Keywords
Bickerstaff brainstem encephalitis, COVID-19, Guillain Barre syndrome, immune mediated neuropathy, Pfizer BioNTech vaccine.
This article is included in the Emerging Diseases and Outbreaks gateway.
This article is included in the Coronavirus (COVID-19) collection.
Bickerstaff brainstem encephalitis, COVID-19, Guillain Barre syndrome, immune mediated neuropathy, Pfizer BioNTech vaccine.
Although vaccinations play a cornerstone role in the prevention of infections, numerous side effects and complications reported have a temporal relationship with inoculation. Fortunately, neurological complications post vaccination are rare, and tend to be monophasic with good recovery. The first neurological complications due to vaccines that were reported were neuroparalytic syndrome following Pasteur’s rabies vaccination in 1889 and encephalitis after smallpox vaccination in 1905. Afterwards, many reports were published on complications such as encephalomyelitis after rabies, measles, and smallpox vaccinations, deafness and neuritis after mumps vaccines, multiple sclerosis with recombinant hepatitis B vaccination and Guillain–Barre syndrome (GBS) after influenza, tetanus, and shingles vaccination, amongst others.1,2 Moreover, brainstem encephalitis was also reported post influenza vaccine.17 More recently, neurological complication post COVID-19 vaccines are increasingly being reported. Although they are usually transient and mild, some of the more debilitating complications include cerebral venous sinus thrombosis, Bell’s palsy, and immune mediated neuropathies. In this paper, we report the onset of GBS and Bickerstaff brainstem encephalitis (BBE) temporally related to coronavirus disease (COVID-19) vaccines.3
The World Health Organization Global Advisory Committee on Vaccine Safety (WHO GACVS) concluded that there have been reported cases of GBS after adenovirus vector COVID-19 vaccines Vaxveria and Janssen, but that increased reports of cases after mRNA COVID-19 vaccines have not been observed. Therefore, it was suggested that more studies should be conducted on the topic, and that it is vital for healthcare professionals to monitor and report cases of GBS after COVID-19 vaccines. Some cases were reported regarding the development of BBE after infection with COVID-19. However, to the best of our knowledge, no cases were reported in relation to COVID-19 vaccination.18
GBS is a collection of clinical syndromes manifesting as an acute immune-mediated inflammatory polyradiculoneuropathy that typically presents as increasing weakness and diminished reflexes. GBS patients usually develop symptoms 1-3 weeks post gastrointestinal and respiratory infections, or post inoculation with the aforementioned vaccines.4 BBE is defined as a rare immune disorder characterized by ataxia, ophthalmoplegia, and a decreased level of consciousness.5
These two cases of GBS and BBE reported at King Fahd Hospital of the University (KFHU) were temporally related to the Pfizer BioNTech vaccine. This report will add to the growing literature regarding possible autoimmune neurological complication post COVID-19 vaccine.
A health 38-year-old right-handed man, attended the emergency department complaining of muscle weakness and imbalance for one week. The weakness was followed by gradually worsening generalized fatigue and weakness in all limbs associated with lower back pain. The weakness ascended from his lower limbs to his upper limbs over the course of a few days, leading to interference with activities involved in daily living. The patient also reported constipation, choking, slowing of movement, and hypophonia. The patient had no history of recent respiratory or gastrointestinal infections, and had not recently received other vaccines apart from the first dose of Pfizer BioNtech vaccine 10 days before the onset of symptoms. The patient had no sphincter dysfunction, sensory disturbances, behavioral change, loss of consciousness, visual manifestations, sleep disturbances, memory impairment, seizure, abnormal movements, or trauma.
During physical examination, his Glascow Coma Scale (GCS) score was 9. On motor examination, his upper limbs were bilaterally hypotonic. Based on the Medical Research Council (MRC) scale, his upper limb power was +4/5 bilaterally, and 4/5 for his lower limbs. The patient’s reflexes were +2 all over, and they were negative for Babinski signs. The rest of the neurological exam was unremarkable.
Routine laboratory investigations were unremarkable. The autoimmune profile including anti-thyroid peroxidase (560.62 IU/mL), anti-thyroglobulin (65.55 IU/mL), and antinuclear antibodies (160) were all elevated. The patient also tested positive for GD1a antibody. Lumbar puncture revealed a normal glucose level (67 mg/dl), a high protein level (180.2 mg/dl) with high albumin (89.55 mg/dl), and no pleocytosis (Table 1).
The patient also underwent neuroimaging studies. Plain head computed tomography (CT) and magnetic resonance imaging (MRI) of the brain with contrast were normal. MRI of the spine with contrast showed faint T2 hyperintensity within the distal spinal cord/conus medullaris with diffuse enhancing nerve roots of the conus medullaris and cauda equina, which was suggestive of GBS (see Figure 1).
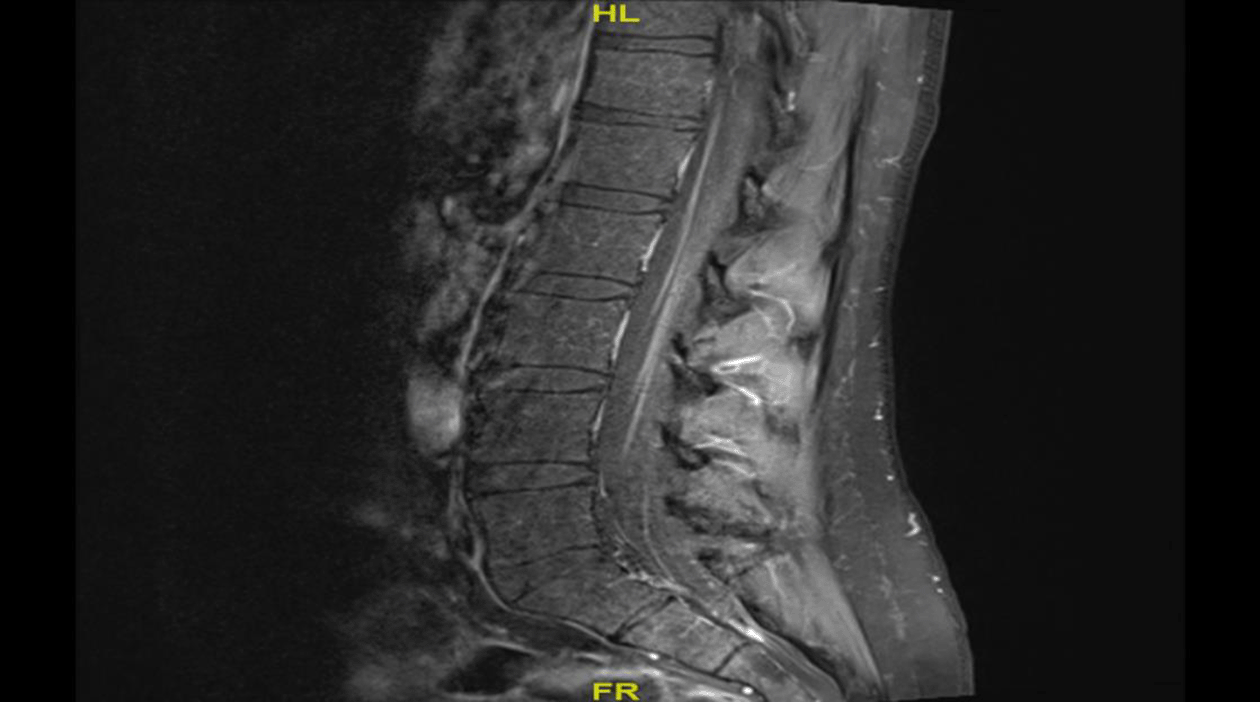
Diffuse enhanced nerve roots of the conus medullaris and cauda equina suggestive of GBS.
Nerve conduction study (NCS) of the median, ulnar, tibial, and peroneal nerves showed significantly reduced compound muscle action potential (CMAP) amplitude, with normal distal latencies, and conduction velocities. F wave responses were significantly delayed in median and tibial nerves, and non-recordable in peroneal nerves. Sensory responses of the median, ulnar, and sural nerves showed normal onset latency, normal sensory nerve action potential (SNAP) amplitude and normal conduction velocities. These findings in the electrophysiologic study were consistent with pure motor axonal polyneuropathy.
Based on clinical findings and above results, the patient was diagnosed as a case of acute motor axonal neuropathy (AMAN). He was treated with a five-day course of intravenous immunoglobulin (IVIG) but showed no significant improvement in his weakness. Three weeks later, his condition deteriorated in the form of bulbar dysfunction, respiratory compromise, and worsening of his extremity weakness, which became 3/5 in his upper limbs, and 2/5 in his lower limbs. Therefore, he was transferred to the intensive care unit (ICU) where he was intubated and managed with seven plasmapheresis sessions and corticosteroids pulse therapy for five days. Due to prolonged intubation, he was tracheostomized during his ICU stay. After a one month stay in the ICU, his condition stabilized, his upper and lower limb power improved to 4/5 in the upper limbs, and 3/5 in the lower limbs, and he was transferred back to the ward with a tracheostomy which did not require oxygen supplementation and a nasogastric tube. During his stay in the general ward, he underwent percutaneous gastrostomy tube placement. His plan is to continue management and rehabilitation in a long-term care facility.
A 54-year-old Saudi gentleman and a known case of uncontrolled hypertension and diabetes mellitus type 2 attended the emergency department complaining of dysphagia, an altered mental status and progressive body weakness. He was functionally independent until two weeks after he received the second dose of COVID-19 vaccine Pfizer BioNTech, at which point he started to experience generalized fatigue and subjective fever associated with headache and blurry vision. Symptomatic treatment was given as primary health care and accordingly he was discharged. Two days later, he started to develop difficulty in swallowing associated with a cough. A diagnosis of aspiration pneumonia was made, and he received treatment accordingly. He progressively worsened and was admitted to the ICU for further care. During his admission, he was noted to have developed limb ataxia along with a disturbed conscious level.
Upon further assessment, his Glasgow coma score (GCS) was 8/15 (E 4, M 3, V 1). His pupils were 2 mm in diameter with sluggish reactions. Additionally, he had extraocular muscle limitation in all directions which could not be overcome with oculocephalic reflex. He also exhibited facial diplegia which was more prominent on the right side. Regarding his motor examination, assessment of muscle strength showed a power of 1/5 in his lower limbs, and 2/5 in his upper limbs, with overall exaggerated deep tendon reflexes +3, and upgoing plantar reflexes.
During the patient’s hospital course many investigations were performed as shown in Table 2. Cerebrospinal fluid (CSF) analysis revealed lymphocyte-predominant pleocytosis, and positive oligoclonal bands in the CSF along with positive GQ1b antibodies, while the rest of the investigations did not reveal anything further.
Further imaging techniques, including plain CT brain and MRI studies, were undertaken as shown (Figures 2 and 3). Ancillary testing like electroencephalogram (EEG) and NCS/electromyogram (EMG) were done. The EEG showed no posterior background rhythm, with continuous generalized slowing of 4-6 Hz. No epileptiform discharges were detected. This result was consistent with moderate diffuse encephalopathy with no epileptiform discharges. Motor responses in the NCS showed significantly reduced CMAP amplitude, with normal distal latencies and conduction velocities in median, tibial and peroneal nerves. F wave responses were non-recordable in the median and peroneal nerves, and borderline in the tibial nerve. Sensory responses showed a mildly reduced SNAP amplitude, with normal conduction velocity in the median, ulnar, and sural nerves. These findings were consistent with mixed motor-sensory axonal polyneuropathy.
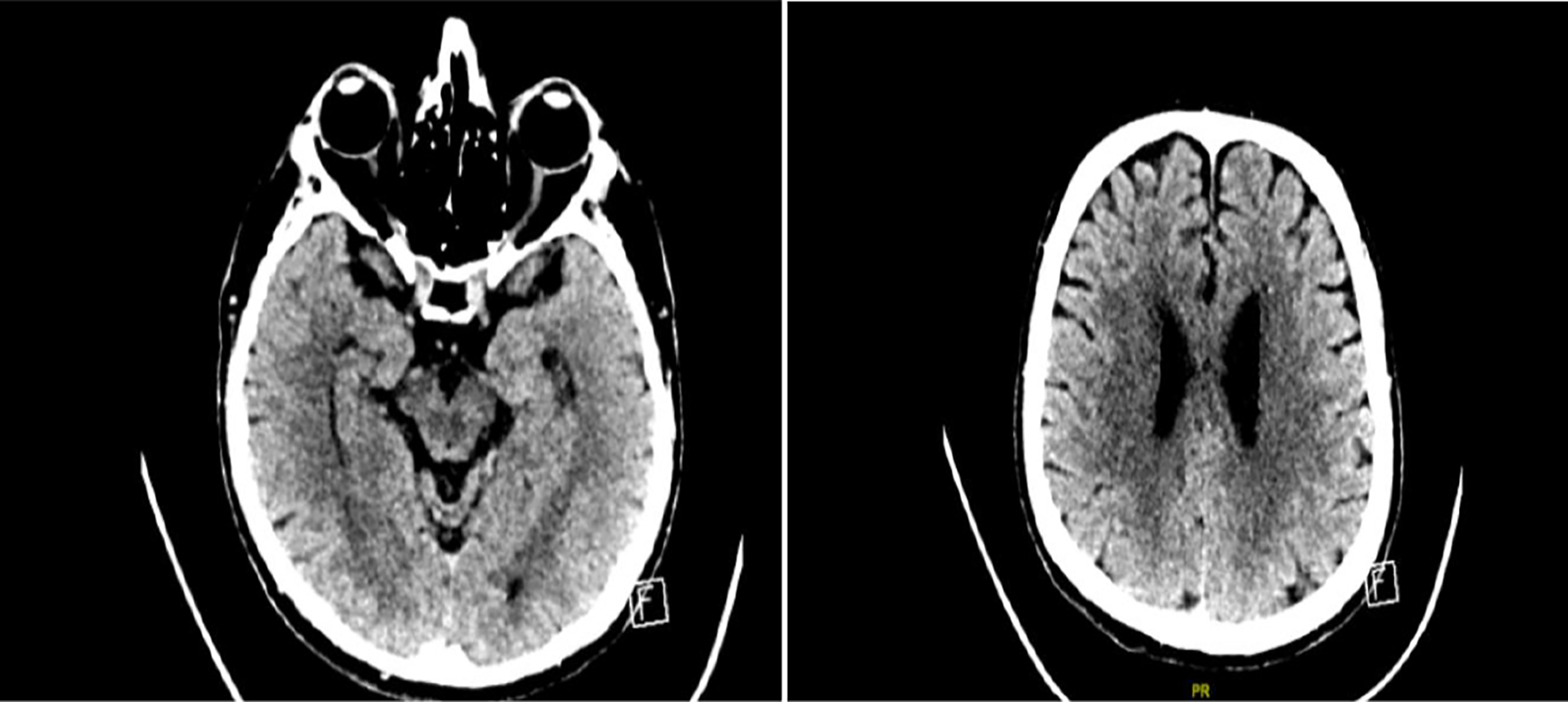
Scattered foci of hypodensity seen at the level of deep white matter region of bilateral corona radiata and posterior part of the external capsule bilaterally, more prominent in the right side with no evidence of mass effect seen.
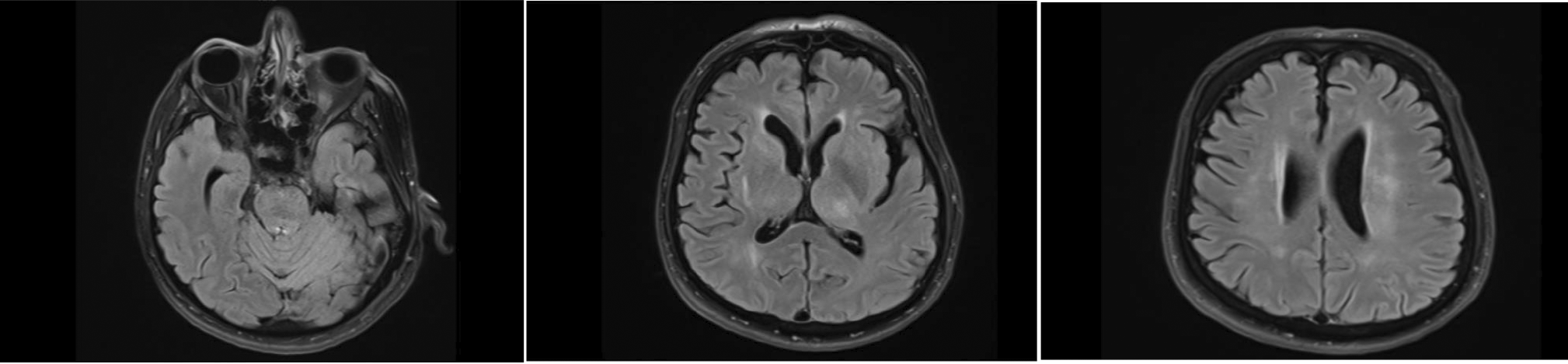
High signal intensity involving the left thalamic region. Interval newly abnormal signal intensity seen in the dorsal aspect of the pons and anterior aspect of the medulla oblongata. Multiple discrete confluent high signal intensity seen within the periventricular white matter region cortical and deep white matter region of bilateral frontotemporal area bilateral thalamus right putamen bilateral external capsule right anterior horn of internal capsule.
Based on above mentioned investigations, the patient was diagnosed with BBE, and he was started on the standard IVIG therapy course for five days, along with full courses of antiviral therapy. However, there was no noticeable improvement despite therapy. Two weeks after the IVIG therapy, he underwent seven plasmapheresis sessions. Consequently, the patient’s condition was stabilized. During his ICU stay, he was tracheostomized as he required prolonged intubation. He was then transferred to the ward with a tracheostomy which required oxygen support and nasogastric tube. Therefore, long-term immune biological therapy with Rituximab was initiated and the patient received three doses. The patient has shown minimal improvement in his consciousness level, being able to move his head and protrude his tongue on command. His ophthalmoplegia also improved to the point where he became able to move his eyes horizontally. He underwent placement of a percutaneous gastrostomy tube, and he is planned to receive extensive rehabilitation in a long-term care facility, to continue his supportive care.
Many reports were published regarding the neurological sequelae associated with different types of COVID-19 vaccinations. Fortunately, most of the neurological side effects reported were mild and transient, such as headache, arthralgia, myalgia, and local injection site pain. On the other hand, cerebral venous sinus thrombosis, Bell’s palsy, acute transverse myelitis, acute demyelinating polyneuropathy and acute disseminated encephalomyelitis were some of the more devastating complications reported.3 In this report, we discussed two cases that experienced GBS and its variants, and BBE temporally related to a COVID-19 vaccine.
GBS is characterized by ascending symmetrical motor weakness and sensory symptoms, such as paresthesia or numbness, associated with hyporeflexia or areflexia that rapidly progress and reach maximum severity in four weeks. Patients may present with ataxia, back pain, and cranial nerve involvement. GBS is a heterogeneous illness with various subtypes, the most common amongst them is acute inflammatory demyelinating polyneuropathy (AIDP). Other subtypes are AMAN, acute motor sensory axonal neuropathy (AMSAN), and Miller Fisher syndrome (MFS), which is characterized by ophthalmoplegia, ataxia and areflexia. Nerve conduction studies and cerebrospinal fluid analysis support the clinical diagnosis of GBS. The pathogenesis of GBS involves antiganglioside antibodies, molecular mimicry, and complement activation. Effective treatments for GBS include intravenous immunoglobulin and plasma exchange. GBS is associated with onset of illness 2-4 weeks post respiratory and gastrointestinal infection, or after vaccination. Thus far, it has been linked with shingles vaccines, tetanus containing vaccines, and influenza vaccines.4,6
In our first patient, the ascending motor weakness with lack of sensory symptoms and neurophysiological evidence point to a diagnosis of AMAN. Our patient also experienced back pain and ataxia. Although no history of infection was present, the patient experienced the onset of symptoms 10 days post vaccination. The presence of GM1 and GQ1D antibodies, the MRI findings, the NCS and lumbar puncture results all confirmed the diagnosis. The patient showed improvement with the standard treatment of intravenous immunoglobulin and plasma exchange.
BBE was initially reported in the 1950s by Cloake and Bickerstaff.7 It is a rare neurological disorder, and because of its rarity, there is controversy regarding considering BBE as a variant of GBS or MFS.5,7,8 However, Koga et al. have proposed that BBE is a different disease entity with a distinguishable etiology of antiganglioside antibodies, and that it is not merely defined by symptomatic entities.9 The mandatory diagnostic criteria of BBE include ophthalmoplegia, an altered level of consciousness or hyperreflexia, and ataxia.5 Also, there are other supportive features that help in outlining this disorder, such as antecedent infection, positive anti GQ1b antibodies, and presence of albumin-cytological dissociation in CSF.7
It is important to highlight that anti GQ1b antibodies are not necessarily positive in all the published cases in literature.5,9,10 Nevertheless, the detection of the autoantibodies against GQ1b ganglioside have prompted a marked increase in the diagnosis of BBE.9 Therefore, Koga et al. have defined BBE with typical and atypical cases. The typical BBE has similar presentation to MFS, and it is associated with spontaneous recovery and a good prognosis while the atypical BBE, which is characterized by abnormal CSF and MRI findings along with negative anti GQ1b antibodies, is associated with delays in recovery.9
The second patient showed symptoms of fever and progressive fatigue and weakness weeks before his admission to our center. This supports the fact that BBE is associated with antecedent infection. Also, they were positive for anti GQ1b antibodies, indicating a diagnosis of typical BBE. Additionally, it has been postulated that BBE is underdiagnosed because of the overlap with MFS and GBS, and the incomplete definition of BBE.11,7 Because of this overlap, initial diagnosis of the patient has required extensive investigations and repetition of lumbar punctures several times. The hallmark of the disorder in our patient was the rapid and sudden deterioration in the level of consciousness along with hyperreflexia, ocular muscle movement limitations which prompted review of initial diagnosis of viral encephalitis vs GBS. Moreover, no definite treatment for BBE has yet been found and it is primarily treated with IVIG, steroids or plasma exchange, though no clinical trials have been performed to determine the effectiveness of this approach.9,11,12 Additionally, Hardy et al. reported a case of a patient with BBE who didn’t show improvement on either IVIG or plasma exchange therapy. Therefore, Rituximab treatment was initiated, and subtle improvements associated with GQ1b antibody reduction were noted.16 Thus, we have pursued a similar approach for our patient. We started him first on IVIG, then multiple sessions of plasma exchanges. Because the improvement noticed after plasma exchange was incomplete, we opted to initiate the patient’s treatment on Rituximab to maintain the achieved improvement and possibly achieve further improvements.
At the time of this report, 24,359,489 people have received two doses of COVID-19 vaccines in Saudi Arabia, and the total cases are 748,489. The four vaccines currently approved in Saudi Arabia are: Pfizer- BioNTech, Moderna, Janssen, and Vaxzervria.13 The WHO GACVS has issued a statement on reports of GBS following adenovirus vector COVID-19 vaccines, specifically Janssen and AstraZeneca. It was advised that healthcare professionals should be alert to any symptoms of GBS developing after adenovirus vector vaccination, and that the individuals taking the vaccines should also be aware of the symptoms and seek immediate medical attention if symptoms develop. No statements regarding mRNA vaccines such as the Pfizer-BioNtech vaccine have been issued by the GACVS, but it was emphasized that more rigorous studies should be conducted, and that healthcare professionals should report all adverse events including GBS. Unfortunately, no statements regarding BBE were issued by the GACVS as no prior cases highlighting a possible temporal relationship were reported.
Globally, there are numerous published reports of GBS post Vaxzevria and Janssen vaccines.14 GBS cases after the Pfizer-BioNTech vaccine, although less common, have also been reported.15 It is worth mentioning that there are no reports regarding the relationship between COVID-19 vaccines and BBE. Although it is hard to make a causal relationship between these two events, further investigations and studies should take place to alert healthcare practitioners about these potential side effects. In this study, we reported the first case of GBS after COVID-19 vaccines in Saudi Arabia and highlighted the first BBE case temporally related to a COVID-19 vaccine in all published literature. Since a temporal relationship does not signify causation, we cannot draw any conclusions regarding the significance of the association between COVID-19 vaccines and these neurological disorders. However, it is vital that new cases are reported so that the knowledge base is built and to increase healthcare workers’ vigilance for early signs of GBS or BBE.
All data underlying the results are available as part of the article and no additional source data are required.
Written informed consent for publication of their clinical details and clinical images was obtained from the relatives of the patients.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the background of the cases’ history and progression described in sufficient detail?
Partly
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Partly
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Partly
Is the conclusion balanced and justified on the basis of the findings?
Yes
References
1. Maramattom BV, Krishnan P, Paul R, Padmanabhan S, et al.: Guillain-Barré Syndrome following ChAdOx1-S/nCoV-19 Vaccine.Ann Neurol. 2021; 90 (2): 312-314 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Clinical neurology, neuroimmunology, epilepsy
Is the background of the cases’ history and progression described in sufficient detail?
Partly
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Partly
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
No
Is the conclusion balanced and justified on the basis of the findings?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: neuromuscular diseases
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 25 Aug 22 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)