Keywords
Soya-saponins, adjuvants, immune response, Tumour Necrosis Factor, Interleukin-6
This article is included in the Plant Science gateway.
Soya-saponins, adjuvants, immune response, Tumour Necrosis Factor, Interleukin-6
Vaccination is one of the most successful and cost-effective public health interventions globally, preventing 3.5–5 million deaths annually. It was estimated that vaccination prevented 14.4 million deaths from coronavirus 2019 (COVID-19) globally between Dec 8, 2020, and Dec 8, 20211 and it could prevent 709,411 deaths from pneumococcal disease in China alone over the initial 10-year period after vaccination.2 Vaccination against hepatitis B virus (HBV) infection, which affects more than 296 million people and causes more than 523,000 deaths globally per year due to disease-related cirrhosis and liver cancer,3 can prevent more than 80% of HBV-related deaths.4
The currently licensed hepatitis B vaccines have limitations including hypo-responsiveness in older adults and the extended time for most people to develop sero-protection.5 The aluminium-hydroxide-adjuvanted hepatitis B vaccines require three doses over 6 months to achieve high rates of protection in adults.6 Despite the alum adjuvant inducing a good antibody response (Th2), it has little capacity to stimulate the cellular (Th1) immune response.7 Moreover, although licenced and used in many human vaccines, the alum adjuvant can induce swelling and allergic reactions in the injection sites.8
Several vaccines require adjuvants to realize their optimal immune response. Vaccine adjuvants stimulate and enhance the immune response’s magnitude, durability, and breadth toward their co-administered antigens.9 They are divided into delivery systems and immune-stimulatory adjuvants, based on their principal mechanisms of action.10 Adjuvants with specific properties are necessitated by the desired qualities of the vaccine and few adjuvants are effective for most vaccine applications.11,12
Among the classes of vaccine adjuvants that are gaining renewed interest are saponin-based adjuvants (SBAs) that stimulate both cell-mediated immunity (CMI) and humoral immunity.13 They have been shown to induce strong Th1 and Th2 and moderate cytotoxic T lymphocytes (CTL) responses to particular proteins, mainly due to the formation of protein-saponin micelles.14 Saponins from Quillaja saponaria have proved to be effective adjuvants in human cytomegalovirus and influenza vaccines as well as polysaccharide vaccines.15 A combination of 3-O-desacyl-4′-monophosphoryl lipid A (MPL) and QS21 (a Matrix M saponin-derived immunologic adjuvant) has been found to increase the immune response to the HBV vaccine.16 Soybean (Glycine max (L.) Merr.) is known to have more than nine saponins: Aa, Ab, Af, Ba, Bb, Bb′, and three soya-saponin Ab-derivatives (AbDs), which have been found to elicit substantial immune responses to ovalbumin in mice with minimal toxicity.17 Despite the promising results of this study by Qiao et al.,17 the use of soya-saponin has not been explored extensively with other vaccines. Yet, if more uses of SBAs could be found, it could be economically feasible to produce them since soya beans are food crops that are easy to cultivate.18 Hence the design of this study.
Approval to carry out this study and ethical clearance was obtained from KEMRI Scientific Ethics Review Unit (SERU), protocol No. KEMRI/CBRD/SERU/005/500/4641, on the 2nd of February, 2023. All animal work was carried out per the relevant national and international standards as approved by KEMRI-Animal Care and Use Committee and as specified in the above protocol number. The commercial HBV vaccine, Revac B™ (Bharat Biotech International, India) was used in this study solely for research and not for modification or commercialization.
This experimental laboratory-based research study was conducted at the Innovation & Technology Transfer Division (ITTD) of Kenya Medical Research Institute. The sample size (N=51) was determined using the ‘Resource Equation (E)’.19 Treatments (in triplicate) for the various experimental groups are shown in Table 1.
Female BALB/c mice (20 ± 2 g, 7–8 weeks old) were used in this study. They were procured from the Institute of Primate Research (IPR) Kenya and acclimatized for 7 days at the temperature of 21 ± 3 °C, a humidity of 40–70%, 12-hour light/dark cycle, and ad-lib access to appropriate mice chow and water. All efforts were made to ensure minimal harm and suffering to the mice by treating them according to the obligations enlisted in the 3Rs; replacement, reduction, and refinement.20
The organic extraction of saponins was done by soaking three portions of 500 g of soybean meal in one litre of 80% methanol (Sigma-Aldrich, Switzerland) for one hour and then filtered with Whatman filter paper (Merck, Darmstadt, Germany). The final volume of the combined filtrate was transferred into a round-bottomed flask and concentrated using a rotary evaporator (Goel Scientific Glass Works, India) at 65 °C. The solvent was partitioned sequentially using diethyl ether, distilled water, n-butanol, and acetone as described by Kim & Park.21 Briefly, 150 mL of diethyl ether (Merck, Darmstadt, Germany) and 50 mL of distilled water were mixed with the extract, and the resulting slurry was transferred into a separatory funnel (Duran®, New Jersey, USA). Diethyl ether was discarded after the water layer was washed. This process was repeated twice. The water layer was combined with 100 μL of n-butanol (Merck, Darmstadt, Germany) in the separatory funnel (Duran®, New Jersey, USA), and the mixture was vigorously shaken. After setting still, the resulting n-butanol was collected. The process was repeated once more and the combined n-butanol fraction was concentrated at 82 °C. Acetone (Merck, Darmstadt, Germany) was added to the n-butanol fraction and the resulting slurry was shaken vigorously and centrifuged (Beckman Coulter, USA) at 1,250 rpm for 15 minutes. The resulting supernatant was concentrated in vacuo at 56 °C.
A standard foam test was carried out on the extract of Glycine max (L.) Merr. as described by Harbone.22 Using 300 mL of hot distilled water in a beaker, three grams of the extract were weighed and extracted. After filtration, 5 mL of the filtrate was placed in a test tube and diluted with 5 mL of distilled water. This mixture was shaken vigorously for two minutes and observed for the appearance of persistent foam. Three drops of olive oil were added to the mixture and shaken to examine the formation of an emulsion.
A Fourier transform infrared (FTIR) analysis was also done on the extract as described by Wangia et al.23 by mixing 2 mg of the extract with 300 mg of spectroscopic grade KBr in a crucible and compressing it into a disk. The disk was subjected to FTIR spectroscopy, and the infrared spectra were recorded using an FTIR spectrophotometer (Shimadzu, Japan) in the range of 4500 cm-1 to 500 cm-1.
On day 0, the mice were intramuscularly injected with either 50 μL of vaccine alone, or 50 μL of vaccine + 50 μL of saponin extract, 50 μL HBsAg + 50 μL of saponin extract or 50 μL of 1X phosphate buffered saline (PBS) (Bioneer, Korea). On day 14, blood samples were collected through the tail vein. The blood was centrifuged at 5000 rpm for 10 minutes to obtain clear sera, which was stored at -20 °C, awaiting the ELISA test. On day 15, the mice were euthanized by the method of cervical dislocation, by a trained researcher, ensuring a painless animal sacrifice. Spleens were harvested and placed in sterile 1.5 mL centrifuge tubes and stored at -80 °C before they were used for total RNA extraction. Through a cardiac puncture, 500 μL of whole blood for haematological analysis was collected using a 1 mL syringe (BD Luer-Lok™, Canada) and a 22 gauge needle and collected in 1.5 mL EDTA-containing blood collection tubes (BD Vacutainer®, Canada) and stored at 8 °C awaiting analysis.
The total murine HBsAg antibodies (HBsAb) were evaluated using an adapted ELISA method as described by Wolters et al.24 Briefly, 100 μL of HBsAg (a working standard donated by a KEMRI Scientist, Dr James Kimotho) was diluted 2-fold serially with 100 μL murine generated HBsAb (also donated by Dr James Kimotho). The experimental samples were tested with the HBsAg ELISA test kit (Creative Biolabs, NY, US) to develop a calibration graph. From the serum obtained, 50 μL were mixed with 50 μL of the working standard HBsAg and tested using the ELISA kit following the manufacturer’s instructions. The reciprocal optical densities (ODs) were then computed.
The total RNA from the murine splenocytes was extracted using the Direct-zol™ RNA Miniprep (Zymo Research, CA USA) according to the manufacturer’s instructions. Briefly, 1 μL of proteinase K was added to 100 μL of the sample and incubated at room temperature for 15 mins. Then, 300 μL of the TRI Reagent was added to the mixture and mixed thoroughly. To the lysed sample, 400 μL of molecular grade ethanol (Merck, Darmstadt, Germany) was added and mixed thoroughly. The mixture was then transferred into a Zymo-spin column in a collection tube and centrifuged at 16000 × g for 1 min. The column was transferred to a new collection tube. To the column, 400 μL of RNA wash buffer was added and centrifuged at 16000 × g for 1 min. A mixture of 5 μL DNases and 75 μL DNA digestion buffer was added to the column matrix and incubated at room temperature for 15 mins. This was followed by the addition of 400 μL of Direct-zol RNA pre-wash to the column and centrifuging at 16000 × g for 1 min. This step was carried out twice. RNA wash buffer, 700 μL, was added to the column and centrifuged at 16000 × g for 1 min. This step was also carried out twice to ensure the complete removal of the wash buffer. The column was transferred into an RNase-free tube and 50 μL of DNase/RNase-free water was added directly to the column matrix for elution. The tube was centrifuged at 16000 × g for 1 min. The RNA purity and concentration were assessed using the NanoDrop™ 2000/2000c spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) at absorbance 260/280. The samples were stored at -80 °C awaiting analysis.
cDNA for each of the samples was synthesized and subsequently amplified in a single tube using the Accuris™ One-Step RT-PCR Kit (Accuris, NJ, USA) according to the manufacturer’s instructions. Briefly, the following quantities of qPCR reaction components; Accuris 2X One-Step Mix 10 μL, forward primer (10 μM) 0.8 μL, reverse primer (10 μM) 0.8 μL, 20X RTase Blend 1 μL, and, PCR grade water 4.4 μL, were mixed with 3 μL of template RNA to a total reaction volume of 20 μL.
The reaction was performed using the Applied Biosystems Quant Studio 5 qPCR machine (PE Applied Biosystems, USA). The thermocycling conditions used in the experiment were as follows; reverse transcription at 55 °C for 10 mins, pre-denaturation at 95 °C for 2 mins, then 40 cycles of denaturation and annealing at 95 °C for 5 seconds and 60 °C for 30 seconds, respectively.
Relative quantification analysis to determine the expression of IL-6 and TNF-α mRNA levels was performed with QuantStudio™ 5 Real-Time PCR system (Applied Biosystems) using cDNA synthesized from the RNA from the spleen. All levels of expression quantities were expressed in fold-changes, comparing the animal groups using the 2-ΔΔCt method, formula ΔCt=Ct (gene of interest) - Ct (housekeeping gene).25 The TATA binding protein (TBP) gene was used as the housekeeping for the normalization of the genes of interest. The sequences of the primers used in the experiment are shown in Table 2.
Whole blood count analysis was performed using the HumaCount 30TS (Human Diagnostics Worldwide, Wiesbaden, Germany) haematology analyzer machine following the manufacturer’s instructions. Briefly, 100 μL of the anti-coagulated whole blood sample was aliquoted into sterile micro-centrifuge tube. Then, 25 μL of the blood was automatically aspirated by the machine for analysis. The results were recorded and expressed as mean of experiments using Microsoft Excel (2016).
The optical densities from the ELISA analysis were expressed as the mean of experiments using Microsoft excel (2016). The p-value was calculated using Pearson correlation coefficient (r) for immune response values. Relative quantification of gene expression was calculated using the delta-delta threshold cycle (ΔΔCt) formula. Cytokine expression levels were expressed in fold-changes, and a fold change <0.5 or >2 was considered as differentially expressed. Microsoft excel (2016) was used to create the bar charts to display the fold change in expression levels from the experimental groups. The data obtained for the haematological parameters were expressed as mean values using Microsoft excel (2016) and displayed in bar charts. A p-value of <0.05 was taken as significant for all statistical analyses.
The methanol extract of Glycine max (L.) Merr. demonstrated the presence of saponins by producing characteristic saponin foam and FTIR spectra with the hydroxyl group (-OH) at 3382 cm-1, C-H bond at 2928 cm-1, C=C absorbance at 1635 cm-1, and C-O-C bond at 1055 cm-1, which correlated well with the spectra for soya-saponin that were obtained by El-Keiy29 (Figure 1).30
Comparison of the humoral immune response of the BALB/c mice that were immunized with HBsAg showed a higher immune response (mean reciprocal OD = 0.788 ± 0.007) than those treated with Revac B™ vaccine (mean reciprocal OD = 0.747 ± 0.007). However, the difference was not statistically significant (P-Value < 0.3704). When the two immunogens (HBsAg and Revac B™ vaccine) were co-administered with Glycine max (L.) Merr. extract, the immune responses were slightly higher (0.799 ± 0.013 for HB and 0.758 ± 0.012) than with the two immunogens when used alone. However, the difference in immune response between these concentrations was not statistically significant (p-value < 0.467 and 0.416 respectively) (Figure 2).
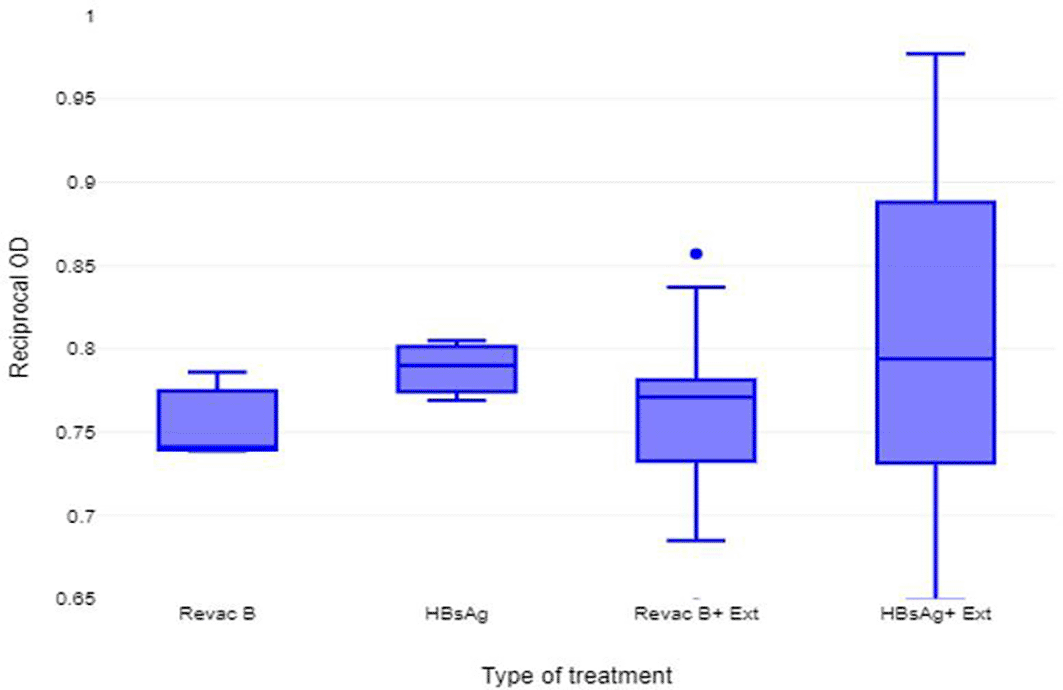
From this experiment the saponin extract of Glycine max (L.) Merr. did not demonstrate significant adjuvant effect in the humoral immune response in BALB/c mice immunized with HBV vaccine (Revac B™ vaccine) 14 days post-immunization.
The BALB/c mice immunized with the vaccine only expressed less IL-6 (fold change in transcription (FCT) = 0.603) than the untreated mice (FCT = 1.468). Addition of saponin extract of Glycine max (L.) Merr. to Revac-B™ vaccine increased the levels of IL-6 suppressed by the vaccine with the addition of a 50% concentration of extract increasing the FCT values from 0.603 in the vaccine-only immunization to a fold change value of 1.00 (an increase of 150% increase). Administration of 50% Revac-B™ vaccine and 50% extract almost restored the expression levels of IL-6 to the untreated levels (FCT 1.423 and 1.469 respectively) (Figure 3A).
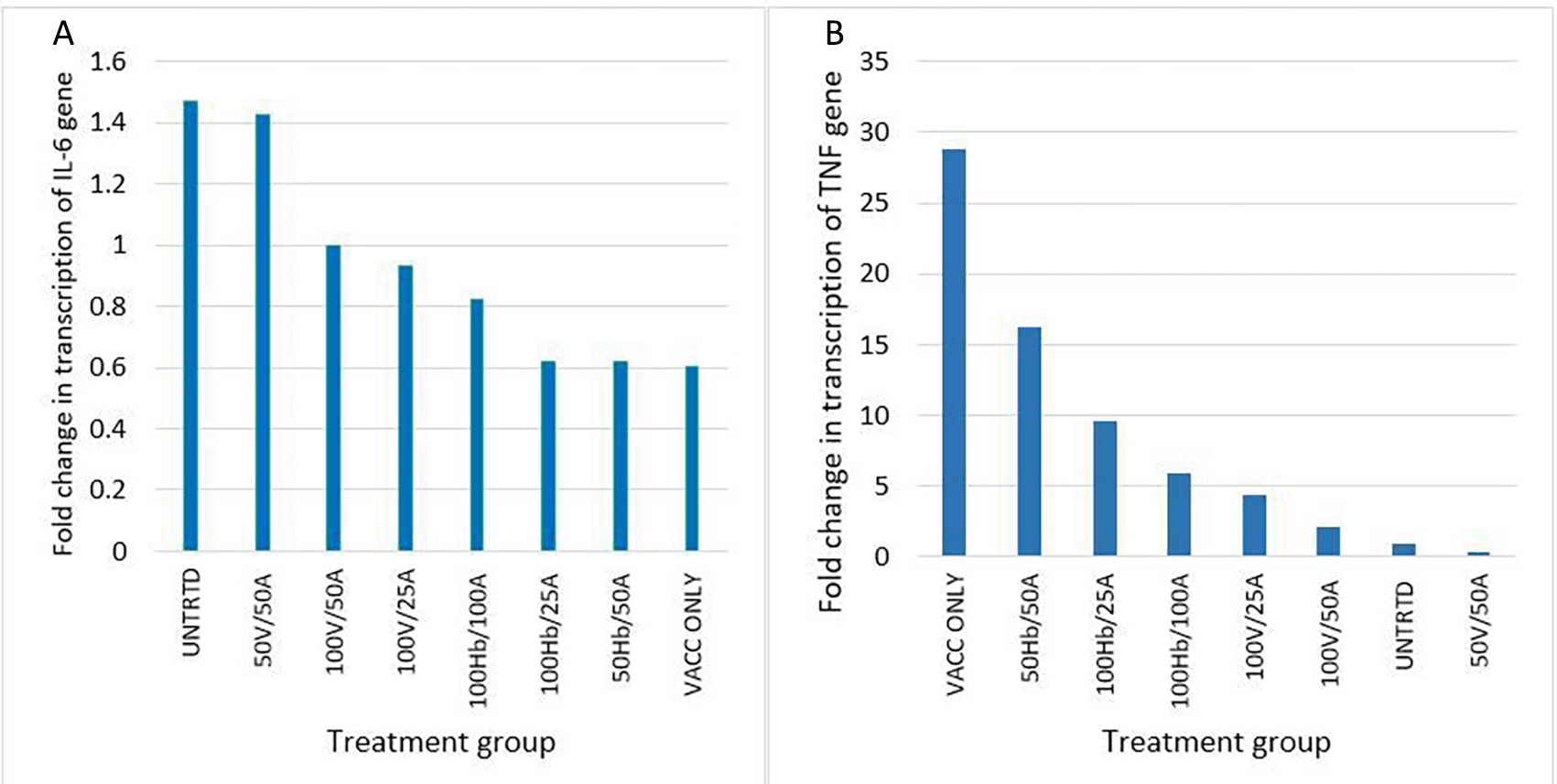
Key: UNTRTD = Untreated, Vacc - Revac-B™ vaccine, HB - HbsAg, and A - Glycine max (L.) Merr. extract. Figure A: IL-6 gene expression, Figure B: TNF-α gene expression.
BALB/c mice treated with vaccine alone, HBsAg/extract at 50%/50%, and 100%/25% proportions presented high expression levels of the TNF-α gene (FCT 28, 16.2 and 9.7 respectively) in comparison to all the other treatment groups (Figure 3B). The mice treated with the vaccine and extract at 50%/50% proportions had the least expression of the TNF-α gene (FCT=0.370). The group treated with the vaccine alone showed the highest expression levels (FCT 28) of TNF-α than any other group. In both animal groups that were treated with HBsAg and vaccine the addition of the extract significantly reduced the expression of TNF-α (p < 0.063).
The relationship between the expression of the IL-6 gene and the TNF-α gene was a moderately negative correlation (R = -0.7444, P = 0.0343, at p < 0.05).
Administration of Revac-B™ vaccine, HBsAg and Glycine max (L.) Merr. extract to BALB/c mice did not affect haematological parameters apart from white blood cell (WBC), neutrophil and platelet counts (Figure 4). The WBC counts increased by 33.3% in mice immunized with Revac-B™ vaccine alone and even increased (by 94.4%) in mice immunized with the Revac-B ™ vaccine that was mixed with 50% Glycine max (L.) Merr. extracts immediately before injection. There was no significant change in WBC count in mice immunized with HBsAg. However, immunization with HBsAg combined with 50% Glycine max (L.) Merr. extracts reduced WBC count by 73.6% though not statistically significant (p-value = 0.333). The neutrophils count increased by 33.3% in mice immunized with Revac-B™ vaccine alone and by 8.33% in mice immunized with the Revac-B™ vaccine that was mixed with 50% Glycine max (L.) Merr. extract immediately before injection. There was a 62.5% decrease in neutrophil count in mice immunized with HBsAg. Moreover, immunization with Revac-B™ vaccine combined with 50% of the Glycine max (L.) Merr. extract significantly reduced neutrophil count by 66.7% (p < 0.026).
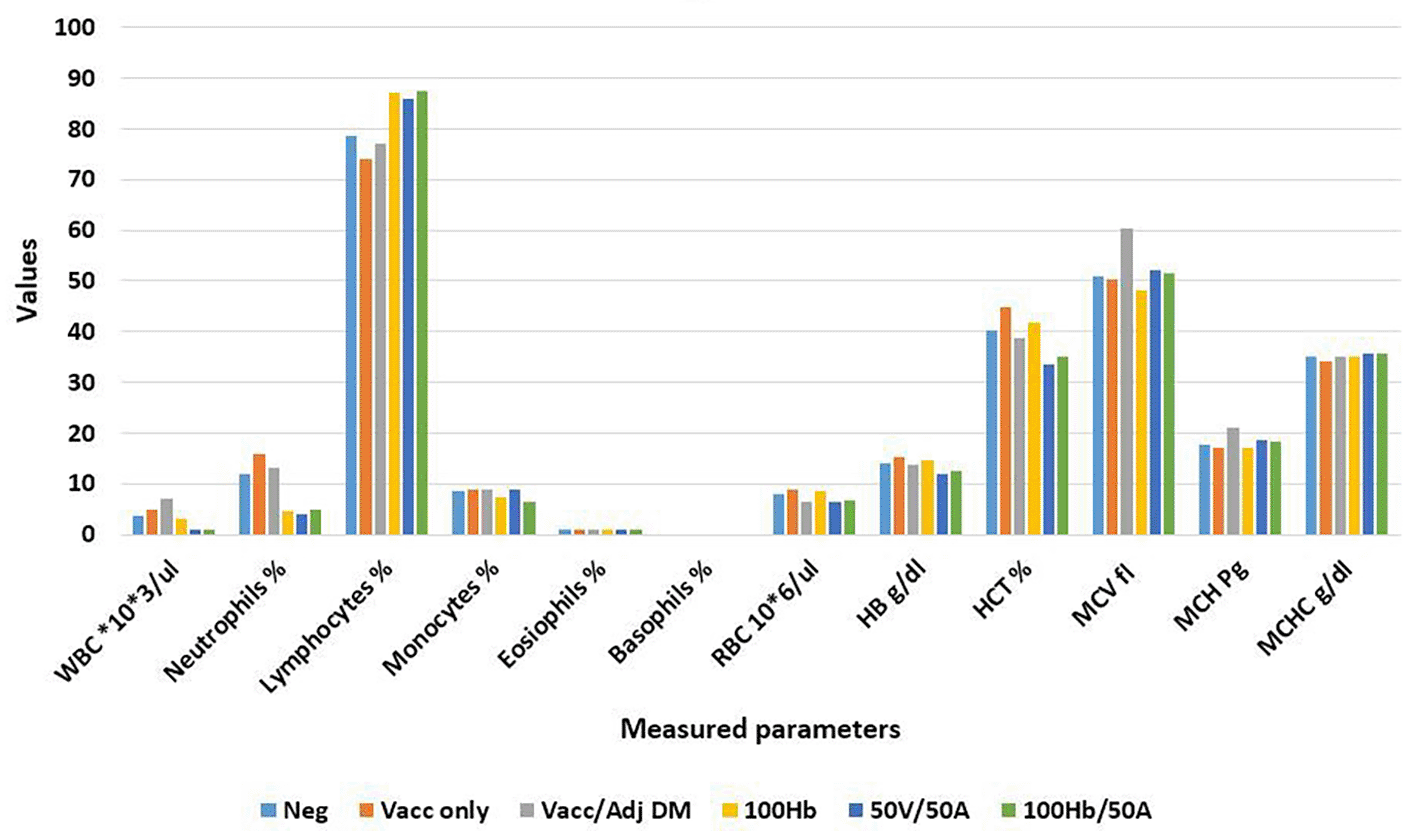
Key: WBC - White blood cells, RBC - Red Blood cells, HB - Hemoglobin, HCT - hematocrit, MCV - mean corpuscular volume, MCH - mean corpuscular hemoglobin, MCHC - mean corpuscular hemoglobin concentration.
The main contributor to the reduced WBC and neutrophils count was the immunization with 50% Revac-B™ vaccine/50% Glycine max (L.) Merr. extract and 100% HBsAg/50% Glycine max (L.) Merr. extract. The Pearson’s correlation coefficient between WBC and neutrophil counts was moderate but there was a significant correlation (R = 0.724, P-Value = 0.027 at p < 0.05).
The platelet count increased by 18.3% in mice immunized with the Revac-B™ vaccine alone and increased by 116.0% in mice immunized with the Revac-B™ vaccine that was mixed with 50% Glycine max (L.) Merr. extract immediately before injection and 155.3% in mice immunized with HBsAg only (Figure 5). The study demonstrated a weak relationship between the variation of neutrophils and platelet counts (R = 0.1213, P-Value = 0.756, at significance level of 0.05).
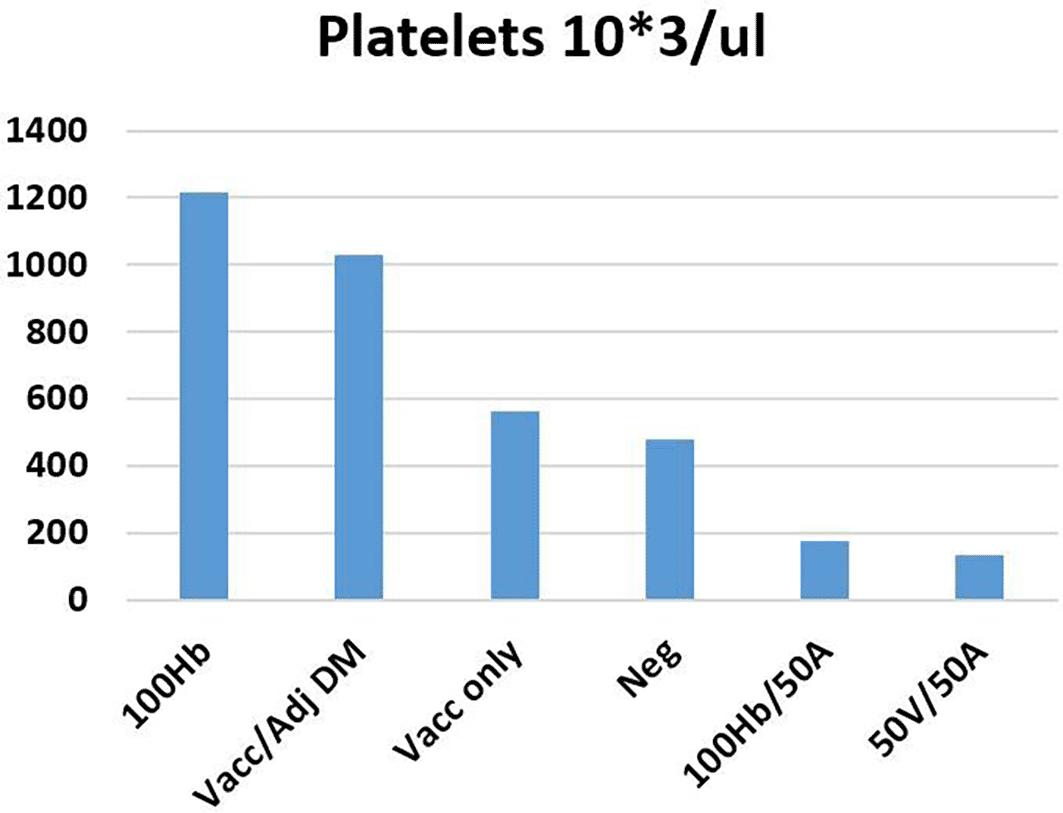
Key: WBC - White Blood Cells, Mean Neg - Mean of negative control (2), Vacc - Revac-B™ vaccine, Vacc/adj DM - Revac-B™ vaccine mixed with Glycine max (L.) Merr. extract 50%, HB- HbsAg, and A - Glycine max (L.) Merr. extract.
The study confirmed the presence of saponins in Glycine max (L.) Merr. extract by the characteristic saponin foam and FTIR spectra as earlier reported by El-Keiy et al.29 The extract of Glycine max (L.) Merr. demonstrated a slightly more enhanced immune response in BALB/c mice that were immunized with HBsAg and Revac B™ vaccine (reciprocal OD = 0.799 ± 0.013 and 0.758 ± 0.012) than with the two immunogens when used alone (reciprocal OD = 0.788 ± 0.007 and 0.747 ± 0.007). However, the difference in immune response between these concentrations was not statistically significant (p-value < 0.467 and 0.416 respectively). The extract was expected to enhance the humoral immune response more than what was observed. Saponin-based adjuvants (SBAs) have been known to stimulate both cell-mediated immunity and humoral immunity.13,14 Saponins from Quillaja saponaria are effective adjuvants in human cytomegalovirus, influenza vaccines, polysaccharide, and COVID-19 vaccines.15,31 Soybean extracts have been found to elicit substantial immune responses to ovalbumin in mice.17 One possible reason why the soybean extract in this study did not show substantial humoral immune response with the HBV vaccine is that the extract adjuvant is not innately effective with this vaccine as adjuvants are not universal and they often tend to be specific with a vaccine or a group thereof.11,12 A Matrix M saponin-derived immunologic adjuvant (QS21) could not raise adequate immune response in HBV vaccine alone, but its adjuvant activity was markedly boosted upon the addition of 3-O-desacyl-4′-monophosphoryl lipid A (MPL).16
The decreased expression of IL-6 (FCT = 0.603 against FCT = 1.468 of unimmunized) noted in this study was contrary to several studies32,33 that have found an increase of IL-6 expression levels upon immunization. Increased IL-6 levels were associated with systemic reactogenicity following vaccination with HBsAg AS01B/Alum vaccine in humans.34 The study noted a significant increase in expression of TNF-α gene (p < 0.063).35 According to Murphy et al.,35 the cytokine profile induced by trained immunity differs depending on the microbial stimulus. For example, Bacillus Calmette-Guerin (BCG) vaccination increased TNF-α production while Mycobacteria tuberculosis stimulation decreased TNF-α production.36,37 Other studies have not observed changes with the expression of IL-6 and TNF-α.38 Increased expression of TNF-α could be exploited to develop a mucosal adjuvant consisting of a HBV vaccine leveraging on the observation by Kayamuro et al.39 that a mutant TNF-α has substantial mucosal adjuvant activity.
The study observed an increased neutrophil count (by 33.3%) in mice immunized with Revac-B™ vaccine alone, while there was a 62.5% decrease in neutrophil count in mice immunized with HBsAg. However, immunization with Revac-B™ vaccine combined with 50% Glycine max (L.) Merr. extract significantly reduced the neutrophil count by 66.7% (p < 0.026). This finding is contrary to the one of Lin et al.40 who showed the increase in neutrophils and decrease in lymphocytes in female rats after treatment with quinoa saponins. It was also noted that the platelet count increased in mice immunized with Revac-B™ vaccine and HBsAg and reduced by more than three times when the extract was added. A study by Qiao et al17 noted that the soya-saponin was generally safe in mice and the only adverse effect noted was a slight haemolytic effect to 0.5% of the total red blood cell. Other studies have established that saponins, such as saponins from quinoa,40 Amaranthus cruentus seeds,41 and Momordica dioica,42 have limited acute toxicity effects.
The study established that Glycine Max (L.) Merr. contains significant levels of saponins, although the saponin extract did not demonstrate remarkable adjuvant activities in BALB/c mice immunized with HBV vaccine. However, it was established that when combined, the HBV vaccine and soybean extract reduced platelet counts, it suppressed the expression of IL-6 gene while it promoted the expression of TNF-α gene. These findings could be researched further to develop new therapeutics such as mucosal vaccine adjuvants. Some limitations of this study included a small sample size, and limited funds, leading to analysis of only representative groups.
Mendeley Data: Preliminary assessment of adjuvant activities of Glycine Max (L.) Merr. saponin extract in BALB/c mice immunized with hepatitis B virus vaccine, https://doi.org/10.17632/d3rzx39sy4.1. 30
This project contains the following underlying data:
• ELISA Results.png (the mean optical densities obtained from the different treatments).
• FTIR Results.png (results obtained from the infrared analysis doe on the extract).
• Hematology results.png (results from haematological analysis of the whole blood from the mice).
• PCR Results.png (results from the real-time PCR for the target genes; IL-6 and TNF-α and housekeeping gene, TBP).
Figshare: ARRIVE checklist for ‘Preliminary assessment of adjuvant activities of Glycine Max (L.) Merr. saponin extract in BALB/c mice immunized with hepatitis B virus vaccine’. https://doi.org/10.6084/m9.figshare.23790246.
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
I wish to express my sincere appreciation to Mrs Lucy Nungari and Mr Steven Kaniaru for their expertise and assistance throughout the various aspects of this study.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)