Keywords
Empathy, ERP, performance, psychotropic medication, social cognition
Empathy, ERP, performance, psychotropic medication, social cognition
Empathy is involved in several aspects of social cognition. It has been proposed to notably contribute to prosocial behavior1,2 and to regulate aggression.3 Empathy is defined as the ability to experience and understand the feelings of another person, while also understanding the origin of the feelings, that is, whose belong to whom.4 Previous research based on psychometric data has defined different facets of empathy: empathic concern, personal distress, perspective-taking and fantasy.5,6
Perspective-taking enables us to make inferences, understand and foresee other’s mental and emotional states – an ability known as Theory of Mind7 as well as to have empathic ability. The ability to take the perspective of another person is known as emotional perspective-taking and is determinant for the success of social interactions.8
The use of benzodiazepines has been reported to facilitate aggressive9 and violent behavior,10,11 leading to suspicion of their inhibitory effects on empathic responses. Bearing this in mind, Nilsonne and colleagues12 investigated the effects of oxazepam on emotional mimicry and empathic responding. Using an experiment on empathy for pain, the authors found that oxazepam did not inhibit empathic responses to others’ pain.12 Benzodiazepines are often added to antidepressants in initial depression treatment but previous research showed that the simultaneous use is long term.13 Nonetheless, a previous study suggested that antidepressant treatment reduces behavioral and neural responses to pain empathy14 and may have a cumulative effect to that caused by the use of benzodiazepines.
To our knowledge, these are the only studies examining the effects of benzodiazepines and antidepressants, when used alone, on empathy. Furthermore, there is no information on the neural temporal dynamics of empathy processes under the use of such medication. The current study examines the effects of concomitant benzodiazepines and antidepressants long-term use on perspective-taking using event-related potentials (ERPs). To this purpose, we used a task previously adapted by our group15 in which scenarios presenting two persons engaged in social interactions, depicting emotional and neutral scenes, are shown to participants. In each scenario, one person has the face masked. In the next display, a target facial expression of emotion (FEE) is presented and participants are asked to judge whether this FEE is congruent or not with the emotion expected to be portrayed by the masked person. Thus, in each scenario, participants must infer the affective mental state of the masked intervener. Then, to an accurate decision, participants also have to compare the inferred emotion to the emotion presented in the target FEE, and decide wether they were congruent or incongruent. Using this experimental manipulation, we will be able to investigate perspective-taking abilities through the behavioral performance and, with simultaneous EEG recording, to assess how these abilities modulate two ERP components - the N170 and the late positive potential (LPP). These components are known to be typically influenced by the affective and evaluative congruency.
The N170 is a negative deflection usually peaking at ~170 ms post-stimulus in occipito-temporal sites. This component seems to reflect the earliest stage of facial structure encoding16 and is sensitive to the emotional content of a face.17,18 Concerning the influence of context, higher N170 amplitudes are usually recorded in congruent trials when pictures are used as contextual stimuli.19–22
The LPP is a positive deflection usually peaking at 300-700 ms after the stimulus onset in centro-parietal sites.23 This component represents facilitated attention to emotional stimuli and has larger amplitudes after emotionally arousing pictures in comparison to neutral ones.24 In the present study, the congruency between a prime and a target is manipulated, and previous studies using a similar experimental manipulation, reported that LPP indexes the processing of affective or evaluative congruency, and exhibits larger amplitudes to incongruent targets compared to congruent ones.24 Furthermore, cognitive appraisal and motivated attention,25 as well as morally good actions26 and helping scenes27 also modulate the LPP component.
The research on this field is essential considering that benzodiazepines and antidepressants are the most prescribed drugs in the world28,29 despite their unwanted effects (e.g., psychomotor, and cognitive impairments; see Ref. 30). Benzodiazepines act at the limbic system, including at the thalamic and hypothalamic levels of the central nervous system31 through GABAA receptors.32 Antidepressants were shown to increase the activation of dorsolateral, dorsomedial and ventrolateral prefrontal cortices and to decrease the activation of the amygdala, hippocampus, parahippocampal region, ventral anterior cingulate cortex, orbitofrontal cortex, and insula.33 Since some of these structures are involved in perspective-taking processes,34 we may expect impaired performance of long-term concomitant benzodiazepines and antidepressants users on our perspective-taking task. Regarding electrophysiological results, as we expected a decreased perspective-taking ability in the group of long-term concomitant benzodiazepines and antidepressants users, we anticipated that N170 and LPP modulations would be absent in this group. Additionally, neurocognitive measures were collected to explore whether performance on the perspective-taking task is related to cognitive performance.
A total of 60 participants were recruited from the community and local University to two groups: a long-term concomitant benzodiazepines and antidepressants users group (experimental) and a control group, matched on age and years of formal education. The experimental group consisted of subjects who had a minimum period of concomitant benzodiazepines and antidepressants use of one year. We excluded participants with scores inferior to 22 (cutoff for mild cognitive impairment35) in the Montreal Cognitive Assessment (MoCA36; n = 1), as well as participants who reported uncorrected visual impairments (n = 1), history of brain injury and neurological diagnosis (n = 2). Participants reporting use of psychotropic medication besides benzodiazepines and antidepressants (n = 4), and psychiatric diagnosis aside from anxiety and depression (except severe conditions rendering study participation impossible) were also excluded from the experimental group. We also excluded participants from the control group if they reported use of psychotropic medication (n = 13) and psychiatric diagnosis (n = 4). Additionally, 9 participants dropped out the study at the end of the neuropsychological assessment. Thus, the final sample was composed of 13 experimental subjects (all female; Mage = 44.1, SD = 10.0; Myears of education = 15.9, SD = 2.4) and 13 control subjects (12 female; Mage = 46.5, SD = 10.9; Myears of education = 16.9, SD = 4.9). They each gave written, informed consent and received EUR 20 (gift card). The study was approved by the local Ethics Committee.
Self-report measures
Anxiety and depression traits were measured by the Hospital Anxiety and Depression Scale (HADS37; Portuguese version by Pais-Ribeiro et al.38), and psychopathological symptomatology by the Brief Symptom Inventory (BSI39; Portuguese version by Canavarro40).
Neuropsychological measures
Executive functioning was assessed using the Trail Making Test (TMT41; normative data by Cavaco et al.42), and the INECO Frontal Screening (IFS43; Portuguese version by Moreira et al.44). Semantic fluency and phonemic fluency tests were used to assess non-motor processing speed, language production and executive functions (see Ref. 45; Portuguese versions by Cavaco et al.46). Short-term memory was assessed by the Corsi Block-Tapping Task (CBTT47).
Emotional perspective-taking task
This task was adapted from a previous fMRI study that assessed perspective-taking8 as described in our prior work15 examining perspective-taking ability during an ERP experiment. Succinctly, the protocol used by Derntl et al.8 was adapted according to experimental designs of previous studies addressing contextual congruency.19–23,48,49 Contextual congruency implies the matching between a target facial expression of emotion (FEE) and the emotion portrayed by a masked face in a previous scenario.15 Thus, participants had to recall perspective-taking abilities to accurately judge the contextual congruency: they had to see the scenario and examine it from another’s perspective, and then judge whether a FEE shown next was congruent or not with the emotion expected to be portrayed by the masked person.
To this purpose, participants viewed 360 pictures representing scenes of two persons engaged in social interactions. Each scenario presented one of the actors’ faces masked, and participants were instructed to infer the respective emotional expression. The scenarios depicted emotional (anger, fear, disgust, sadness, happiness) and neutral scenes and all actors were adult Caucasians. After the scenario display, a target FEE was presented, and participants were asked to judge it as congruent or incongruent with the inferred FEE of the masked person. Next, an unlimited duration screen with the response options was presented until key press. During this screen, participants used two response buttons held in the right and left hand. Participants were asked to respond only during this response screen, to prevent overlap of preparatory response potentials with the ERP components of interest. The structure of each trial is depicted in Figure 1.
As the original set of stimuli,8 ten scenarios for each emotional condition were included, with six repetitions for each one. For congruent conditions, the FEE was randomly selected from all alternative actors with the congruent emotion; the incongruent conditions included FEE selected from all the incongruent alternatives. This results in 30 congruent trials and 30 incongruent trials for each emotional condition. The FEE was selected from the NimStim Face Stimulus Set,50 picking the five most accurately identified facial expressions for each emotional category (see Ref. 50) which led to 30 female and 30 male facial stimuli. Participants viewed the images on a 17-in. screen from a distance of 115 cm, with 6.67° × 8.55° visual angle, and a refresh rate of 60 Hz. E-Prime 2.0 (Psychology Software Tools, Inc., Sharpsburg, PA, USA) was used to control the experiment and collect responses. After a block of six practice trials, participants completed two experimental blocks of 180 trials each, with a pause between them.
All participants were tested individually in two experimental sessions to avoid fatigue effects. In the first session, a semi-structured interview and the MoCA were conducted to assess inclusion criteria. The remaining neuropsychological tests and self-report measures were then administered in a random order between participants. Participants meeting the inclusion criteria were invited to participate on a second session, in which the emotional perspective-taking task was performed simultaneously to EEG recording. This second session was part of a larger research protocol, demanding all experimental tasks to be performed in random order. After the placement of the EEG cap, participants read the instructions and completed a practice block.
The eletroencephalographic (EEG) data were recorded on the acquisition software V4.5.2 (2008, Electrical Geodesics Inc., Eugene, OR, USA – EGI) using a 128-electrode Hydrocel Geodesic Sensor Net, connected to a Net Amps 300 amplifier (EGI). Impedances were kept below 50 kOhm for all electrodes since this is a high-input impedance system. Data were recorded with a sampling rate of 500 Hz, filtered with a notch filter of 50 Hz and the electrodes were referenced to the vertex (Cz).
The EEG raw data were pre-processed in the EEGLAB version 13,51 a MATLAB toolbox (2017, The Math-works Inc., Natick, MA, USA). Continuous EEG signal was downsampled to 250 Hz, bandpass filtered (0.2−30 Hz), and submitted to an Independent Components Analysis (ICA) decomposition. Eyeblinks, saccades, and cardiac activity artifacts were corrected, by subtracting the corresponding Independent Component activity from the data, followed by visual inspection to ensure the correction did not alter the signals outside the time windows of the artifacts. Channels with artifacts were interpolated (up to a maximum of 10% of the sensors) using the spherical spline interpolation method.52 The EEG signal was re-referenced to the average of all electrodes. EEG records were segmented into epochs (-200 to 800 ms) time-locked to the onset of the target FEE and visually inspected for manual artifact rejection. Trials in which participants gave incorrect responses were also excluded. All epochs were baseline corrected (200 ms pre-stimulus) and averaged by congruency (congruent, incongruent) and emotion (anger, fear, disgust, sadness, happiness, neutral).
According to previous studies, and visual inspection of ERP grand-average and topographical maps, three time windows and three regions of interest (ROIs)2 were chosen for statistical analysis. The higher amplitude of the N170 component is exhibited bilaterally at occipitotemporal regions, precisely at P7/P8 and PO7/PO8, being more marked at the inferior locations as P9/P10 and PO9/P10 (Rossion and Jacques 2008). A negative maximum over these regions was recorded in our topographical maps, leading us to set a ROI containing these and a cluster of surrounding electrodes, to increase the signal-to-noise ratio.53,54 Accordingly, the peak amplitudes for the N170 were extracted in the [140, 240] ms time window after FEE onset, at right (electrodes 83, 84, 89, 90 [PO8], 91, 95 [P10], 96 [P8]) and left (70, 66, 69, 65 [PO7], 64 [P9], 58 [P7], 59) ROIs. Regarding the LPP component, other studies reported that its higher amplitude occurs over centro-parietal sites such as CPz and Pz.23 Our topographical maps are in accordance with this result, so we extracted the mean LPP amplitudes at the centro-parietal ROI (54, 55 [CPz], 61, 62 [Pz], 78, 79). The time window of this component was divided into an early (LPPe; 300–500 ms after FEE onset) and a late component (LPPl; 500–700 ms after FEE onset) given its temporally broad distribution (e.g., Ref. 48).
Student’s t-tests were used to analyze group differences in neuropsychological performance and self-report measures. Whenever necessary, non-parametric tests were performed. Perspective-taking results were obtained from accuracy rates (percentage of correct responses in relation to the total number of trials) calculated by participant and condition. The effects of emotion, congruency, and group on perspective-taking results were analyzed in a mixed ANOVA. The group (experimental, control) was used as between-participants factor, whereas emotion (anger, fear, disgust, sadness, happiness, neutral) and congruency (congruent, incongruent) were used as within-participants factors. The same analysis was performed for reaction times.
The electrophysiological data were analyzed by mixed factors ANOVAs with group as between-participants factor, and emotion and congruency as within-participant factors. For N170, the hemisphere (left, right) was also used as within-participant factor.
To test the influence of cognitive abilities in behavioral performance and ERPs modulation, a Linear Regression Model was computed for each group. Scores of cognitive tests (raw scores) in which differences between groups were detected were entered as main predictors of behavioral measures and N170, and LPPs modulation (independent models). For N170, LPPe and LPPl modulation, the model was applied to each electrode or electrode cluster examined in the ERP data analysis.
The threshold for statistical significance was set at α = .05, and the p-values reported for t-tests are from one-tailed tests. Statistical analysis was performed using SPSS 24 (IBM Corp., Armonk, NY, USA). Violations of sphericity in ANOVA were corrected via the Greenhouse-Geisser method.
Neuropsychological data analysis revealed no differences between groups in IFS, CBTT, TMT, PF, nor SF (all p > .313). However, significant group differences were observed on depression, t(24) = -3.735, p < .002, anxiety, t(24) = -3.156, p = .004, and psychopathological symptomatology, t(24) = -4.450, p < .001. Experimental subjects had higher scores in these self-report measures (see Table 1).
Accuracy rates and reaction times are shown in Table 2. These were examined using mixed factors ANOVAs. The results showed that experimental and control subjects were equally accurate in their responses, F(1, 24) = 0.68, p = .417. However, a main effect of emotion, F(5, 120) = 28.2, p < .001, η2p = 0.541, ε = 1.000, revealed accuracy rates significantly higher following scenarios portraying happiness (all p < .001), sadness (all p < .033), and neutral scenes (all p < .027), without significant differences between the latter (p > .998). Scenarios portraying anger, disgust, and fear elicited similar accuracy rates (all p > .988). We also found a significant emotion x group interaction, F(5, 120) = 3.32, p = .008, η2p = .121, along with a significant emotion x congruency interaction, F(5, 120) = 9.31, p < .001, η2p = .280. No other significant interactions emerged (all F < 0.78, p > .387).
Regarding reaction times, no significant differences were found between the groups F(1, 24) = 2.22, p = .149. Also, no main effects were found for emotion, F(5, 120) = 1.21, p = .309, or congruency, F(1, 24) = 0.91 p = .350, factors. The analysis also showed that none of the interactions – emotion x group, congruency x group, emotion x congruency, and emotion x congruency x group – was statistically significant for reaction times (all p > .150).
The Linear Regression analysis was significant for accuracy rates of scenarios portraying happiness in control subjects F(5, 7) = 10.9, p = .002. Anxiety (Adj R2 = .712, β = - .42, p = .035) and psychopathological symptomatology (Adj R2 = .712, β = - .53, p = .008) were main predictors of accuracy rates for happiness scenarios. For participants from the experimental group, the model was not significant (all p > .234).
Peak amplitudes for N170 component are presented in Table 3 and mean amplitude for LPPs components are presented in Table 4.
The repeated measures ANOVA revealed no significant differences on the N170 amplitude between groups, F(1, 22) = 0.33, p = .571. A main effect of emotion emerged, F(5, 110) = 2.60, p = .029, η2p = .106, ε = .782, but the main effects of congruency and hemisphere were not significant (all F < 1.96). Pairwise comparisons revealed that faces portraying sadness elicited higher N170 amplitudes than faces portraying anger (p = .050) and neutral faces (p = .012). Additionally, the analysis showed that none of the interactions was statistically significant (all p > .150).
For the LPPe component, no significant differences were found on the mean amplitude between groups, F(1, 22) = 0.22, p = .647. A main effect of emotion was not found on the LPPe mean amplitude, F(5, 110) = 1.15, p = .338, but the main effect of congruency was significant F(1, 22) = 18.5, p < .001, η2p = .457, ε = .984. Additionally, a significant emotion x congruency interaction emerged, F(5, 110) = 4.32, p = .004, η2p = .164. Pairwise comparisons revealed that congruent conditions elicited higher LPPe amplitudes than incongruent conditions (p < .001) (Figure 2). No other significant interactions emerged (all F < 0.95, p > .435).
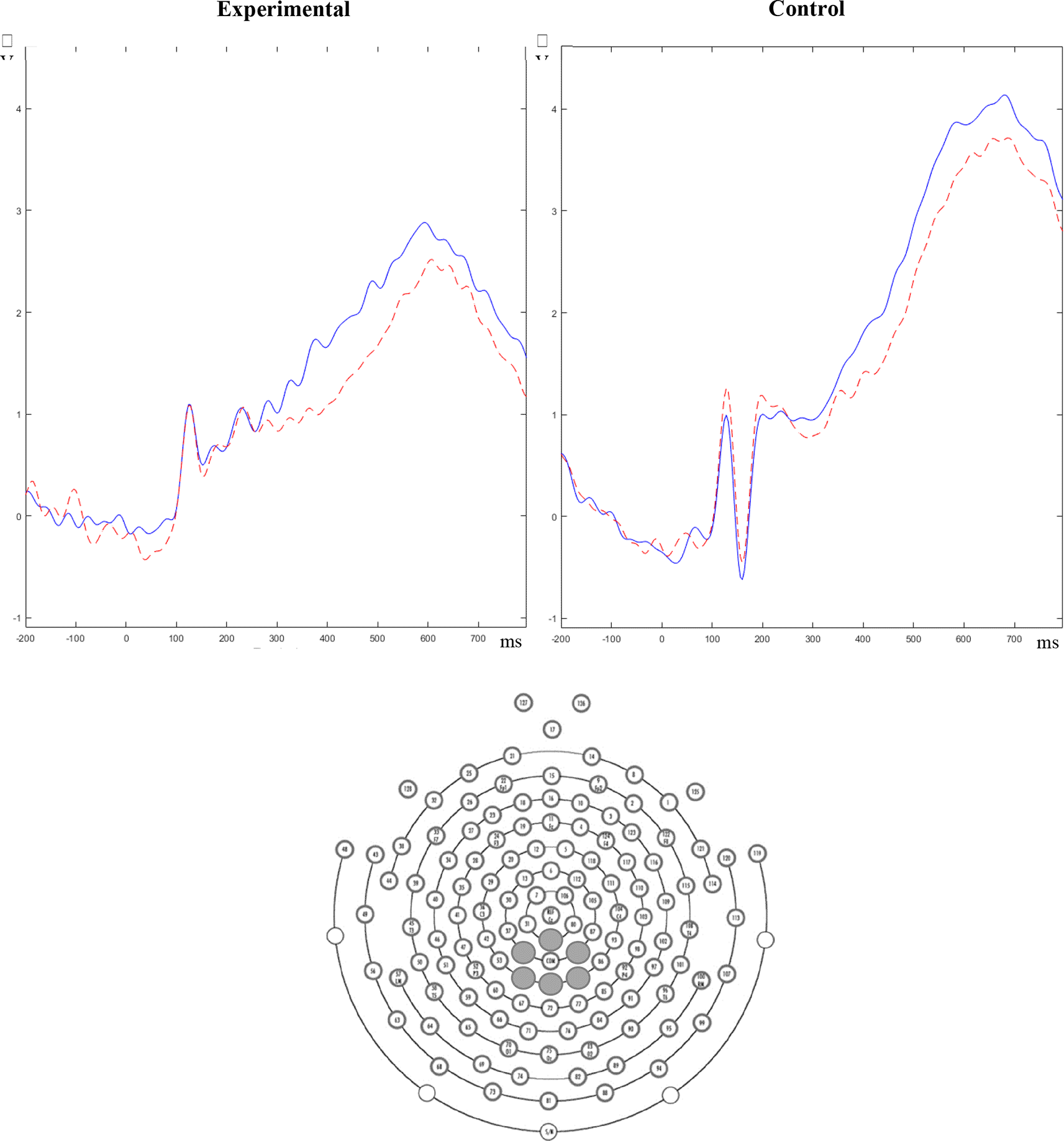
Solid lines (blue) = congruent condition, broken lines (red) = incongruent condition.
Regarding the LPPl, the pattern of results found was similar to the LPPe: no significant differences were found on the mean amplitude between groups, F(1, 22) = 1.80, p = .194; the main effect of emotion was not significant, F(5, 110) = 1.59, p = .168, but a main effect of congruency emerged, F(1, 22) = 12.5, p = .002, η2p = .363, ε = .923. The analysis also revealed a significant emotion x congruency interaction, F(5, 110) = 2.81, p = .038, η2p = .113. Pairwise comparisons revealed that congruent conditions elicited higher LPPe amplitudes than incongruent conditions (p = .002) (Figure 2). No other significant interactions emerged (all F < 1.92, p > .096).
The Linear Regression model was neither significant for N170 (all p > .089) nor LPPe (all p > .056) components in both groups. The model was significant for LPPl amplitudes in control subjects, F(5, 6) = 5.36, p = .026. Anxiety was a marginally main predictor of LPPl amplitudes for congruent conditions (Adj R2 = .543, β = -.504, p = .054). For experimental subjects, the model was not significant (all p > .680).
In the present study, concomitant benzodiazepines and antidepressants users and control subjects were presented with scenarios presenting two persons (one person with the face masked) engaged in social interactions, depicting emotional and neutral scenes, and asked to judge whether a FEE shown next was congruent or not with the emotion expected to be portrayed by the masked person.
Our results did not reveal group differences in perspective-taking ability, contrary to what we expected. Nonetheless, these results are consistent with the neural responses, revealing no differences in the processing of perspective-taking information as we will discuss later. The only studies12,14 examining the effects of benzodiazepines and antidepressants on empathy did it for each class of drug isolated, using an experiment on empathy for pain. One study examined the effect of one single administration of oxazepam on empathy for pain and showed that empathic responses to other’s pain were not inhibited.12 Another study examined the effect of a three months antidepressant therapy on the responses to an empathy for pain task, in patients with major depressive disorder.14 The results suggested that antidepressant treatment reduces behavioral and neural responses to pain empathy.14 Bearing im mind that the ability of identifying FEE is required in the present task, we should mention the results of a previous meta-analysis by our group.55 The meta-analysis examining how benzodiazepines administration affects the identification of FEE showed that participants receiving benzodiazepines were less accurate at identifying FEE of anger compared with those receiving placebo.55 The identification of the remaining facial expressions (disgust, sadness, fear, surprise, and happiness) appears to be unaffected by benzodiazepines administration. However, these studies are methodologically far from our study which makes any comparison of the results impossible.
A main effect of emotion emerged consistent with the results of a previous study conducted by our group15: greater accuracies were reported for scenarios portraying happiness, sadness, and neutral scenes. Similar to the identification of happy faces,56–58 happiness scenarios seem to be recognized more accurately than any other scenarios.
The current sample of concomitant benzodiazepines and antidepressants users scored higher in self-report measures of anxiety, depression, and psychopathological symptomatology. Anxiety and psychopathological symptomatology predicted accuracy rates for happiness scenarios in control subjects. This result was unexpected since it was not found in our previous study.15
Regarding electrophysiological data, we did not find significant differences between groups in the N170 component, but a main effect of emotion emerged similar to the pattern found in behavioral data. Furthermore, previous studies found a N170 modulated by emotion59,60 providing evidence that early face processing can reflect processing of the emotional expression. In accordance with our previous study,15 this component was not modulated by the congruency between the emotional contexts and the target’s FEE. As noted by the authors,15 this was unexpected since previous studies using pictures as contexts reported a systematically higher N170 in congruent trials.19–22 However, a congruency modulation in N170 was previously not found in studies using verbal instead of visual stimuli.48,49
Consistent with our previous work,15 the results obtained in the LPPs were similar in the early and late time-windows, revealing higher amplitudes in congruent than in incongruent trials. This pattern is opposite to the one reported by previous studies using context-target congruency tasks, i.e., larger LPPs after incongruent FEE.48,61 However, while higher LPP amplitudes elicited by incongruent trials were reported in tasks requiring an evaluative congruity between primes and facial23,62 or verbal targets,63 our task required attending the scenes, inferring the mental state of the masked interveners, and deciding if the FEE displayed after the scene matched the one previously inferred. The recognition of the masked person’s emotion in the stimuli displayed by the target FEE may explain the increased amplitudes to congruent stimuli since the LPP is also modulated by the explicit recognition of stimuli, exhibiting larger amplitudes in response to recognized stimuli vs. new.64,65
Noteworthy, there are several factors related to the combined use of psychotropic medication that may influence the perspective-taking ability, namely the type of antidepressants (tricyclic antidepressants (TCAs), selective serotonin reuptake inhibitors (SSRIs), serotonin-noradrenaline reuptake inhibitors, noradrenergic and specific serotonergic antidepressants, monoamine oxidase inhibitors, and other antidepressants) and benzodiazepines (short-acting or long-acting), the dose of each drug, and the percentage of days of concomitant drug use. However, we were not able to test these variables as potential moderators of any concomitant drug use effects observed, due to the small sample of the present study. The literature in the present field is still very limited and multi-center studies pursuing the influence that long-term use of benzodiazepines and antidepressants may have on social cognition, including perspective-taking ability, are needed.
Contrary to our hypothesis, the modulation of N170 and LPPs components was present in the group of concomitant benzodiazepines and antidepressants users. Overall, the results seem to indicate that long-term use of these psychotropic substances together do not affect perspective taking abilities and neither the processing of related information. Although the evidences of deficits in several general cognitive abilities in long-term benzodiazepine users,31,66 the combined use of benzodiazepines and antidepressants on long-term seem to preserve at least the perspective-taking ability of social cognition. Nevertheless, the lack of previous studies with a similar sample, makes any interpretation difficult to support. We hope, however, to have brought important evidence to light, which may help future research on social cognition under the influence of benzodiazepines and antidepressants.
DANS: Psychotropic long-term use and perspective taking: behavioral and neural dataset, https://doi.org/10.17026/dans-xjs-kje4.
This project contains the following underlying data:
- Accuracy.xlsx
- LPPe_Congruent.xlsx
- LPPe_Incongruant.xlsx
- LPPI_Congruent.xlsx
- LPPI_Incongruent.xlsx
- N170_Congruent.xlsx
- N170_Incongruent.xlsx
- Reaction_times.xlsx
- Sociodemographic_neuropsychological.xlsx
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
References
1. Field A, Miles J, Field Z: Discovering Statistics Using R. Sage Publications, Ltd. 2012.Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Empathy, perspective taking / theory of mind, neuroimaging (fMRI), placebo analgesia
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 2 (revision) 07 Nov 22 |
read |
|
Version 1 13 Jul 22 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)