotic concentration or a resistant bacterial strain)26. Persistent/resistant bacterial subpopulations can also be inferred from time-kill curve regrowth27–29. Antibiotic degradation in the test suspension also plays a role; decreased active antibiotic amount during the final hours of testing may render inhibition ineffective, allowing regrowth to occur30.
Meropenem and ampicillin-sulbactam are time-dependent beta-lactam antibiotics31. The synergism may be due to the distinct penicillin-binding proteins (PBP) binding mechanisms, hence enhancing the activity of beta-lactams in bacteria32. Meropenem has a high affinity for PBP 2, PBP 3, PBP 1a, and PBP 1b, ampicillin has a high affinity for PBP 4, and sulbactam has a high affinity for PBP 1 and PBP 333,34. The downregulation of native and subsequent synthesis of altered PBPs is one of the mechanism behind A. baumannii's resistance to beta-lactam antibiotics35–37. In addition to its simultaneous action on PBP, sulbactam's beta-lactam inhibitory activity can boost meropenem's affinity and, consequently, activity38,39. Numerous investigations have demonstrated that subinhibitory concentrations of beta-lactam antibiotics can alter the shape of bacteria's cell walls40. In theory, it has the potential to augment the intake of other antibiotics41.
Meropenem in combination with ampicillin-sulbactam at a concentration twice the MIC was bactericidal against isolates intermediate to ampicillin-sulbactam. Moreover, it had a lower rate of regrowth than the meropenem and amikacin exposure groups. Differences in resistance levels are believed to have an effect on the efficiency of antibiotic combinations42–44. It should be anticipated that the distinct resistance mechanisms held by various strains resulted in different responses to combination antibiotic exposure20,45,46.
Additionally, this study found that isolates treated at sub-MIC concentrations of antibiotics had a higher colony count than the growth control group. This finding merits additional investigation to ascertain the underlying mechanism. Antibiotics have a selection and inducer effect on antibiotic resistance, which demonstrates the importance of using them prudently.
Meropenem in combination with ampicillin-sulbactam at a concentration twice the MIC was bactericidal against isolates resistant to meropenem and intermediate to ampicillin-sulbactam. Meropenem and ampicillin-sulbactam in combination demonstrated bacteriostatic activity against isolates resistant to both antibiotics. Meropenem and amikacin in combination had no bactericidal effect on isolates that were either intermediate or resistant to meropenem and amikacin. Combined administration of meropenem and ampicillin-sulbactam can be considered in cases of A. baumannii infection that is not susceptible to any antibiotics. Higher doses show better results and should be attempted when clinical circumstances allow.
Figshare: Colony Count and Turbidity Data from Time-Kill Assay of Acinetobacter baumannii exposed to Meropenem-based Antibiotic Combinations. https://doi.org/10.6084/m9.figshare.20024270.v324.
This project contains the following underlying data:
Data are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
We thank Dr. Soetomo General Academic Hospital and Department of Microbiology, Faculty of Medicine, Sriwijaya University, for providing all necessary support in this research and Daniel Edbert, MD, Clin. Microbiol., for editorial assistance.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Microbiology and epidemiology
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: antimicrobial resistance, MDRO
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Microbiology and epidemiology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 28 Nov 22 |
read | |
|
Version 1 08 Jul 22 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)