Keywords
shock index, modified shock index, acute myocardial infarction, mortality, major adverse cardiac events
shock index, modified shock index, acute myocardial infarction, mortality, major adverse cardiac events
According to all constructive revisions from reviewer, we have revised all the grammatical errors in our manuscript.
See the authors' detailed response to the review by Dyana Sarvasti
ACM: all-cause mortality
AMI: acute myocardial infarction
BP: blood pressure
CI: confidence interval
DBP: diastolic blood pressure
HR: heart rate
I2: inconsistency index
LBBB: left bundle branch block
LVEF: left ventricular ejection fraction
MACE: major adverse cardiac events
MAP: mean arterial pressure
MSI: modified shock index
N/A: not available
NOS: Newcastle-Ottawa Scale
NSTEMI: non-ST-segment elevation myocardial infarction
PCI: percutaneous coronary intervention
RR: risk ratio
SBP: systolic blood pressure
SI: shock index
STEMI: ST-segment elevation myocardial infarction
TIMI: thrombolysis in myocardial infarction
Acute myocardial infarction (AMI) with or without ST-segment elevation is a life-threatening disorder that continues to be a fount of lofty mortality and morbidity rate, resulting in a various severe detrimental effects, including a reduction in patient quality of life.1 Pursuant to data from the American Heart Association (AHA), nearly 18% of men and 23% of women over forty will die within a year of being diagnosed with AMI.2 Tertiary prevention through risk stratification is thus the key to clinical decision-making in this patient population, thereby increasing patient prognosis due to potential menace.
Blood pressure (BP) and heart rate (HR) are commonly considered to have essential roles in the prognosis of AMI. These parameters are critical for measuring risks using several risk stratifications, most notably the TIMI (Thrombolysis in Myocardial Infarction) risk score, in which lower systolic blood pressure (SBP) and greater heart rate (HR) are associated with poorer prognoses.3,4 Thus, when compared to relying on only one metric, combining these two components of vital signs yields results with higher validity in predicting poor prognosis in AMI patients.
Shock index (SI) (defined as heart rate divided by systolic blood pressure) and modified shock index (MSI) (defined as heart rate divided by mean arterial pressure (MAP)), are simple yet forefront tools that were originally used to assess critically ill patients, such as those with sepsis, trauma, pulmonary embolism, and hemodynamically unstable patients.5,6 Several observational studies suggested that elevated admission SI in patients with AMI was associated with worse prognoses, including increased risk of mortality and major adverse cardiac events.7–13 MSI was later introduced since diastolic blood pressure is also of undeniable importance when determining AMI patients’ clinical severity, given that coronary perfusion occurs mostly during the diastolic phase.12,14 A meta-analysis conducted in 2017 by Zhang et al. demonstrated that high SI might increase the risk of in-hospital mortality, short-term, and long-term major cardiac events in AMI patients.15 However, due to the small number of studies included in the analysis, lack of subgroup analyses, demoded year of publication, and the fact that no meta-analysis of MSI regarding this issue has ever been conducted, the association between SI and MSI with poor prognoses in AMI patients remain obscure. Therefore, we conducted a systematic review and meta-analysis to determine the effect of elevated SI and MSI on the prognosis of AMI patients, particularly in terms of all-cause mortality (ACM) and major adverse cardiac events (MACE).
This meta-analysis was recorded in the PROSPERO (International Prospective Register of Systematic Reviews) database under registration number CRD42022329558 and adhered to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines.15,51
From conception until 21st May 2022, when the last search was conducted, two independent authors performed a systematic literature search using several online databases: PubMed, ProQuest, EBSCO Host, and Europe PMC. We utilized the following search terms: (“shock index” OR “modified shock index”) AND (“myocardial ischemia” [MeSH Terms]) AND (“all-cause mortality” OR “major adverse cardiac events”). The duplication removal process was conducted using Mendeley software version 1.19.8. Afterwards, the remaining studies were manually screened for eligibility based on their title and abstract, and eventually the full-text eligibility was determined. Disagreement between two independent authors was resolved through discussion.
The current meta-analysis included all retrospective and prospective cohort studies of adult participants (age ≥18 years old) with acute myocardial infarction who were assessed for SI or MSI that captured our outcomes of interest, namely ACM and/or MACE.
AMI is classified as either ST-segment elevation myocardial infarction (STEMI) or non-ST-segment elevation myocardial infarction (NSTEMI). STEMI was diagnosed when a patient presented with severe chest discomfort accompanied with electrocardiographic findings of sustained ST-segment elevation >1 mm in two or more contiguous leads or new onset left bundle branch block (LBBB).16 NSTEMI was detected in a patient who had severe chest pain, with no ST-segment elevation on electrocardiography, and an elevated level of a cardiac enzyme (troponin or creatinine kinase myocardial band).17
The key exposure was high SI or MSI, defined as an increment in SI or MSI beyond the cut-off point reported in each study. SI is determined by dividing heart rate by systolic blood pressure.18 Whereas the formula of MSI is HR divided by mean arterial pressure.10,12,19–21 SI and MSI were assessed at the time of admission. Our outcomes of interest were poor composite outcomes, which included ACM and MACE. ACM was defined as any cause of death, regardless of its etiology. MACE, on the other hand, was described as the combination of cardiac mortality, reinfarction, stroke, heart failure, cardiogenic shock, and malignant arrhythmias (e.g., ventricular tachycardia, ventricular fibrillation, atrial fibrillation, and atrial flutter).8,10–12,19,20,22–24
A meta-analysis, clinical trials studies, cross-sectional studies, case-control studies, case report studies, comments, editorials, conference abstracts, and all papers written in languages other than English were excluded from this study.
The relevant data from the selected studies were then retrieved by two separate authors using a predesigned table. Extracted data comprised baseline and methodological features of the studies involving the first author’s name, year of publication, the country in which the study was conducted, study design, total participants, study population, age, sex, type of index, high index criterion, and study outcomes. Any values presented using the median (interquartile range) were transformed into mean ± SD (standard deviation) for statistical analysis purposes.25,26
Quality assessment was conducted by two independent authors using the Newcastle-Ottawa Scale (NOS).27 A study with a total score of seven or above was determined to be a high-quality publication with a low chance of bias, and hence would be included in the analysis. Nonetheless, studies with a total score of less than seven indicate a low-quality publication with a significant risk of bias, and therefore will be removed from the analysis.
All statistical analyses were performed using STATA (Software for Statistics and Data Science) software version 17.0. The effect size in the current analysis was calculated as risk ratios (RRs) with 95% confidence intervals (CIs). Any included studies that used hazard ratios (HRs) to calculate effect size were transformed into RRs for the purposes of the analysis. We used random-effects models and the restricted maximum-likelihood method to calculate the overall effect size regardless of the heterogeneity status. The statistical significance was obtained when two-tailed P-values were attained at ≤0.05. The inconsistency index (I2) was used to quantify heterogeneity among studies, and an I2 value above 50% or P-value <0.10 indicated significant heterogeneity. To identify the sources of heterogeneity and obtain more robust results across studies, sub-group analysis was done by classifying the outcomes in a time-specific manner, study population, and cut-off points. Nonetheless, a median-split approach was performed to stratify the included studies in the MSI group based on the MSI cut-off in each study. We used Begg's funnel plot analysis and Egger's test to examine the publication bias both qualitatively and quantitatively.
The preliminary search yielded 1414 studies in total. After the duplication removal process, 1297 studies remained. Moreover, only 24 studies were retained following the screening procedure based on the title and abstract. Furthermore, the remaining studies were subjected to the full-text eligibility procedure, and eight studies were removed for various reasons, including missing data, dichotomization that was not based on high and low SI/MSI, and outcomes of interest that were irrelevant. Based on NOS bias risk assessment, 13 studies7,9–13,19–22,28–30 receive ratings of 9, 2 studies8,23 receive scores of 8, while another study24 obtains a score of 7. Conclusively, this meta-analysis comprised 16 studies7–13,19–24,28,29 with a total of 80,195 subjects (Figure 1) with males predominated among all participants (73.7%). Of the 16 included studies, 10 studies applied only SI, four studies used both SI and MSI, and two studies utilized only MSI. In the SI set of studies, six studies used a cut-off of 0.7, and the other studies used a variety of cut-offs. Whereas in the MSI group of studies, all the studies utilized completely diverse cut-offs. The detailed information on the included studies is provided in Table 1.
| No. | Author (year) | Country | Study design | Total participants | Study population | Age (years) | Male (%) | Index | High index cut-off | Outcome(s) | NOS |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Abe (2016)11 | Japan | Retrospective cohort | 680 | AMI patients who had PCI | 67.2±12.4 | 533 (78.4) | SI | ≥0.66 | 9 | |
| 2 | Abreu (2017)12 | Portugal | Retrospective cohort | 1158 | STEMI | 61.70±13.5 | 949 (82) | MSI | ≥0.93 | 9 | |
| 3 | Bilkova (2011)7 | Czech Republic | Retrospective cohort | 644 | STEMI | 63±11.7 | 467 (72.5) | SI | ≥0.8 | 9 | |
| 4 | Chunawala (2021)28 | United States | Retrospective cohort | 18,301 | NSTEMI | N/A | 10,831 (59.2) | SI | ≥0.7 | 9 | |
| 5 | El-Menyar (2019)23 | Qatar | Retrospective cohort | 24,636 | AMI | 56.68±12.61 | 19,059 (77.7) | SI | ≥0.8 | 8 | |
| 6 | Hemradj (2017)13 | The Netherlands | Prospective cohort | 7412 | STEMI patients who received primary PCI | 63.2±12.7 | 5376 (72.5) | SI | ≥0.7 | 9 | |
| 7 | Huang (2014)8 | China | Retrospective cohort | 7187 | STEMI | 62.5±11.9 | 5,105 (71) | SI | ≥0.7 | 8 | |
| 8 | Kobayashi (2016)24 | United States | Retrospective cohort | 481 | NSTEMI | N/A | 298 (62) | SI | ≥0.7 | 7 | |
| 9 | Ndrepepa (2019)29 | Germany | Retrospective cohort | 1369 | STEMI | 64.3±9.7 | 1003 (73.3) | SI | ≥0.67 | 9 | |
| 10 | Pramudyo (2022)30 | Indonesia | Retrospective cohort | 1306 | AMI | 58.1±11 | 1009 (77.3) | MSI | ≥1 | 9 | |
| 11 | Reinstadler (2016)22 | Germany | Retrospective cohort | 791 | STEMI | 62±3.2 | 600 (76) | SI | ≥0.62 | 9 | |
| 12 | Schmitz (2022)19 | Germany | Retrospective cohort | 10,174 | AMI | N/A | 7423 (73) | SI MSI | >0.58 >0.85 | 9 | |
| 13 | Shangguan (2015)20 | China | Retrospective cohort | 160 | STEMI patients who received primary PCI | 65.5±13.1 | 132 (82.5) | SI MSI | ≥0.7 ≥1.4 | 9 | |
| 14 | Spyridopoulos (2014)9 | United Kingdom | Retrospective cohort | 3049 | STEMI patients who received primary PCI | N/A | 2149 (70.5) | SI | >1 | 9 | |
| 15 | Yu (2017)21 | China | Retrospective cohort | 1864 | AMI patients who underwent PCI | 61.8±11.9 | 1321 (70.9) | SI MSI | >0.51 >0.51 | 9 | |
| 16 | Zhou (2019)10 | China | Retrospective cohort | 983 | STEMI patients who underwent PCI | 63.1±13.0 | 793 (80.7) | SI MSI | In-hospital ACM SI>0.74 MSI>0.74 6-month ACM SI>0.87 MSI>0.87 Long-term ACM SI>0.73 MSI>0.73 In-hospital MACE SI>0.87 MSI>0.87 | 9 |
Due to five studies8–10,13,29 presented with at least two outcomes regarding period of mortality, the calculation of pooled RRs could not be obtained. Furthermore, we performed sub-group analyses based on the study population (AMI, STEMI, and NSTEMI), period of ACM (long-term (≥1-year) mortality, 1-month to 6-month mortality, and in-hospital to 7-day mortality), and SI cut-off (≥0.7 and others). High SI significantly increased the risk of ACM in the AMI population compared to low SI (RR=2.90 (95% CI=1.43-5.88); P=0.003; I2=91%, P-heterogeneity<0.001). This significance was also obtained in the STEMI and NSTEMI populations, with summary risk ratios of (RR=1.65 (95% CI=1.29-2.10); P<0.001; I2=91.6%, P-heterogeneity<0.001) and (RR=1.90 (95% CI=1.31–2.74); P=0.001; I2=68.4%, P-heterogeneity=0.042) (Figure 2A).
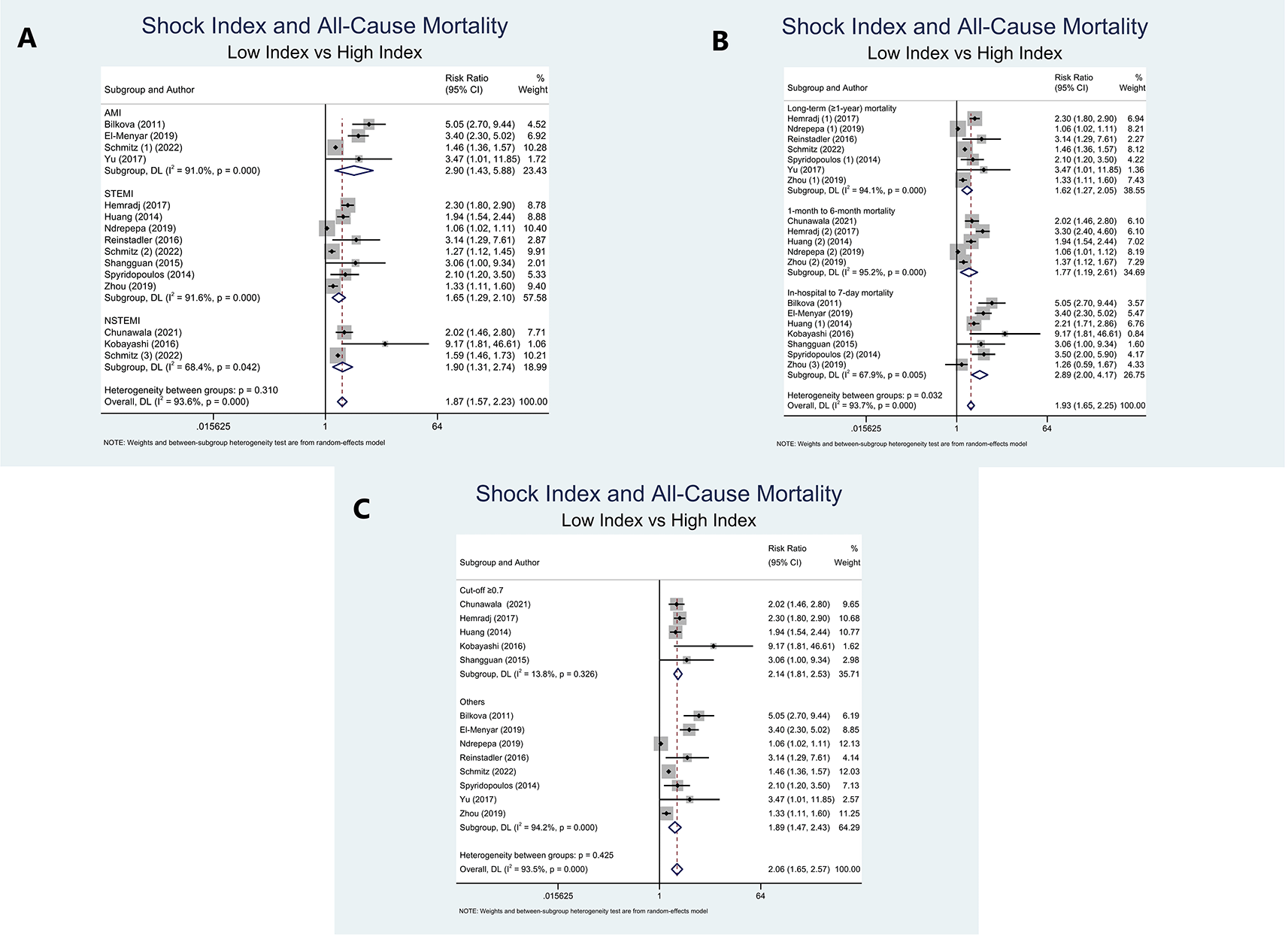
Long-term (≥1-year) mortality was significantly increased in the high SI group in comparison to the low SI group (RR=1.62 (95% CI=1.27-2.05); P<0.001; I2=94.1%, P-heterogeneity<0.001). In addition, as opposed to the low SI population, the high SI population had a substantially higher risk of 1-month to 6-month mortality (RR=1.77 (95% CI=1.19-2.61); P=0.004; I2=95.2%, P-heterogeneity<0.001). Moreover, high SI patients were notably associated with a higher risk of in-hospital to 7-day mortality in contrast to low SI patients (RR=2.89 (95% CI=2.00–4.17); P<0.001; I2=67.9%, P-heterogeneity=0.005) (Figure 2B).
In terms of SI cut-off, sub-group analysis of 5 studies with cut-off ≥0.7 was linked to a higher risk of mortality and remarkably reduced the heterogeneity (RR=2.14 (95% CI=1.81-2.53); P<0.001; I2=13.8%, P-heterogeneity=0.326). Furthermore, a sub-group analysis of 8 studies with cut-off other than 0.7 also revealed a higher mortality risk (RR=1.89 (95% CI=1.47-2.43); P<0.001; I2=94.2%, P-heterogeneity<0.001) (Figure 2C).
Our pooled results of 9 studies suggest that populations with high SI were strongly associated with MACE when compared to low SI (RR=2.36 (95% CI=1.76-3.18); P<0.001; I2=81.3%, P-heterogeneity<0.001) (Figure 3).

With reference to the study population, our merged analysis showed that AMI (RR=1.74 (95% CI=1.34-2.26); P<0.001; I2=0%, P-heterogeneity=0.487), STEMI (RR=2.39 (95% CI=1.50-3.83); P<0.001; I2=89%, P-heterogeneity<0.001), and NSTEMI patients (RR=2.99 (95% CI=2.09-4.28); P<0.001; I2=0%, P-heterogeneity=0.650) with high SI substantially increased the risk of MACE compared to those with low SI (Figure 3A).
Sub-group analysis based on MACE period revealed that a high SI population had a significantly increased risk of long-term (≥1-year) MACE (RR=2.22 (95% CI=1.43-3.45); P<0.001; I2=0%, P-heterogeneity=0.881), 7-day MACE (RR=1.66 (95% CI=1.39-1.98); P<0.001; I2=0%, P-heterogeneity=0.331), and in-hospital MACE (RR=2.64 (95% CI=1.69-4.11); P<0.001; I2=86.7%, P-heterogeneity<0.001) as opposed to the low SI population (Figure 3B).
Further sub-group analysis of 6 studies with cut-off values ≥0.7 revealed that high SI patients had a higher risk of MACE and significantly reduced the heterogeneity to 43% (RR=2.08 (95% CI=1.28-3.39); P<0.001; I2=43%, P-heterogeneity=0.173). Additionally, a sub-group analysis of three studies with cut-offs other than 0.7 demonstrated that high SI was correlated with MACE (RR=2.44 (95% CI=1.68–3.54); P<0.001; I2=83.1%, P-heterogeneity<0.001) (Figure 3C).
As two studies10,12 yielded at least two mortality period outcomes, pooled RRs could not be evaluated. On the basis of study population, we discovered that high MSI was associated with a higher risk of ACM in AMI (RR=2.25 (95% CI=1.18-4.26); P=0.013; I2=81.8%, P-heterogeneity=0.004), STEMI (RR=1.74 (95% CI=1.28-2.37); P<0.001; I2=81.8%, P-heterogeneity<0.001), and NSTEMI population (RR=1.52 (95% CI=1.40-1.66); P<0.001; I2=0%, P-heterogeneity=0.968) (Figure 4A).
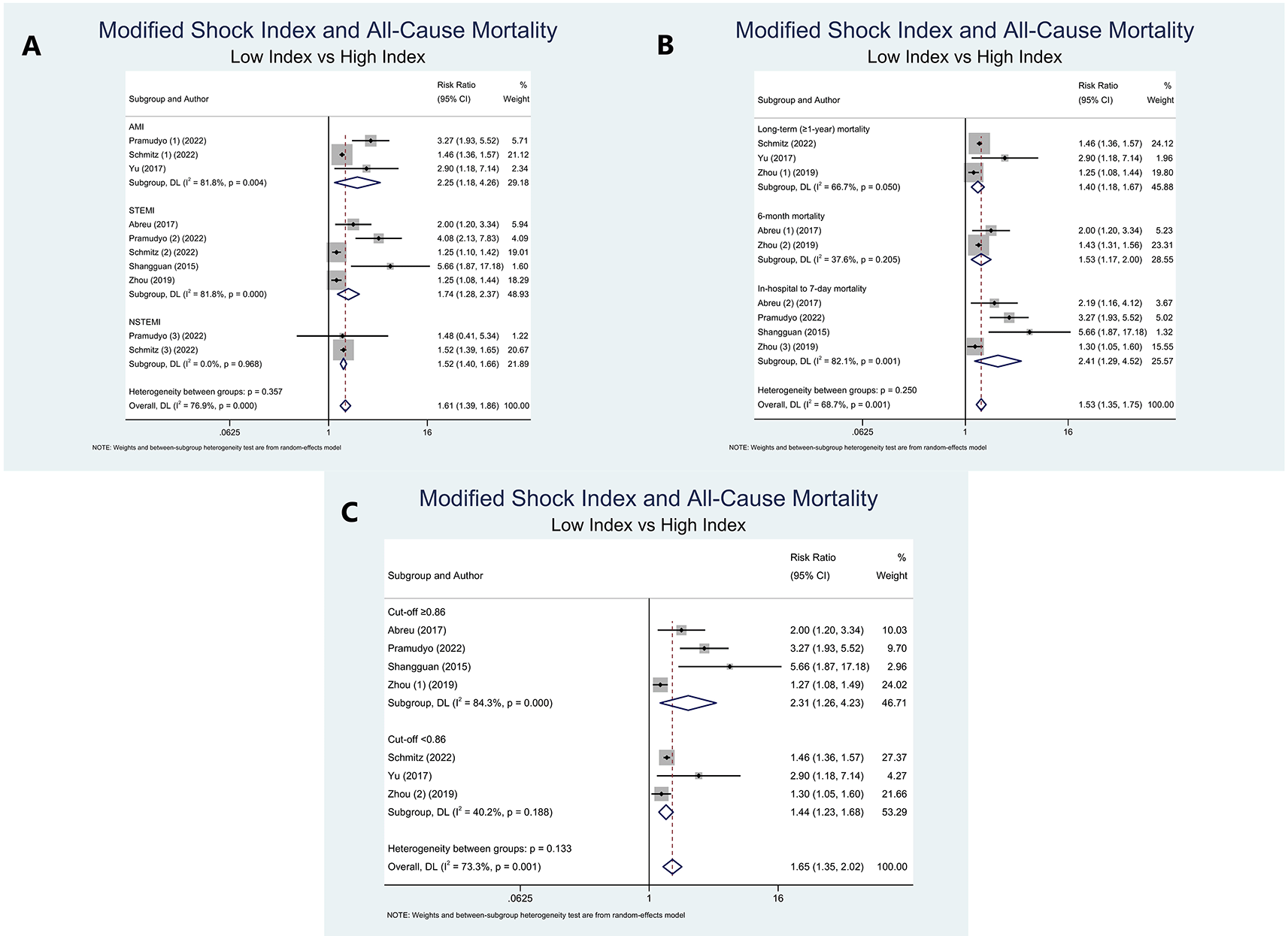
Consistently, in connection with mortality period, sub-group analysis disclosed that high MSI was related to an elevated risk of long-term (≥1-year) mortality (RR=1.40 (95% CI=1.18-1.67); P<0.001; I2=66.7%, P-heterogeneity=0.050), 6-month mortality (RR=1.53 (95% CI=1.17-2.00); P<0.001; I2=37.6%, P-heterogeneity=0.205), and in-hospital to 7-day mortality (RR=2.41 (95% CI=1.29-4.52); P<0.001; I2=82.1%, P-heterogeneity=0.001) (Figure 4B).
Since the cut-off of MSI varies greatly across all included studies, we classified the studies based on the cut-off resulting from the median-split approach. A sub-group analysis of three studies with cut-off values of <0,86 revealed that high MSI patients were associated with ACM and markedly reduced the heterogeneity within the included studies (RR=1.44 (95% CI=1.23-1.68); P<0.001; I2=40.2%, P-heterogeneity=0.188). ACM risk was also substantially more elevated in patients with high MSI (cut-off ≥0.86), compared to those with low MSI (RR=2.31 (95% CI=1.26–4.23); P=0.002; I2=84.3%, P-heterogeneity<0.001) (Figure 4C).
A meta-analysis comprising five studies showed that high MSI was substantially correlated with MACE in distinction to low MSI (RR=2.55 (95% CI=1.56-4.16); P<0.001; I2=92.4%, P-heterogeneity<0.001). Moreover, due to the limited number of included studies, we were unable to conduct additional sub-group analysis based on the aforementioned subsets (Figure 5).
The Begg’s funnel plot could not be drawn to identify publication bias across the included studies since the number of studies in each subgroup was relatively small. However, Egger’s test showed no indication of small study effects amid all outcomes and subsets of this meta-analysis (P>0.05).
This comprehensive meta-analysis highlighted that both high SI and MSI were significantly associated with an increased risk of poor outcomes that comprise all-cause mortality and major adverse cardiac events in patients with acute myocardial infarction. Higher mortality within the high indexes’ populations was observed, regardless of the periods of mortality and study populations during observation. In addition, the difference in major adverse cardiac events was significant between the two groups, denoting that elevated SI and MSI might play a crucial role in prognosticating the risk of MACE. Our findings of the main outcomes are strengthened by the fact that subgroup analyses based on outcome period, study population, and cut-off values did not alter the results, although it was only obtained in the SI group, but not in the MSI group, particularly in the MACE outcome due to a lack of studies (Table 2). Thus, based on the current study’s results, we suggest the use of SI and MSI as a convenient index in patients with AMI, STEMI, or NSTEMI at admission to predict the risk of ACM and MACE. Nevertheless, since the association between MSI and MACE is still inconclusive in each study population and according to outcome period, more cohort studies are needed to better evaluate the correlation between these two variables.
SI and MSI were originally used as simple metrics for gauging the degree of hypovolemia in several critical states as they increased proportionally with progressive loss of circulating blood volume and have proven to be a good predictor of mortality in trauma patients.18,31 These indices are commonly used in critically ill patients to assess disease severity, treatment response, and need for intensive care admission. Thus, the use of SI and MSI in AMI patients is thought to be relatively reasonable, as each component of SI and MSI, including heart rate (HR), systolic blood pressure (SBP), and mean arterial pressure (MAP), was previously known to be an independent risk factor of mortality in patients with AMI.3,4,32–35
Although there are several plausible explanations for the nature of such an association, the precise pathophysiological relationship between SI, MSI, and adverse outcomes remains undecipherable. First, it is widely known that AMI patients generally experience sympathetic nervous system hyperactivity, which regulates HR and SBP.36 Stimulation of the sympathetic nervous system can cause sustained a release of catecholamine to compensate for the decreased cardiac output (CO) derived from the ongoing ischemic myocardial tissue process, as well as pain and anxiety due to chest pain.37,38 In other words, higher SI or MSI may reflect sympathetic overactivity circumstances. Second, an observational study conducted by Petersen et al. discovered that catecholamine levels were inversely correlated with left ventricular ejection fraction (LVEF) in AMI patients, demonstrating that sympathetic hyperactivity is also linked to the degree of left ventricular dysfunction.39 Several studies19,22 support this evidence, stating that patients with a higher shock index on admission had significantly larger infarcts, the higher peak of myocardial damage parameters, leading to further extensive left ventricle dilatation (remodelling) and heart failure, which are associated with worse prognoses in the latter phase. Therefore, SI and MSI may reflect an integrated cardiovascular and neuroendocrine system to maintain stroke volume and neurohormonal responses. Moreover, sympathetic stimulation in AMI can increase calcium production from the sarcoplasmic reticulum, resulting in catecholamine-induced tissue lipolysis and a surge in plasma free fatty acids levels. This may overburden the acutely ischemic myocardium, impair glucose utilization, and cause abnormal electrophysiological conduction and refractoriness, potentially leading to irreversible ventricular tachyarrhythmias.8,12,28,40,41 Hence, according to the aforementioned hypotheses, HR elevation, as indicated by high SI and MSI, was associated with an increased risk of mortality and MACE in AMI patients.
The majority of myocardial tissue receives blood supply primarily during the diastolic phase, and several studies42–49 have suggested that extremely low diastolic blood pressure (DBP) may damage coronary autoregulation and lead to an even worse adverse prognosis in AMI patients. Hence, theoretically, MAP in the MSI variable, which is primarily controlled by CO and systemic vascular resistance (SVR), may be an even better prognosticator than SI in predicting poor outcomes. However, no difference was observed in the predictive value for adverse outcomes between the SI and MSI in patients with AMI. Schmitz et al., for example, discovered that the area under curve (AUC) values for MSI were significantly higher than those for SI in their large study involving over 10,000 patients.19 Still the graphs appear to be very comparable, with no obvious superiority of the MSI could be noticed. Furthermore, a study conducted by Reinstadler et al. also found no significant differences in SI and MSI for predicting poor outcomes in a STEMI population.22 Our meta-analysis shared the same results, indicating that SI and MSI are still comparable in predicting mortality and MACE in AMI cases.
Our analysis of SI and MSI as predictors for mortality and MACE in AMI patients was done in a sub-group manner, particularly based on the study population, cut-off values, and lastly, the outcomes periods. Therefore, not only were we able to evaluate potential causes of heterogeneity among included studies, but we were also able to achieve more transparent results by separating them into in-hospital and longer-term outcomes periods. Our current study showed that high SI and MSI were consistently linked to a higher risk of adverse outcomes not only in the short-term period, but also in the mid- and long-term subgroups. Moreover, it was found that the predictive value of these indices attenuated over time as shown in the outcome’s period subgroup in Table 2. This trend can be explained by the fact that peri-hospital complications are par for the course given that our main outcome was mortality from all-causes, namely the risk of getting a hospital-related infection, major medications side effects, iatrogenic causes, that can lead to a worse prognosis, which may partially explain the increased risk of poor outcomes in the short-term period.50 Aside from that, AMI patients are always given medications that can improve prognosis after hospital discharge, which may mitigate the unfavourable effect during long-term follow-up. Still and all, cardiac remodelling, sympathetic hyperactivity caused by ischemic injury, begins in the relatively early stages of AMI and may persist afterwards, and has always been associated with poorer short- and long-term prognoses.36
Despite its positive findings, there are a few key points to consider before drawing any conclusions from our study. First, high heterogeneity is noted in the analysis, which is primarily caused by different SI and MSI cut-offs, the difference in the study population, and diverse outcome periods. Hence, validation of fixed cut-off of SI and MSI is required in order to confirm the significant association between high SI and MSI along with mortality and MACE in AMI patients. Second, conferring about mortality could not be separated from several well-known independent confounding factors including age, comorbidities (e.g., hypertension, stroke, DM, peripheral artery disease, hyperlipidemia, kidney disease), high KILLIP classification (≥2), cardiogenic shock, anterior myocardial infarction, multivessel disease, and malignant arrhythmia, all of which may contribute to an increased risk of ACM in patients with AMI. Fortunately, the majority of included studies7–12,20,21,23,28,29 adjusted these variables by performing multivariate analysis, thus as we collected adjusted RRs from all included studies, thereby diminishing confounding bias. Third, as SI and MSI are highly dependent on HR, long-term use of beta-blockers, calcium channel blockers, and ivabradine prior to admission may affect SI and MSI. Additionally, because most of the included studies were initiated in tertiary referral hospitals, many patients may have received drugs that affected their hemodynamic status (e.g., vasopressor, inotropic, beta-blocker). Nonetheless, none of the included studies provide any information regarding participants’ prior medication use, thereby raising the risk of bias.
Several limitations are noted in this meta-analysis. For starters, most of the included studies are retrospective cohorts, which may lead to recall and selection bias. Secondly, significant heterogeneity was discovered in the analysis as a result of various cut-offs for SI and MSI, outcome, and study population. Fortunately, after we conducted subgroup analysis, the heterogeneity was significantly reduced, possibly due to more distinct conditions in the STEMI population, shorter-term outcome periods, and each subset of cut-off values. Lastly, further prospective cohort studies with a fixed cut-off of SI and MSI, longer outcome periods, and analyses of each AMI phenotype are mandatory to better understand the association between these indices along with mortality and MACE in patients with AMI.
Current evidence indicates that high SI and MSI upon admission are associated with a higher risk of ACM, most notably in the short-term period, followed by mid-, and long-term periods in patients suffering from an AMI. Moreover, AMI patients with high SI and MSI had a substantially elevated risk of MACE, and the significance was consistent across all MACE periods and study populations in the SI group. Whereas, due to a lack of studies, no sub-group analysis of the relationship between MSI and MACE was performed. To assess the prognostic value of these indices in AMI patients, prospective cohort studies with a validated and fixed cut-offs of SI and MSI, longer outcomes periods, and evaluation of each study population are still needed. Lastly, currently, we highly suggest the usage of SI and MSI in AMI patients at first medical contact, to predict the risk of ACM and MACE.
All data underlying the results are available as part of the article and no additional source data are required.
figshare: PRISMA checklist and flowchart for ‘Elevated shock index and modified shock index are associated with mortality and major adverse cardiac events in patients with acute myocardial infarction: A systematic review and meta-analysis’, https://doi.org/10.6084/m9.figshare.20380641.v5.51
MP: Conceptualization, Data Curation, Formal Analysis, Writing – Review & Editing, Supervision, Validation.
ICSP: Conceptualization, Data Curation, Formal Analysis, Investigation, Methodology, Project Administration, Software, Validation, Visualization, Writing – Original Draft Preparation.
WK: Data Curation, Formal Analysis, Investigation, Methodology, Project Administration, Software, Validation, Visualization, Writing – Original Draft Preparation.
HSP: Writing – Review & Editing, Supervision, Validation.
AS: Writing – Review & Editing, Supervision, Validation.
MRA: Writing – Review & Editing, Supervision, Validation.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
Yes
Are the conclusions drawn adequately supported by the results presented in the review?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Cardiovascular Prevention and Rehabilitation, Clinical Cardiology.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 2 (revision) 30 Aug 22 |
|
|
Version 1 11 Aug 22 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)