Keywords
anti-virulence, natural compounds, vibriosis, food safety
This article is included in the Pathogens gateway.
Background: Vibrio parahaemolyticus is a pathogenic bacterium that affects shrimp aquaculture; its infection can lead to severe production losses of up to 90%. On the other hand, plant phenolic compounds have emerged as a promising alternative to combat bacterial infections. The antibacterial and anti-virulence activity of the plant phenolic compounds quercetin, morin, vanillic acid, and protocatechuic acid against two strains of V. parahaemolyticus (Vp124 and Vp320) was evaluated.
Methods: The broth microdilution test was carried out to determine phenolic compounds' antibacterial activity. Moreover, the biofilm-forming ability of V. parahaemolyticus strains in the presence of phenolic compounds was determined by total biomass staining assay using the cationic dye crystal violet. The semisolid agar displacement technique was used to observe the effect of phenolic compounds on the swimming-like motility of V. parahaemolyticus.
Results: Results showed that phenolic compounds inhibited both strains effectively, with minimum inhibitory concentrations (MICs) ranging from 0.8 to 35.03 mM. Furthermore, at 0.125 – 0.5 × MIC of phenolic compounds, V. parahaemolyticus biofilms biomass was reduced by 63.22 – 92.68%. Also, quercetin and morin inhibited the motility of both strains by 15.86 – 23.64% (Vp124) and 24.28 – 40.71% (Vp320).
Conclusions: The results suggest that quercetin, morin, vanillic, and protocatechuic acids may be potential agents for controlling V. parahaemolyticus.
anti-virulence, natural compounds, vibriosis, food safety
A statement on the importance of Vibrio parahaemolyticus infection in shrimp was added to the introduction section.
Information about the manufacturers of reagents and equipment was included in the materials and methods section.
A comparison between effective doses of antibiotics and phenolic compounds, as well as the challenges of using phenolic compounds in aquaculture, were included in the discussion section.
See the authors' detailed response to the review by Chumporn Soowannayan
See the authors' detailed response to the review by Saharuetai Jeamsripong
According to the Food and Agriculture Organization of the United Nations (FAO), shrimp farming represents 53% of world aquaculture production of crustaceans.1 In recent years, stocking densities have increased, which has led to the proliferation of diseases and the appearance of new pathologies that directly affect the profitability of shrimp production systems. Twenty percent of the diseases that appear during shrimp farming are related to bacteria, including the genus Vibrio.2 Vibrio parahaemolyticus is considered of veterinary importance in shrimp farming, as some strains can infect shrimp, causing various diseases such as vibriosis. Strains that have acquired the pVA-1 plasmid, encoding the PirAB toxin, are of special concern because they cause Acute Hepatopancreatic Necrosis Disease (AHPND), initially defined as Early Mortality Syndrome (EMS).3 This atypical vibriosis has been responsible for losses of about 90% of production and, in some cases, up to 100%.3,4 The increasing prevalence of antibiotic-resistant strains has made the control of V. parahaemolyticus infections progressively challenging.
Once effective in controlling these infections, traditional antibiotics are now facing limitations due to the emergence of resistant strains and the negative environmental impacts resulting from their extensive use in aquaculture.5 Moreover, the complex and dynamic nature of aquatic environments makes preventing the spread of bacterial infections in these systems difficult. Waterborne pathogens, such as V. parahaemolyticus, can quickly disseminate throughout aquaculture facilities, contaminating water sources and other organisms.
In addition, the biofilm-forming capacity of V. parahaemolyticus further complicates the control measures, as biofilms provide protection against antimicrobial agents and enable the bacteria to persist in the environment.6 Biofilms are communities of microorganisms attached to biotic or abiotic surfaces embedded in a self-produced matrix consisting of polysaccharides, proteins, and nucleic acids.7 In the inner part of the biofilms, the oxygen concentration is lower. Hence, an anaerobic atmosphere with a low pH develops, which causes a decrease in the activity of some antibiotics.8 Other phenomena caused by these altered microenvironment conditions are decreased bacterial metabolism and dropped replication times.9 Therefore, once the biofilm is formed, its eradication becomes complicated. This challenge requires the development of novel strategies and compounds capable of effectively inhibiting bacterial growth, biofilm formation, and motility, without causing significant environmental harm or promoting antibiotic resistance.
The use of natural compounds, such as phenolic compounds, offers a promising alternative due to their antimicrobial properties and potential to address the limitations of traditional antibiotics.10 Phenolic compounds, which are secondary metabolites produced by a wide variety of plants, have shown antibacterial activity against Gram-negative (Escherichia coli, Salmonella spp., Vibrio spp.) and Gram-positive (Listeria monocytogenes and Staphylococcus aureus) pathogenic bacteria.11–13 They are widely distributed in plants as defense mechanisms against microbial pathogens. Owing to their structural diversity and biological activity, phenolic compounds have gained increasing interest as potential antibacterial agents in various applications, including food preservation, agriculture, and human health.14,15 For example, the flavonoids quercetin and myricetin showed a minimum inhibitory concentration (MIC) against V. parahaemolyticus of 0.125 and 0.25 mg/mL, respectively.16 In comparison, catechin and isorhamnetin showed MIC values of 0.05 and 0.025 mg/mL, respectively, against V. cholerae.17 Moreover, some of them, such as quercetin, catechin, ferulic acid, vanillic acid, and protocatechuic acid, have been shown to disrupt biofilm formation of pathogenic bacteria through different mechanisms of action, including inhibition of motility and EPS synthesis or/and disruption of intercellular communication.18–21 However, the effect of these phenolic compounds on V. parahaemolyticus virulence factors, such as motility and biofilm formation capacity is unknown. Therefore, the present study aimed to evaluate (quercetin, morin, vanillic acid, and protocatechuic acid) phenolic compounds’ antibacterial and anti-virulence activity against a V. parahaemolyticus reference strain (CAIM 320; Vp320), and a strain isolated from white shrimp samples (Vp124). It is hypothesized that the selected phenolic compounds will significantly inhibit the growth, biofilm formation, and motility of V. parahaemolyticus strains, thus highlighting their potential as alternative agents for controlling infections and the persistence of this pathogen. Furthermore, the flavonoids (quercetin and morin) are expected to exhibit different mechanisms of action compared to the phenolic acids (vanillic and protocatechuic acids), which may offer unique advantages in tackling V. parahaemolyticus.
The human pathogenic reference strain CAIM 320 (Vp320) harboring the virulence genes tlh (thermolabile haemolysin), tdh (thermostable direct haemolysin), trh (TDH-related haemolysin), T3SS1: VP1680 (type III secretion system 1) and VPI and a strain isolated from shrimp, designated as Vp12422 were donated by the Laboratory of Clinical Microbiology of the University of Sonora. The studied strains were seeded on Thiosulfate Citrate Bile Sucrose agar (TCBS; BD Difco, Sparks, MD, USA), reseeded in Trypticase Soy Broth (TSB; BD Bacto, Sparks, MD, USA) with 3% NaCl, and incubated at 37°C during the assays.
Standards (purity >95%) of gallic acid (G7384), vanillic acid (94770), protocatechuic acid (03930590), rutin (R5143), morin (M4008), and quercetin (Q4951) were purchased from Sigma-Aldrich (St Louis, MO, USA).
The antibacterial activity of phenolic compounds against V. parahaemolyticus strains was determined as described by Alvarez et al.23 An inoculum of each strain (Vp124 and Vp320) was prepared from exponential phase cultures (16 h in TSB + NaCl 3% at 37 °C), adjusting the optical density (600 nm) to 0.1 absorbance units, which is equivalent to 1 × 108 CFU/mL. Concentrations of 0.8 mM - 40 mM of each compound (quercetin, morin, vanillic acid, protocatechuic acid, gallic acid, and rutin) were prepared in TSB + NaCl 3% at 37°C from dimethyl sulfoxide (DMSO; Sigma-Aldrich, St Louis, MO, USA) stocks. Subsequently, 5 μL of the adjusted inoculum and 295 μL of each concentration of the compounds were taken and placed in microplate wells (Costar 96; Corning, NY, USA) and incubated for 24 h at 37°C. The positive control was the same bacterial inoculum in TSB + NaCl 3% + DMSO 5%. As negative controls, each concentration of the tested compound and TSB + NaCl were placed in microplate wells without bacterial inoculum. The lowest compound concentration at which no visible growth was observed was considered the minimum inhibitory concentration (MIC). In addition, each compound's minimum bactericidal concentration (MBC) was determined by placing 50 μL aliquots from wells with three concentrations above the MIC on Mueller-Hinton agar (BD Difco, Sparks, MD, USA) + NaCl 3% plates. The MBC was considered the minimum concentration of each compound where no growth of V. parahaemolyticus was observed on the plate.23
The biofilm-forming ability of V. parahaemolyticus strains in the presence of phenolic compounds was determined by total biomass staining assay using the cationic dye crystal violet according to Beltran-Torres et al.24 An inoculum of each strain at an optical density of 0.1 units at 600 nm was prepared from exponential phase cultures. Each compound was dissolved in DMSO (5% of final volume) and added to TSB at 0.125, 0.25, and 0.5 × MIC. Subsequently, 5 μL of the inoculum and 295 μL of each concentration were taken, placed in microplate wells, and incubated for 24 h at 37 °C.
At the end of the incubation, the culture medium was removed from the microplate wells by aspiration. Three consecutive washes were performed with distilled water to remove weakly adherent cells, and then the microplate was allowed to dry at 50°C. After that, 300 μL of a 0.1% crystal violet (Sigma-Aldrich; St Louis, MO, USA) solution was added to each well and allowed to stand for 45 min to stain the biomass of the formed biofilms. The crystal violet solution was then carefully removed, and three consecutive washes were carried out to remove the excess dye with distilled water, followed by drying at 50°C. Finally, 300 μL of 20% acetic acid was added to each well to solubilize the crystal violet for 15 min, and the absorbance of the solution was read at 594 nm. The absorbance of the solubilized violet crystal is proportional to the total biomass of the biofilms formed in each well. Each experiment was performed in triplicate, and the results were expressed as a percentage of the reduction of total biomass compared to the positive control (biofilms formed without the presence of phenolic compounds) according to the following equation:
The semisolid agar displacement technique was used to observe the effect of phenolic compounds on the swimming-like motility of V. parahaemolyticus following the protocol of Beltran-Torres et al.24 Phenolic compounds were added to TSB + NaCl 3% medium at a final concentration of 0.5 × MIC. An inoculum of each V. parahaemolyticus strain from exponential phase cultures (18 h at 37°C in TSB + NaCl 3%) was prepared at a final 1 × 108 CFU/mL concentration. Subsequently, 10 μL of bacterial suspension were taken and placed in the center of a Petri dish with semi-solid TSB + NaCl 3% agar (0.42% agar) and incubated for 16 h at 37°C. Finally, the diameter of motility halos was measured. The experiment was performed in triplicate, and cultures of each V. parahaemolyticus strain on TSB + NaCl 3% without phenolic compounds were used as positive controls.
A complete randomized experimental design was used for all experiments. In the biofilm formation assay, the factors were the type of compound (quercetin, morin, vanillic acid, and protocatechuic acid) and the tested concentration (0.125, 0.25, and 0.5 × MIC), and the response variable was biomass reduction (%). In the motility assay, the factors were also the type of compound and concentration, and the response variable was motility inhibition (%). An analysis of variance (ANOVA) was performed, and where differences were found, multiple comparisons of means were performed by the Tukey-Kramer method at 95% confidence using the statistical software NCSS 2007.
The MICs of the assessed compounds are shown in Table 1, where four of the six compounds were able to inhibit the growth of both Vibrio strains. The flavonoid quercetin was the most effective, presenting the lowest MIC (0.8 mM for both strains). The flavonoid morin and protocatechuic acid showed the same effect on both Vibrio strains, with MICs of 1.6 and 28.43 mM, respectively. On the other hand, vanillic acid presented a MIC equal to 31.73 mM for the Vp124 strain and 35.03 mM for the Vp320 strain. In contrast, rutin and gallic acid did not inhibit the growth of V. parahaemolyticus at the evaluated concentrations, 0 - 4 mM and 0-41.12 mM, respectively. For this reason, they were discarded in the following determinations. The minimum bactericidal concentration was also evaluated; however, this effect was not observed at any tested concentrations. The observed MICs of quercetin, morin, protocatechuic acid, and vanillic acid against both Vibrio strains support our hypothesis that certain phenolic compounds can effectively inhibit the growth of V. parahaemolyticus.
To determine the effect of phenolic compounds on V. parahaemolyticus biofilm formation, sub-inhibitory concentrations of each compound were selected based on the MIC (0.125, 0.25, and 0.5 × MIC). Figure 1 shows the percentage of total biomass of V. parahaemolyticus biofilms formed at different concentrations of quercetin and morin. Both compounds were able to interfere with the biofilm formation process. For quercetin at 0.125 × MIC, a 71.97% reduction (p<0.05) was observed on the Vp124 strain, while no significant differences were found between 0.25 and 0.5 × MIC, achieving a reduction of 77.97-80.85% (p<0.05) respect to control. A similar pattern was observed in strain Vp320, where quercetin at 0.125 × MIC reduced 48.44% of the total biomass of V. parahaemolyticus biofilms, and for 0.25-0.5 × MIC, total biomass reduction was 85.31 – 91.66%. Regarding the effectiveness of morin, a reduction (p<0.05) in the total biomass of biofilms of strain Vp124 of 73.39%, 84.08%, and 89.07% was observed at concentrations of 0.125, 0.25, and 0.5 × MIC, respectively. While in the Vp320 strain, no differences were observed in the percentages of reduction at the evaluated concentrations (p>0.05), showing a reduction of 88.84-89.93 % compared to the control. The significant decrease of V. parahaemolyticus biofilm formation observed at sub-inhibitory concentrations of quercetin and morin supports our hypothesis that certain phenolic compounds can effectively disrupt biofilm formation processes.
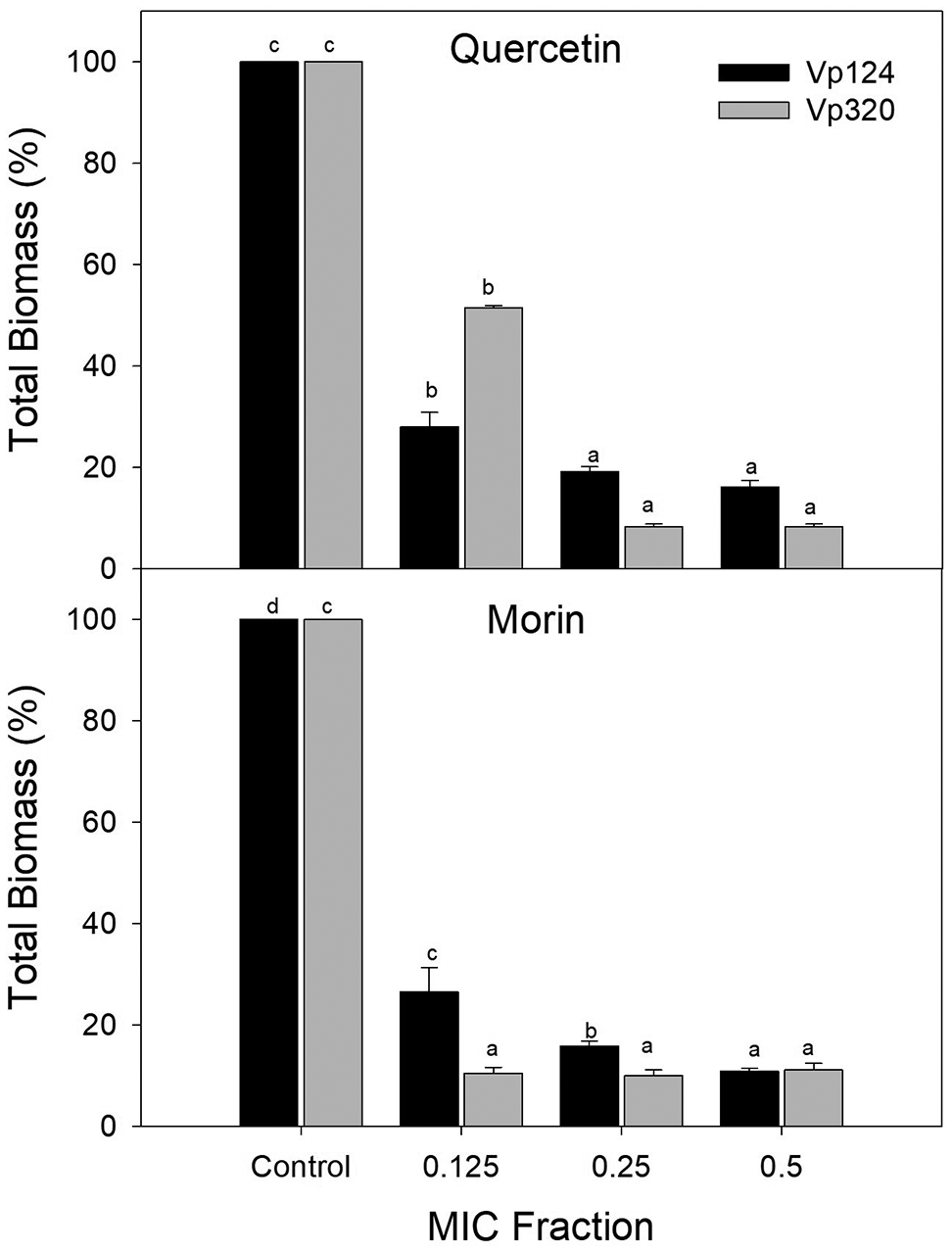
Values represent mean ± standard error (n = 3). Different literals represent differences (p<0.05) between the evaluated concentrations for each strain.
Figure 2 shows the total biomass of biofilms formed by V. parahaemolyticus in the presence of vanillic and protocatechuic acids. Both phenolic acids inhibited the biofilm formation capacity of both strains compared to the control. However, no differences were found (p>0.05) between the evaluated concentrations. Vanillic acid reduced the total biomass of Vp124 biofilms by 85.92% to 92.68% and 89.55% - 90.27%for Vp320 strain. In comparison, protocatechuic acid inhibited 91.61% - 93.12% and 91.04% - 92.82% of the biofilm formation of Vp124 and Vp320 strains, respectively.
Table 2 shows the effect of quercetin, morin, vanillic and protocatechuic acids on the motility of Vp124 and Vp320 strains. At the tested concentrations, the flavonoids quercetin and morin inhibited the motility of both strains on soft agar (0.42% agar). In this assay, differences (p<0.05) were observed in the inhibition percentages of each strain. For the Vp124 strain, quercetin inhibited 15.86% of the displacement of the bacteria on soft agar; while an inhibition of 23.63% was observed for the Vp320 strain. In the presence of morin, a similar trend was observed, where Vp320 showed the highest (p<0.05) percentage of motility inhibition (40.71%), while for the Vp124 strain, the percentage of inhibition was 24.28%. However, phenolic acids at the evaluated concentration did not show the capacity to interfere with the motility of V. parahaemolyticus strains (p>0.05). These results demonstrated that the flavonoids quercetin and morin effectively inhibited the motility of V. parahaemolyticus strains Vp124 and Vp320, following the hypothesis that certain phenolic compounds can impact bacterial motility. On the other hand, phenolic acids, such as vanillic and protocatechuic acids, did not exhibit the same inhibitory effect on motility, highlighting the varying impacts of different phenolic compounds on V. parahaemolyticus motility.
Phenolic compounds are known for their antimicrobial activity against various pathogenic bacteria, including Gram-negative and Gram-positive species.10,11 In this study, vanillic and protocatechuic acids, along with the flavonoids quercetin and morin, effectively inhibited the growth of V. parahaemolyticus. Notably, flavonoids exhibited 20-40 times stronger antibacterial activity than phenolic acids, as evidenced by their lower MICs, which aligns with findings from previous research. Abuga et al.16 demonstrated that quercetin and myricetin inhibited V. parahaemolyticus, with MICs of 0.4 and 0.8 mM, respectively. Similarly, Tinh et al.12 evaluated the antibacterial activity of phenolic compounds against 96 V. parahaemolyticus isolates from Pacific white shrimp (L. vannamei) in Thailand. Among the tested compounds, vanillic acid displayed significant bactericidal activity, with MICs and MBCs ranging from 6.09 to 12.18 mM. Pyrogallol, however, exhibited the strongest antibacterial properties, with MICs and MBCs between 0.25 and 2.02 mM. Wu et al.25 also investigated the MIC of 3-p-trans-coumaroyl-2-hydroxyquinic acid against various pathogenic bacteria, including V. parahaemolyticus. Their results showed MICs ranging from 7.06 to 28.24 mM for all evaluated pathogenic bacteria, with a MIC of 14.12 mM for V. parahaemolyticus. In conjunction with our current research findings, the outcomes of these earlier studies underscore the potential of phenolic compounds as effective antibacterial agents against V. parahaemolyticus. Our study contributes to the growing body of evidence by demonstrating the comparative effectiveness of flavonoids and phenolic acids, highlighting the promising role of these natural compounds in combating V. parahaemolyticus infections.
MIC values of the evaluated phenolic compounds were higher than MICs of commonly used antibiotics in aquaculture. For example, enrofloxacin, florfenicol, and oxytetracycline showed MIC values against V. parahaemolyticus strains isolated from diseased white shrimp ranging from 33 – 44.51 μM, 2.9 – 11.6 μM, and 0.55 – 17.4 μM, respectively.26 However, MIC values of antibiotics in the millimolar range have also been reported, as in the case of oxytetracycline hydrochloride, which showed MICs against V. parahaemolyticus strains of 0.257 – 0.515 mM.27 Despite the differences in the effective doses to inhibit planktonic growth, common antibiotics not only do not inhibit the formation of biofilms but, in certain bacterial species, promote their development.28–30
One notable aspect of these results is the direct comparison between flavonoids and phenolic acids regarding their antibacterial activity against V. parahaemolyticus. While previous research has separately investigated various phenolic compounds for their antimicrobial properties, our study offers a unique contribution by evaluating and contrasting the effectiveness of both flavonoids and phenolic acids. The results of our study emphasize that flavonoids, such as quercetin and morin, exerted stronger antibacterial activity than phenolic acids, as evidenced by their lower MICs. This novel finding highlights the potential advantages of using flavonoids over phenolic acids in developing new strategies to control and prevent V. parahaemolyticus infections in aquaculture systems. Additionally, our study contributes to the growing body of evidence supporting using natural compounds as alternatives to traditional antibiotics, which is particularly important in increasing antibiotic resistance.
The antibacterial activity of phenolic compounds is closely related to their physicochemical properties and the ability to interact with the lipid membrane and intracellular targets. Phenolic acids can alter the morphology of treated cells and cause hyperpolarization of the lipid membrane with its consequent loss of integrity.25,31 While flavonoids can intercalate in different regions of lipid bilayers, causing changes in their fluidity, this effect depends on the number and position of hydroxyl groups.32 Wu et al.33 found a strong and positive correlation (r = 0.921) between the antimicrobial activity of flavonoids against E. coli and their ability to reduce the fluidity of lipid membranes. Furthermore, flavonoids can inhibit essential enzymes for bacterial growth, e.g., quercetin, kaempferol, luteonin, galangin, and myricetin are inhibitors of DNA gyrase (IC50 = 0.037-1.18 mg/mL).34 Also, quercetin and apigenin can inhibit d-alanine:d-alanine ligase, the enzyme responsible for the binding of alanine residues during the assembly of peptidoglycan precursors.35
Biofilms enable pathogenic bacteria to survive in adverse environments, such as nutrient scarcity and exposure to antimicrobial agents.7,36 V. parahaemolyticus can form biofilms on various surfaces in aquaculture-related settings, acting as a reservoir that compromises the safety of aquaculture products.6 Our study reveals that vanillic and protocatechuic acids and the flavonoids quercetin and morin can effectively interfere with V. parahaemolyticus biofilm formation (Figures 1 and 2), contributing new insights into the potential of these compounds. Liu et al.21 demonstrated that vanillic acid reduced the total biomass of V. alginolyticus biofilms by approximately 65% at a concentration of 2.9 mM. Additionally, 2,6-di-tert-butyl-4-methylphenol, found in garlic, green algae, and cyanobacteria, inhibited the biofilm formation of V. harveyi, V. parahaemolyticus, and V. vulnificus by 80%, 83%, and 80%, respectively.37 Moreover, ethanolic extracts of ginger and its major compounds, 6-gingerol, 8-gingerol, and 6-shogaol, effectively inhibited V. parahaemolyticus biofilm formation, with 6-shogaol exhibiting the highest efficacy by reducing biofilm biomass by up to 80% at a concentration of 0.15 mM.38 This study breaks new ground by assessing the anti-biofilm activity of selected flavonoids and phenolic acids against V. parahaemolyticus, demonstrating their potential as anti-biofilm agents in combating infections in aquaculture systems. This research significantly expands on prior findings, offering a deeper and more extensive understanding of phenolic compounds' capabilities in mitigating biofilm-associated risks within the aquaculture industry.
Motility is a key virulence factor in the Vibrio genus, essential in the initial adhesion to abiotic surfaces and subsequent biofilm formation.39,40 Our study reveals that flavonoids quercetin and morin inhibited V. parahaemolyticus swimming motility, while vanillic and protocatechuic acids did not exert any inhibitory effects (Table 2). V. parahaemolyticus exhibits swimming motility in aquatic environments, driven by a single polar flagellum,41 which relies on the expression of approximately 60 genes organized in at least 11 operons.42,43 Roy et al.44 reported that quercetin (0.09-0.36 mM) repressed flaA and flgL genes encoding flagellin, inhibiting V. parahaemolyticus motility and biofilm formation on shrimp and crab tissues. Our results corroborated these findings and further suggested that quercetin and morin may impair biofilm formation due to their motility-inhibiting effects. Notably, Vp124 (shrimp isolate) and Vp320 (clinical isolate) exhibited differential responses to flavonoids, with Vp124 being more resistant. Previous studies have indicated that mutations in sodium-type flagellar motor genes (motX and motY) confer resistance to known bacterial motility inhibitors, such as phenamil and amiloride.45 Investigating a potential similar resistance mechanism in these strains could be worthwhile. In contrast, the lack of inhibitory effects of vanillic and protocatechuic acids on V. parahaemolyticus motility suggests that they may interfere with biofilm formation through alternative mechanisms, such as modifying the physicochemical properties of the bacterial surface.18,20 This study provides insights into the potential role of phenolic compounds as motility inhibitors and enhances our understanding of biofilm disruption strategies in the aquaculture industry.
The different responses observed with flavonoid treatments between the Vp124 strain (isolated from shrimp), and the reference strain (clinical isolate) warrant further investigation to elucidate the underlying resistance mechanisms. It is plausible that genetic or phenotypic differences between the two strains may contribute to their varying susceptibilities to the tested compounds. The disparity in response could also be attributed to differences in gene expression profiles, metabolic pathways, or the presence of specific efflux pumps that confer resistance to these flavonoids.46 Further research employing genomic, transcriptomic, and proteomic approaches could help to identify the key genetic determinants and molecular pathways responsible for these differential responses. By understanding the resistance mechanisms in play, it may be possible to tailor more targeted and effective interventions for controlling V. parahaemolyticus infections in different settings, such as aquaculture and clinical environments.
Despite the valuable insights gained from this study, some limitations should be acknowledged. Investigating a broader range of phenolic compounds and a more extensive and diverse set of V. parahaemolyticus strains, including environmental isolates, would help to create a more complex panorama. In addition, the specific mechanisms exerted by phenolic compounds in motility and biofilm formation could go deeper into exploring the molecular targets and pathways involved in the observed inhibitory effects. Additionally, the potential synergistic or antagonistic interactions between the tested compounds and their combination with other antimicrobial agents should be investigated to identify more effective biofilm disruption and motility inhibition strategies.
Finally, the challenge of using phenolic compounds in aquaculture includes obtaining enough quantities due to resource-intensive extraction processes, the variability in yield and purity based on plant species and extraction methods, and stability issues as these compounds can degrade under environmental conditions such as light, temperature, and pH changes. Effective delivery mechanisms are also needed to ensure the compounds reach their target sites in sufficient concentrations. Addressing these limitations involves developing enhanced extraction techniques to increase yield and purity, employing encapsulation methods to improve stability, and/or creating innovative delivery systems like controlled-release formulations. Exploring synergistic combinations with other antimicrobial agents can also enhance effectiveness and mitigate costs and side effects. Addressing these limitations will deepen our understanding of the potential applications of phenolic compounds in controlling V. parahaemolyticus infections and mitigating biofilm-associated risks in the aquaculture industry.
This study provides valuable insights into the potential of phenolic compounds, particularly quercetin and morin, in controlling V. parahaemolyticus infections by inhibiting biofilm formation and motility. Quercetin and morin exhibited strong antibacterial activity compared to vanillic and protocatechuic acids. Moreover, both flavonoids effectively reduced biofilm formation and motility, supporting the hypothesis that these compounds can interfere with key virulence factors in V. parahaemolyticus. Finally, further research should address the study's limitations and focus on the underlying molecular mechanisms, synergistic or antagonistic interactions between the tested compounds, and in vivo efficacy to fully harness the potential of these phenolic compounds in combating V. parahaemolyticus infections.
Figshare: Underlying data supporting the results of the manuscript “Antimicrobial and Anti-Virulence Potential of Plant Phenolic Compounds Against Vibrio parahaemolyticus” https://doi.org/10.6084/m9.figshare.24123315.v4. 47
Data are available under the terms of the CC BY 4.0 Attribution International license (CC BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: My research area focuses on the pathology of aquatic animals, particularly their immunology. I study the application of immunostimulants to combat diseases in fish and shrimp, exploring how these compounds can enhance immune responses and improve overall health in aquaculture.
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Fish and shrimp diseases
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: antimicrobial resistance, aquaculture, epidemiology, food safety
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 2 (revision) 03 Jul 24 |
read | ||
|
Version 1 02 Oct 23 |
read | read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)