Keywords
Prostate cancer, malignant ascites, tumor lysis syndrome, hemorrhagic ascites, ascites, chylous ascites, metastatic prostate cancer, castrate resistant prostate cancer
This article is included in the Oncology gateway.
Prostate cancer, malignant ascites, tumor lysis syndrome, hemorrhagic ascites, ascites, chylous ascites, metastatic prostate cancer, castrate resistant prostate cancer
After skin cancer, prostate cancer is the most common malignancy in men in most of the Western world. Incidence rates in the US rose through the early 1990s and have subsequently decreased, possibly in association with guidelines recommending against use of prostate specific antigen (PSA) for screening.1,2 It remains the leading cause of cancer death in American men after lung cancer and one in nine American men will be diagnosed with prostate cancer in their lifetime.3 While most cases are localized on presentation, up to 17% of patients may experience metastatic disease associated with increased cancer-specific mortality.4 Bone, distant lymph nodes, and abdominal organ metastases are the most common, but peritoneal carcinomatosis with concurrent malignant ascites is exceedingly rare.5 Only 32 cases of prostate cancer with peritoneal carcinomatosis and ascites have currently been reported in the literature (Table 1). We present the first reported case of prostate cancer with peritoneal carcinomatosis and malignant ascites whose treatment was complicated by tumor lysis syndrome along with a literature review evaluating similar cases.
| Year reported | Author | Ref. | Age, years | Time between first diagnosis and ascites | *Other sites of metastases | Treatment (Pre-ascites) | Treatment (Post-ascites) | Response from diagnosis of ascites | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| 1968 | Rapoport et al. | 43 | 76 | 16 years | Lymph nodes, direct invasion of surrounding organs | Orchiectomy + DES | 5-FU and IP Thiotepa | Progression | Death at 3 months |
| 1968 | Rapoport et al. | 43 | 45 | 1 year | Seminal vesicles, bladder, and surrounding lymphatics | Total cystectomy | Orchiectomy, DES | Progression | Death in weeks to few months |
| 1973 | Megalli et al. | 7 | 58 | Initial presentationδ | None | DES, RT | Remission | Resolved ascites, alive at 6 months | |
| 1990 | Beigel et al. | 8 | 29 | Initial presentationβ | Bones | Patient Refused | Progression | Death at 1 month | |
| 1990 | Disdier et al. | 25 | 78 | Initial presentationα | Lymph nodes | None | Orchiectomy, Nilutamide | Remission | Diminished ascites, survival NR |
| 1992 | Catton et al. | 26 | 63 | Initial presentation | Diffuse visceral, local invasion of periprostatic tissues (on autopsy) | Orchiectomy, hormonal therapy | Remission | Resolution of ascites for 9 months, death at 13 months | |
| 1999 | Saif et al. | 27 | 70 | 4 years | None reported | RT, Leuprolide + Flutamide, Leuprolide + Bicalutamide, Thalidomide | NR | Progression | Alive at 2 months |
| 1996 | Zhau et al. | 28 | 83 | >1 year | Liver, bones | NR | Orchiectomy | Progression | Death at 12 months |
| 2000 | Wynn et al. | 29 | 73 | 9 years | Lymph nodes, sigmoid colon | RT | None | Progression | Death at 3 weeks |
| 2001 | Tsai et al. | 30 | 68 | 15 monthsα | Rectal wall | Gosrelin, Flutamide | Interferon alpha-2b and toremifene | Progression | Death at 4 months |
| 2002 | Amin et al. | 9 | 83 | 5 yearsβ | Lymph nodes | Antiandrogen | Withdrawal of antiandrogen therapy | Progression | Death at 6 weeks |
| 2002 | Kehinde et al. | 10 | 76 | 4 yearsδ | None | TURP | Orchiectomy on recurrence | Remission | Resolved ascites in 3 months, alive at 18 months |
| 2004 | Appalaneni et al. | 16 | 60 | 3 years | Bones, lymph nodes | RT, GnRH Agonist + Antiadrogen, Mitoxantrone + Prednisone | NR | Progression | Death at 6 weeks |
| 2004 | Lapoile et al. | 31 | 80 | 10 years | Bone, others | RT, triptorelin, aminoglutethimide and hydrocortisone | None | Progression | Death at 3 months |
| 2007 | Madaan et al. | 32 | 75 | 3 yearsα | Lymph nodes | Gosrelin | DES and ASA | Progression | Death within 4 months |
| 2009 | Okouo et al. | 33 | 81 | 3 yearsα | Brain | None | Triptorelin, cryproterone | Progression | Resolved ascites, death at 3 months |
| 2009 | Zagouri et al. | 34 | 75 | 5 years | None | Gosrelin + bicalutamide, Gosrelin + estramustine | Docetaxel + Estramustine, Docetaxel + prednisone | Remission | Resolution of ascites, alive at 6 months |
| 2010 | Benedict et al. | 35 | 67 | 4 years | None | Orchiectomy and Bicalutamide | Docetaxel | Remission | Diminished ascites, survival NR |
| 2012 | Talwar et al. | 36 | 59 | Initial presentation | Inguinal and umbilical hernia sacks | Leuprolide | Progression | Death at 3 months | |
| 2013 | Ani et al. | 37 | 57 | Initial presentation | Lymph nodes, bones | Bicalutamide + GnRH agonist | NR | Stable Disease | Stable ascites, decreased PSA, alive at 3 months |
| 2015 | Petrakis et al. | 13 | 76 | 16 years | Lymph nodes, bones | TURP, Bicalutamide + Leuprolide | Docetaxel | Remission | Resolved ascites, alive at 10 months |
| 2015 | Pradhan et al. | 38 | 70 | Initial presentation | Bones, lymph nodes | Hormone therapy | Remission | Ascites response NR, alive at 6 weeks | |
| 2015 | Saini et al. | 11 | 65 | 9 yearsδ | None | Orchiectomy, Bicalutamide, Fosfestrol, Ketoconazole + Prednisolone | Taxotere-base chemotherapy | NR | NR |
| 2016 | Gungor et al. | 39 | 60 | 3 years | Locally advanced | RT, Antiandrogens | NR | NR | NR |
| 2016 | Papadatos et al. | 12 | 69 | 3 yearsδ | Bone | RT, Bicalutamide + Triptorelin | None | Progression | Death at 3 weeks |
| 2017 | Ladwa et al. | 14 | 74 | NR | None | Docetaxel, Abiraterone | Cabazitaxel | Stable disease | Increased PSA, stable imaging, alive at 10 months |
| 2018 | van Roekel et al. | 40 | 65 | 15 months | Ureteral | RT, Bicalutamide, Docetaxel | "Palliative Therapy" | Progression | Death at 3 weeks |
| 2019 | Samankan et al. | 41 | 84 | NR | NR | NR | Docetaxel + Enzalutamide | Remission | Diminished ascites, alive at follow up (time NR) |
| 2019 | Tareen et al. | 17 | 70 | 1 week | Bladder, seminal vesicles, liver | Aborted prostatectomy | Bicalutamide + Leuprolide, Docetaxel, Abiraterone + Prednisone, Cabazitaxel | Progression | Alive at 6 months with transiently diminished symptoms, transitioned to hospice given poor functional status |
| 2020 | Present case | N/A | 78 | 8 yearsα | Lung, bone | Leuprolide + Bicalutamide | Docetaxel + Bicalutamide | Progression | Diminished ascites for 1 month, death at 3 months |
| 2020 | Visconti et al. | 15 | 77 | Initial presentationδ | Bone | None | GnRH agonist + Bicalutamide | Remission | PSA reduction & reduced ascites at 3 months, death NR |
| 2021 | Sagar et al. | 42 | 36 | Initial presentation | Bone, liver, lymph nodes | NR | NR | NR | NR |
| Median age, years | 70 |
We reviewed the literature including cases and postmortem studies of patients with “prostate cancer” AND “malignant ascites” through a PubMed (RRID:SCR_004846) search (human; all languages; 1968–2022; last search 10 May 2022). We performed a manual retrieval of bibliographical information of resulting papers to identify additional literature and collect information regarding age, clinical features, metastatic sites, treatments, outcomes, survival, and cause of death.
A 78-year-old retired Caucasian male patient with diabetes, hypertension, peripheral vascular disease, and stage IV chronic kidney disease due to obstructive uropathy was initially diagnosed with intermediate risk, T2b N0 M0, clinical stage II prostatic adenocarcinoma at another facility where he completed definitive radiotherapy in 2008. He had a 90 pack/year smoking history, which he quit in 1992. Family history was notable for prostate cancer in his paternal uncle. In 2018, a computed tomography (CT) scan of his abdomen revealed bilateral hydronephrosis and right-sided bladder wall thickening. He was found to have PSA elevated to 45 ng/ml and was initiated on combined androgen blockade with intramuscular leuprolide (22.5 mg every three months) and oral bicalutamide (50 mg daily). Cystoscopy at that time was only notable for radiation cystitis.
In March 2019, after being transferred to Scripps Clinic, he was found to have a solitary right upper lobe pulmonary lesion on positron emission tomography (PET)/CT. He completed stereotactic body radiation therapy (SBRT) in June 2019, at which time his PSA level was 38.2 ng/ml. A follow up surveillance PET/CT was without evidence of active metastatic disease.
The patient was admitted to Scripps Green Hospital with severe abdominal distension and shortness of breath on 12 April 2020. Physical exam was notable for palpable fluid wave and shifting dullness consistent with new onset ascites. A therapeutic and diagnostic paracentesis yielded 3L of serosanguinous fluid with 79,000 red blood cells, albumin 1.8 g/dL, protein 3.4 g/dL, glucose 154 mg/dL, lactate dehydrogenase (LDH) 1,004 units/L, pH 7.5, polymorphonuclear cell count 184 cells/mm3, and negative gram stain, ruling out spontaneous bacterial peritonitis (SBP). Serum albumin was 2.7 g/dL with a serum albumin ascites gradient (SAAG) <1, consistent with an exudative process. Ascitic fluid cytopathology revealed metastatic prostatic adenocarcinoma with immunohistochemistry (IHC) positive for prostatic markers homeobox protein Nkx-3.1 (NKX3.1), ETS transcription factor ERG (ERG) and PSA (Figure 1 and Figure 2). Gastrointestinal IHC was negative for CDH17. PSA levels were elevated at 446.60 ng/mL. CT abdomen and pelvis without IV contrast demonstrated severe peritoneal thickening, nodular omental infiltration, ascites, mildly nodular bladder wall thickening, scattered sclerotic osseous foci, and new vertebral osseous metastasis at T12 with interval development of 7.1 × 6.3 cm right cardiophrenic angle tumor (Figure 3). He had stable scattered small nonspecific abdominal mesenteric and retroperitoneal lymph nodes. He required paracentesis every 24-48 hours due to rapid reaccumulation of ascites.
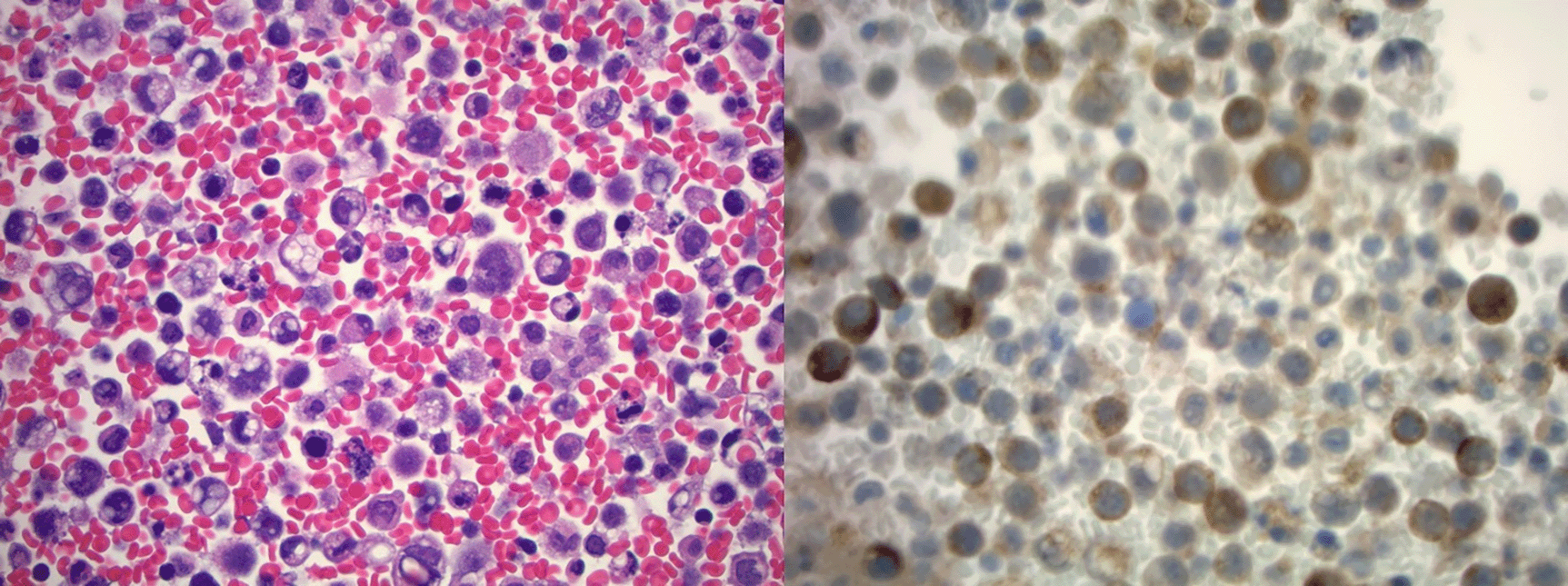
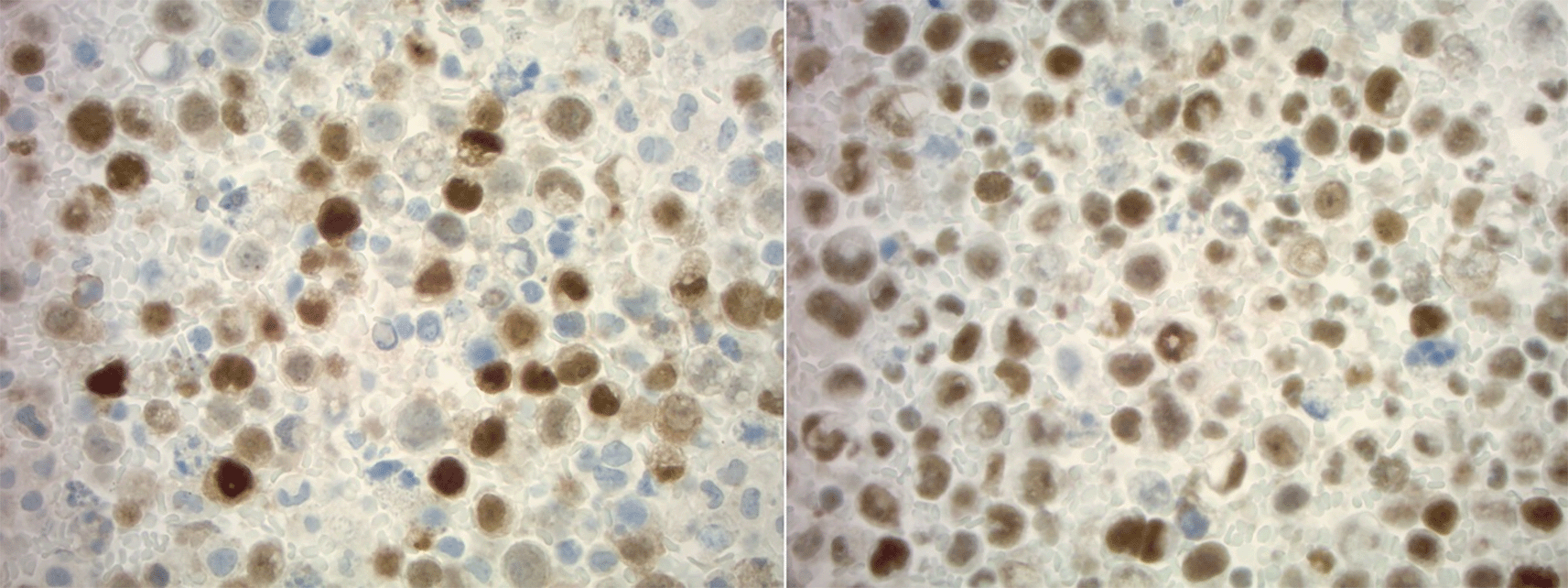
Cytology of ascitic fluid demonstrating highly atypical epithelial cells with somewhat vacuolated cytoplasm, pleomorphic nuclei, and mitotic figures in a background of necrosis and acute inflammation on H&E stain (left) with immunohistochemistry demonstrating positive PSA (right) staining (400×). PSA, prostate specific antigen.
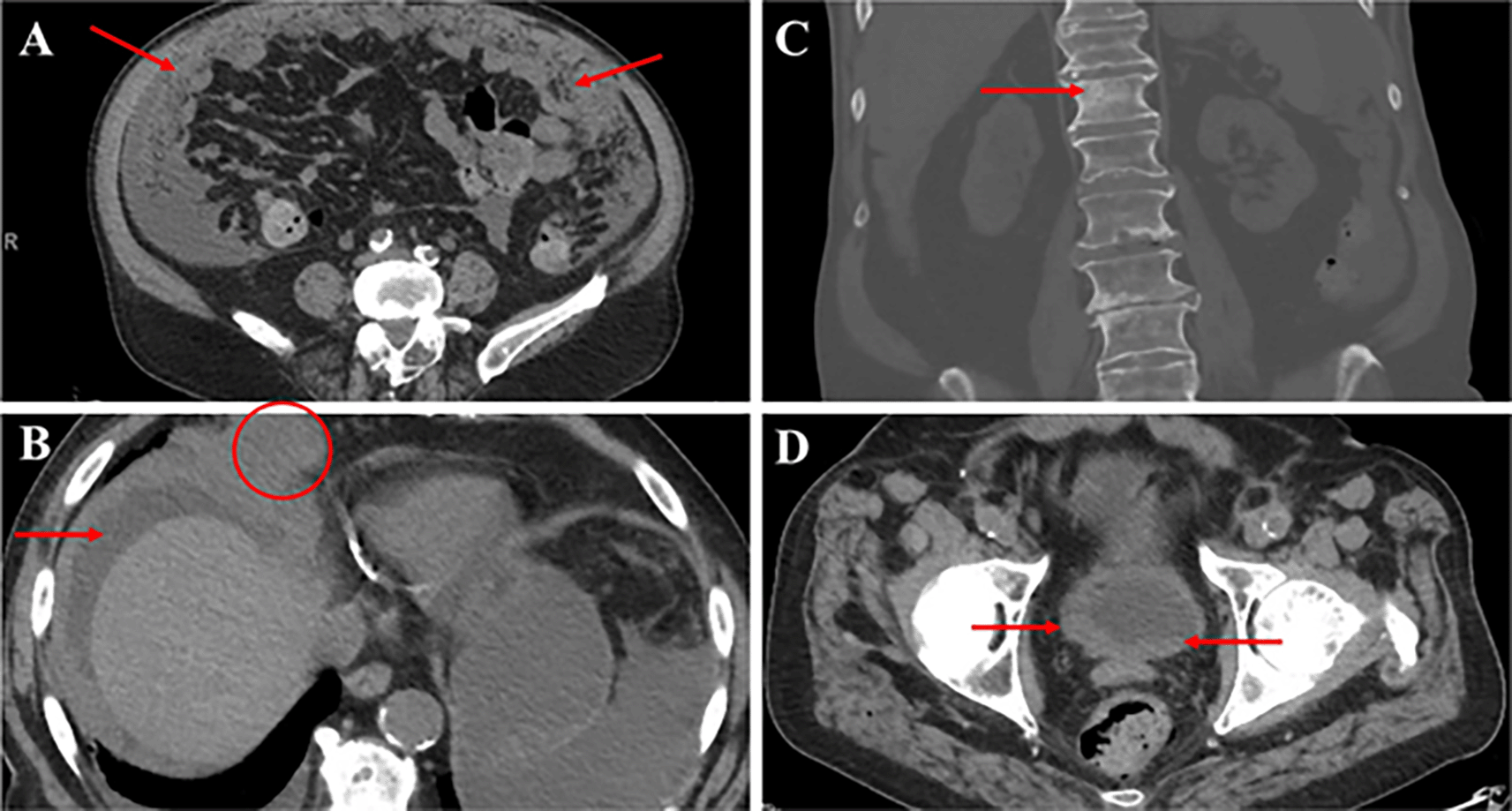
(A) CT abdomen and pelvis without IV contrast showing severe peritoneal thickening with nodular omental deposits (arrows). (B) Heterogeneity of bone density with scattered sclerotic foci and ill-defined sclerotic lesion of T12 vertebral body (arrow). (C) Bulky soft tissue tumor at the right cardiophrenic angle measuring approximately 7.1 × 6.3 cm transversely (circle) with ascites (arrow). (D) Asymmetric and mildly nodular right greater than left bladder wall thickening (arrows). CT, computed tomography.
He was initiated on intravenous docetaxel 75 mg/m2 every 21 days per the androgen ablation therapy vs. combined chemo-hormonal therapy trial for metastatic prostate cancer (CHAARTED) trial.6 Five days later, he developed worsening renal insufficiency secondary to known bilateral hydronephrosis and nephrostomy tubes were placed. Further laboratory workup showed an elevated uric acid (20.6 ng/dL), potassium (5.8 nmol/L), and phosphorus (12.1mg/dL), consistent with tumor lysis syndrome (TLS). He received three hemodialysis sessions with improvement in renal function and was transferred to a skilled nursing facility. He had intermittent improvement in his ascites and did not require therapeutic paracentesis for one month. Cycle two of docetaxel was reduced to 37.5 mg/m2 due to renal insufficiency and fatigue with cycle one. Serum PSA decreased from 446.60 ng/mL to 43.79 ng/ml after two cycles. Unfortunately, he was readmitted on 3 July 2020 for rapidly reaccumulating ascites and encephalopathy, he was then subsequently found to have Escherichia coli bacteremia with acute kidney injury. He eventually expired from obstructive shock due to massive saddle pulmonary embolism during this admission.
Based on our literature review, we describe the 32nd case of prostate cancer with peritoneal metastasis and ascites, and only the 29th case with malignant ascites (Table 1). Of these cases, only four others had hemorrhagic ascites. Six cases (18.8%) were associated with ascites with negative cytology and two of these patients had chylous ascites.7–12 A total of 10 patients (31.2%) achieved documented disease remission with two patients surviving for at least six weeks and five surviving for ≥ six months, four of whom survived at least 10 months in remission.10,12–14 Survival was not reported in four cases. A diagnosis of malignant ascites in two of these cases was made purely based on ascitic PSA elevated above serum PSA levels despite negative cytology.12,15 Elevated ascitic PSA in one case prompted clinicians to repeat a paracentesis, which eventually yielded malignant cytology.16 Time from initial diagnosis of cancer to development of ascites typically ranged from 1-16 years. Ascites occurred one week after aborted prostatectomy in one case, though imaging suggested peritoneal deposits before this.17 There was a documented response of ascites in 14 cases (43.8%), remaining stable in two (6.3%), diminishing in five (14.3%), and resolving in six (18.8%) with remission. Ascites diminished at least transiently in two cases of disease progression, including our own. Responses after development of ascites were elicited by surgical or chemical castration in six cases (18.8%), cytotoxic therapy in four cases (12.4%), and combined therapy in five cases. Ascites was the initial presentation of disease in nine cases (28.1%), occurred late in 20 (62.5%), was not reported in two, and occurred one week after aborted prostatectomy in one case (Table 2). The median age at which ascites presented was 70 years old.
| Number of cases | |||
|---|---|---|---|
| Ascites characteristics (if atypical) | Negative cytology | ||
| Chylous | 2 | ||
| Non-chylous | 3 | ||
| Non-chylous, elevated PSA | 2 | ||
| Positive cytology | Hemorrhagic | 5 | |
| Ascites response to treatment* | If remission achieved | Stable | 2 |
| Diminished | 5 | ||
| Resolved | 6 | ||
| Not reported | |||
| If disease progressed | 2§ | ||
| Response not reported | 1 | ||
| Total | 17 | ||
| Therapy used to elicit response* | Castration | 6 | |
| Cytotoxic | 4 | ||
| Combined | 5 | ||
| Total | 15 | ||
| Time at which ascites presented from initial diagnosis | Initial presentation | 9 | |
| Early (<1 year) | 1 | ||
| Late (≥1 year) | 20 | ||
| Not reported | 2 | ||
| Total | 32 |
In a large population-based analysis by Gandaglia et al., the most common sites of metastasis in prostate cancer were bone (84%), distant lymph nodes (10.6%), liver (10.2%), thorax (9.1%), brain (3.1%) and digestive tract (2.7%).5 Peritoneal and omental metastasis are exceedingly rare with associated ascites being even less common. In determining the pathophysiology of malignant dissemination, Paget first described the “seed and soil” hypothesis, in which cancer cells (seeds) migrate to secondary sites (soil) based on local factors contributing to the proclivity of cell migration.18 Mechanisms of metastasis in prostate cancer have been studied in most detail in osseous disease and describe interactions between chemokines like C-X-C motif chemokine 12 (CXCL12) in bone and chemokine receptors type four and seven (CXCR4, CXCR7) in migration of tumor cells. Other receptors such as annexin II, integrin αvβ3, and receptor-ligand pairs like Notch-Jagged have also been implicated in osseous metastasis.19 While the chylous and transudative nature of fluid in three of these cases suggests ascites was due to lymphangitic carcinomatosis or obstruction, exudative fluid with detectable malignant cells in most other cases suggests lymphangitic carcinomatosis or invasion of the mesothelial peritoneal lining.8–9,16 It has also been hypothesized that robotic-assisted resections have led to port-site metastasis, though not always with concurrent malignant ascites.20,21
In addition to the rarity of our patient’s metastatic spread, we also describe the first documented case of TLS in a patient with prostate cancer with peritoneal carcinomatosis and malignant ascites. TLS is extremely rare in solid tumors, particularly prostate cancer, with only 11 cases reported as of January 2020.22,23 A total of 10 of these cases notably had widespread metastatic disease, typically with extensive bone and liver involvement, though our patient had only a single known vertebral metastasis with few other small, scattered foci of osseous involvement without liver involvement.
Baeksgaard and Sorenson note that the mortality rate of TLS in solid tumor malignancies is significantly higher than in hematological malignancies, further highlighting the importance of early clinical recognition or potential prophylaxis for TLS in patients with metastatic prostate cancer.24
Strengths of our paper include construction of the first comprehensive table of cases of prostate cancer and malignant ascites as well as the characteristics of such cases gained from literature review in all languages (Tables 1 and 2). Furthermore, we describe one of the few known cases of TLS in a patient with prostate cancer. Our conclusions are limited by the inability to make causal inferences regarding the pathogenesis of ascites in all cases, given the retrospective nature of our review.
Our case and review of the literature suggest that (1) the occurrence of ascites in patients with prostate cancer is typically associated with a worse prognosis than non-ascitic variants. (2) More common etiologies of ascites, including other primary malignancy must be evaluated for. (3) Ascitic fluid PSA measurement may aid in the diagnosis of malignant ascites in cases with negative fluid cytology and diagnostic uncertainty. (4) Carefully selected patients with advanced disease may achieve palliative benefit from combined hormonal and cytotoxic therapies. (5) Awareness of the occurrence of TLS in patients treated for prostate cancer or with ascites may help raise clinical suspicion and identify patients in whom prophylaxis would be beneficial.
Written informed consent for publication of their clinical details and/or clinical images was obtained from the daughter of the patient.
We would like to acknowledge and thank Dr. Tridu Huynh for his aid in translating portions of three French papers.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the background of the case’s history and progression described in sufficient detail?
Yes
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Yes
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Yes
Is the case presented with sufficient detail to be useful for other practitioners?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Urological oncology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 18 Aug 22 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)