Keywords
Bile acids, cotransporter, post-translational modification
Bile acids, cotransporter, post-translational modification
Sodium Taurocholate cotransporter polypeptide (NTCP1), codified by the gene solute carrier 10A1 (SLC10A1), is a bile acid transporter dependent of sodium, which modulates bile acid enterohepatic circulation. NTCP1 expression in the basolateral membrane of hepatocytes captures approximately 80% of the conjugated bile acids that come from the enterohepatic circulation.1 The NTCP1 ortholog in mice is a protein with 362 amino acids and seven transmembrane domains.2 This transporter is unidirectional, and with a 2:1 stoichiometry, transporting two Na+ ions for each taurocholate molecule.3
Intracellular and extracellular bile acid concentration regulates the activity and/or the expression of transporters implicated in bile acid enterohepatic circulation. For example, high intracellular concentration of bile acids are harmful to hepatocytes. Thus, high bile acids levels negatively regulate NTCP1 transcription as an adaptive response to decrease bile acid uptake, and alterations in this regulation is directly associated with cholestasis.4,5 Furthermore, NTCP1 expression is positively regulated by a heterodimer conformed by the retinoic acid receptor (RAR) with the retinoid receptor X (RXR), which is disturbed by the nuclear receptor Farnesoid X receptor (FXR). When bile acid concentration rises, FXR forms a heterodimer with RXR and binds to FXR/RXR response element in the SHP promoter. SHP then decreases the transactivation of RAR/RXR in the NTCP1 promoter, resulting in a decreasing NTCP1 expression.5,6
In addition to the transcriptional regulation, NTCP1 can also be regulated through post transductional modifications. In fact, NTCP1 is target of phosphorylation in serine and threonine residues. Specifically, a rise in cAMP leads to NTCP1 dephosphorylation at Ser226 promoting the translocation of NTCP1 to the membrane and its bile acid uptake activity.7,8 NTCP1 dephosphorylation is mediated by the protein phosphatase 2B (PP2B) that is activated by an increase of intracellular calcium.9 Moreover, the phosphorylation/dephosphorylation of the motif Thr225 and Ser226 is critical for NTCP1 plasma membrane localization.10 In addition to phosphorylation, NTCP1 endogenous ubiquitylation sites have been identified in murine tissues by mass spectrometry.11 In fact, NTCP1 is subjected to degradation via the ubiquitination/proteasomal pathway.12 Nevertheless, to our knowledge there has not been an evaluation of whether acetylation could be a post transcriptional modification affecting NTCP1 activity. Therefore, the aim of our work was to evaluate whether nicotinamide, a known general inhibitor of deacetylases that increases the acetylation status of intracellular proteins, could affect bile acid uptake and NTCP1 acetylation.
In silico analyzes were performed for the mouse NTCP1 protein sequence (362 aa), with two software. We first used Prediction of acetylation on internal lysines (PAIL) (http://bdmpail.biocuckoo.org/) as described in previous studies.13 This software facilitates the identification of possible acetylation sites in internal lysines with an accuracy of 85–89%. The internal lysines with a score greater than one predicted by the PAIL program in the NTCP1 amino acid sequence were visualized in the protein structure using the open-source tool Protter (http://wlab.ethz.ch/protter/start/).14
Mouse primary hepatocytes were isolated from male C57BL/6 mice of 8–12 weeks of age obtained from the Departamento de Investigación experimental y Bioterio at Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubiran (INCMNSZ). The procedures were performed in accordance with the Mexican Federal Regulation for Animal Experimentation and Care (NOM-062-ZOO-2001) and approved by the Animal Ethics Research Committee of INCMNSZ (Approval number 1560). Briefly, primary hepatocytes were obtained by in situ perfusion of the liver according to the method by Berry and Friend.15 Firstly, we cannulated and exsanguinated the liver in vivo followed by a continuous perfusion with collagenase. Secondly, we removed the liver and disaggregated the tissue in Hanks balanced salt solution (HBSS). Thirdly, we filtered the cell suspension through a 70 μM mesh and washed the filtered cells twice with HBSS. Finally, we re-suspended the cells in DMEM/F-12 supplemented with 1% antibiotic and 10% fetal bovine serum.
Firstly, we seeded hepatocytes into a 6-well or a 24-well CellBIND plate at 75% confluence and incubated at 37 °C. Unattached cells were removed by medium change after 4 hours. Cells were then treated with Nicotinamide 10 mM for 4 hours. After incubation, we used for western blot analysis cells in the 6-well plate as described below. On the other hand, cells in the 24-well plate were used to perform the taurocholate uptake assay.16 Briefly, hepatocytes were incubated in uptake buffer (D-glucose 11 mM, KCl 5.3 mM, CaCl2 1.8 mM, KH2PO4 1.1 mM, HEPES 10 mM MgSO4 0.8 mM, pH 7.4) with or without sodium 136 mM in presence of sodium taurocholate 10 μM supplemented with 0.1 μCi of [G-3H]-taurocholic acid (specific activity 1–5 Ci/mmol, Perkin Elmer) for 20 min at 37 °C. We then washed the cells three times with PBS 1x, and lysed with SDS 0.05%. An aliquot of cell lysate was placed in a vial with scintillation liquid. We used a beta liquid scintillation counter to quantify the amount of 3H in the cell lysate.
Primary hepatocytes incubated with nicotinamide were homogenized in lysis buffer (Tris-HCl 50 mM, EDTA 1 mM, NaCl 150 mM, NP-40 1%) supplemented with phosphatase inhibitors (NaP2O7 5 mM, Na3VO4 1 mM, NaF 50 mM), protease inhibitors (Complete mini, Roche), and deacetylase inhibitors (Nicotinamide 5 mM, Sodium butyrate 1 mM). Homogenates were centrifuged 10,000 g, 10 minutes at 4 °C, and protein concentration in supernatants was measured using the Lowry method (BioRad). Western blot was performed using 40 μg of total protein. Proteins were separated with a 10% SDS-PAGE and transferred to a PVDF membrane. To evaluate the acetylated status of hepatic proteins, PVDF membranes were incubated with an antibody against acetylated lysines (#9441, Cell signaling). Proteins were detected using ultrasensitive horseradish peroxidase chemiluminescence (Pierce). A tubulin antibody (sc-7396 Santa Cruz Biotechnologies) was used for normalization.
The data are depicted as the mean ± standard error of the mean (SEM). Densitometry analysis of acetylated proteins was quantified by ImageJ PC software. To assure reproducibility, taurocholate uptake experiments were performed four times. Our results were analyzed using a one-way ANOVA or an unpaired t-test using GraphPad Prism program v 7.0a (GraphPad Prism, GraphPad Software, Inc. La Jolla CA). Differences were considered significant at P<0.05.
To determine which internal lysines (K) on NTCP1 could be an acetylation target, the fasta sequence obtained from NCBI Slc10a1 [mus muculus] GenBank: AAH94023.1 was entered into the PAIL software. The in silico analysis showed eleven possible lysines that could be target of acetylation with a precision of 89.21%, four of these lysines had a score greater than one, which were K in positions 81, 113, 309 and 336. To locate these acetylation sites in the amino acid sequence and expected structure of NTCP1, we entered the NTCP1 amino acid sequence obtained from UniProt (UniProtKB-O08705) in Protter. The identified lysines were localized in the intracellular domains of NTCP1, except for K81 that was localized in an extracellular domain (Figure 1A).
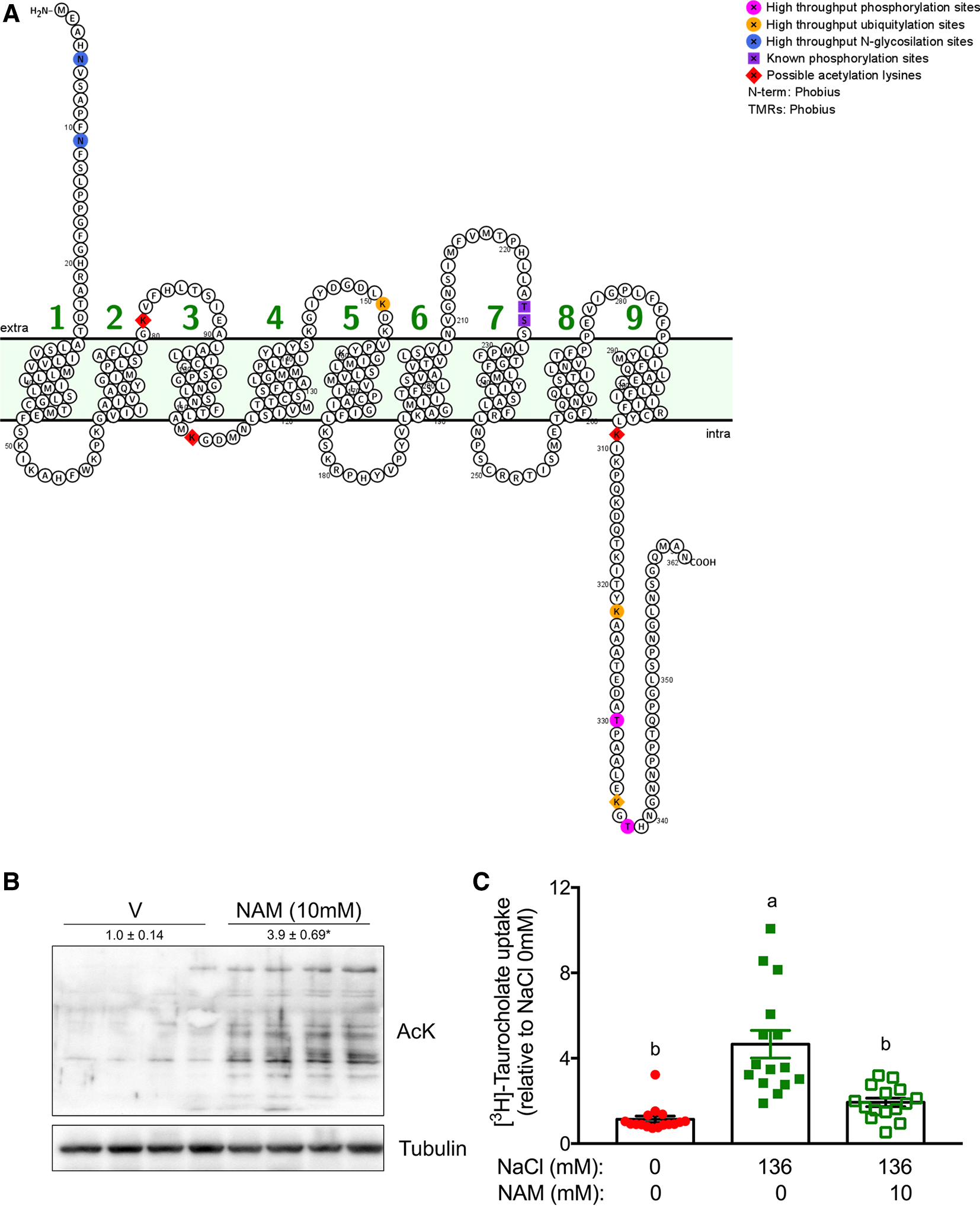
A) Predicted topological structure of mouse sodium and bile acid co-transporter NTCP1 generated using Protter v 1.0. We observe the seven transmembrane domains, and the extracellular amino-terminus and the intracellular carboxy-terminus identified by Phobius. High-throughput phosphorylation, ubiquitylation, and N-glycosylation sites are indicated according to phosphosite.com. The identified internal lysines by the in silico PAIL analysis as potential acetylation sites are highlighted in red. B) Global lysine acetylation in primary hepatocytes incubated with nicotinamide (NAM) 10 mM for 4 h. Lysates were analyzed by western blot using an anti-acetylated–lysine (AcK) antibody. The loading control was tubulin. Data are presented as mean ± SEM and * indicates a significant difference versus vehicle (V) at P < 0.05 by Student’s t-test. C) [3H]-Taurocholate uptake into primary hepatocytes was determined to evaluate NTCP1 activity. We performed four independent biological experiments with primary hepatocytes obtained from different C57BL6 mice and each experiment included at least 4 replicates. Significant difference is designated by letters were a > b at P < 0.05 by One-way ANOVA.
The effect of acetylation on taurocholate uptake was evaluated in mouse primary hepatocytes treated with or without nicotinamide 10 mM for 4 hours. Nicotinamide incubation significantly increased acetylated proteins with respect to the control (Figure 1B). Unfortunately, we were unable to immunoprecipitate NTCP1 and to confirm whether NTCP1 was specifically acetylated. Nevertheless, when we evaluated taurocholate uptake, which depends mainly on NTCP1 activity, it was significantly increased in hepatocytes treated with NaCl 136 mM when compared to those without NaCl. Notably, when hepatocytes were previously treated with nicotinamide, we observed a significant reduction on taurocholate uptake (Figure 1C), suggesting that NTCP1 activity may depend on acetylation.
Our results demonstrate that an increase in acetylated proteins is associated with a decrease in taurocholate uptake by primary hepatocytes. Unfortunately, we were unable to evaluate whether this effect was a result of direct acetylation of NTCP1. However, our result suggest that acetylation is a postraductional modification that could impact hepatic bile acid homeostasis.
Acetylome analysis has provided evidence about the implications of this post-translational modification on physiological and pathological circumstances. For example, acetylation increases in skeletal muscle during aging17 and insulin resistance,18 affecting the activity of several proteins including enzymes, transcriptional factors, among others. However, few reports have thus far reported the acetylation of cotransporters. We have recently showed that acetylation regulates the stability and activity of the potassium-chloride cotransporter 4 (KCC4) in kidney.19 Thus, it is possible that NTCP1 stability or activity may also be modulated directly by acetylation. However, it is also possible that the decrease in taurocholate uptake could be the result of acetylation of FXR, which is known to increase its stability and inhibits its dimerization with RXR,20 possibly affecting NTCP1 expression.
Acetylation of proteins depends on acetyl-CoA levels and the activity of acetylases and deacetylases such as sirtuins, which are dependent of NAD levels. Therefore, maximum acetylation is normally achieved during feeding conditions, and also with minimal mitochondrial activity.21 This suggest that bile acid uptake in the liver will be restricted during feeding condition and promoted during fasting. In fact, Dumaswala et al. reported that hepatic basolateral membrane (BLM) taurocholate uptake is enhanced by 65% in fasted animals.22 Furthermore, the regulation of NTCP1 activity by acetylation may decrease bile acid uptake in pathologies where hyperacetylation of hepatic proteins is also observed, such as fatty liver disease. However, additional research is necessary to demonstrate its implications.
In conclusion, our results demonstrate that acetylation mediates taurocholate uptake in hepatocytes possibly through modulation of NTCP1 activity.
Figshare: Acetylation mediates taurocholate uptake in hepatocytes possibly through modulation of NTCP1 activity. https://doi.org/10.6084/m9.figshare.19226010.v2
This project contains the following underlying data:
1. An excel file with: 1) the raw data of the in-silico analysis performed in PAIL; 2) the raw data of the densitometry analysis of global lysine acetylation; and 3) the raw data of the taurocholate uptake assay.
2. Raw images of western blots for acetylated lysines (AcK) and tubulin.
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Conceptualization, S.Y.L.R. and L.G.N.; investigation, S.Y.L.R. and A.M.L.B.; formal analysis, S.Y.L.R. and L.G.N.; funding acquisition, L.G.N; writing—original draft preparation, S.Y.L.R. and L.G.N.; writing—review & editing, L.G.N.; All authors have read and agreed to the published version of the manuscript.
We thank all members of Fisiología de la Nutrición department for discussion, Sayra Y. López-Ramirez is a doctoral student from Programa de Doctorado en Ciencias Biomédicas, Universidad Nacional Autónoma de México (UNAM) and received fellowship 512595 from CONACYT.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
No
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Bile salt uptake transporters
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Transporters; regulation
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 12 Jul 22 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)