Keywords
Acute submissive pulmonary embolism, Balloon angioplasty, Simultaneous thrombolysis, Maceration
Acute submissive pulmonary embolism, Balloon angioplasty, Simultaneous thrombolysis, Maceration
Acute pulmonary embolism is a common medical condition, and the treatment is often challenging. The incidence of acute pulmonary embolism is in the range of 0.6/1000/year.1 The estimated incidence of deep vein thrombosis is 1-2/1000 persons every year.2 It is the 3rd leading cause of cardiovascular mortality. The burden of deep vein thrombosis and related pulmonary embolism are high.3 These also pose a large economic burden for the health care providers irrespective of the system worldwide.3 The mortality in patients with acute pulmonary embolism is significant, and the exact burden is high when quantified. At present robust data about this condition is not available and the magnitude of the problem is an underestimate. Common challenges are encountered in treating this condition is associated severe breathlessness, co-morbidities and the patient is often moribund. Associated comorbidities or predisposing conditions preclude the treatment methods due to the associated risk of bleeding and mortality. Hence, controversies exist in choosing the method of treatment of this condition, and the best treatment methods at present are catheter directed thrombolysis and low dose thrombolysis. Among the costs involved, the catheter directed thrombolysis is associated with the lowest cost.4 This is by considering the risk of bleeding vs. the benefits achieved, which is primarily the mortality. With the advent of the COVID-19 pandemic, the incidence of venous thrombosis has increased significantly, especially in severe cases of COVID-19.5 At present catheter directed thrombolysis has the best results, and even this is associated with 6.5% cardiac arrest and about 5% hemoptysis.6
Catheter directed thrombolysis is associated with least mortality6 though there are some studies which show definite conclusions regarding the treatment methods is not possible.7 Some studies show similar results with all methods of treatment modalities,8 though many studies claim the benefits9–11 and safety12 of catheter directed thrombolysis. Prompt treatment of this condition is always advised, and treatment delays are associated with increased mortality.13 The current report is descriptive about four male patients encountered with acute sub-massive/severe pulmonary embolism, who were treated with balloon angioplasty/maceration and thrombolysis.
Informed written consent was taken from patients for all procedures. Institutional ethical committee approval has been obtained from Pondicherry Institute of Medical Sciences (PIMS) Institutional ethical review committee, Puducherry. The consent to publish the individual clinical details described in this report was obtained from each patient.
The patient was a 45-year male who developed acute onset of breathlessness and presented to the emergency ward with acute breathlessness and chest pain. Angiogram was performed, and the coronary angiogram was normal. As the patient had persistent tachycardia, the echocardiogram performed showed dilated right atrium, ventricle, and pulmonary artery. Pulmonary angiogram, which showed dilated right atrium and right ventricle and near total occlusion of the left pulmonary artery (Figure 1, panels A). The patient underwent balloon dilatation, and thrombolysis was performed simultaneously (Figure 1, panels B to F). Accuforce balloon, 4.5mm, was used for balloon dilatation through Cordis 6F diagnostic catheter, and thrombolysis was performed with tenecteplase. 35 mg of Tenecteplase was used with boluses given by hand injections simultaneously with balloon dilatations. There was an immediate improvement in breathlessness, reduction in tachycardia, and the oxygen saturation also improved to 99 percent when the procedure was completed in the cardiac catheterization lab. Flow in the left pulmonary artery improved (Figure 1, panels G and H). The patient had improvement in the general condition, and he was discharged on day three. The right atrium and right ventricular dilatation were also reduced. Deep veins were normal by Doppler evaluation, and hence inferior vena-cava filter was not placed in this patient. This patient is under follow-up for one year, and at present on rivaroxaban and currently asymptomatic.
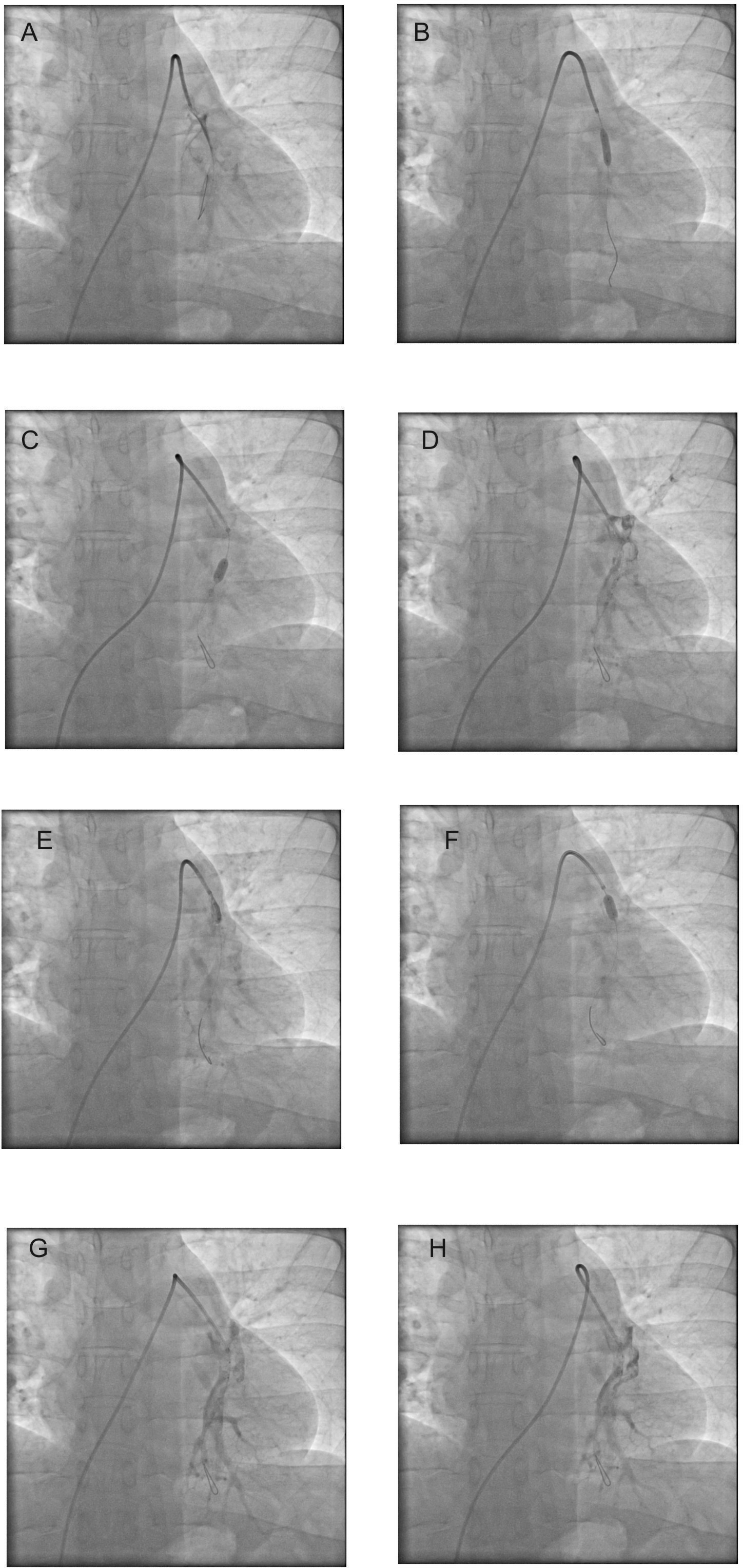
Panel A shows clots in left pulmonary artery. Panels B to F shows balloon dilatation and thrombolysis simultaneously in left pulmonary artery (panels B to F). Results with clearing of the clots and improvement in oxygen saturation (Panels G and H), 4.5 mm Accuforce balloon was used.
The 81-year male patient developed breathlessness and chest pain, which was sudden in onset. The patient recently underwent a hip operation for an intertrochanteric fracture on the right side, and recently he was ambulant. The pulse rate at presentation was 140/min, and the patient had severe breathlessness, and by echocardiography, the right atrium and right ventricle were dilated. A pulmonary angiogram showed near total occlusion of the right pulmonary artery (Figure 2A, panel A). The patient underwent thrombolysis with balloon dilatation in the right pulmonary artery, where the clots were visualized (Figure 2A, panels B to E). A 5 mm quantum apex balloon was used initially, and it was improvised with a 6 mm Sterling balloon (Figure 2, panel F). Tenecteplase 35 mg was used in the procedure and was given as boluses after dilution with saline. At the end of the procedure, the clot volume was reduced, and pulmonary artery blood flow improved (Figure 2A, panels G and H). The patient’s symptoms improved, and tachycardia mildly reduced immediately after the procedure. Subcutaneous low molecular weight heparin was given with good results. On day two, the tachycardia and breathlessness were reduced by about 60%. A repeat pulmonary angiogram performed on the third day showed significant clearance of the clots in the right pulmonary artery (Figure 2B). The patient is on regular follow-up for one year and 1 m.
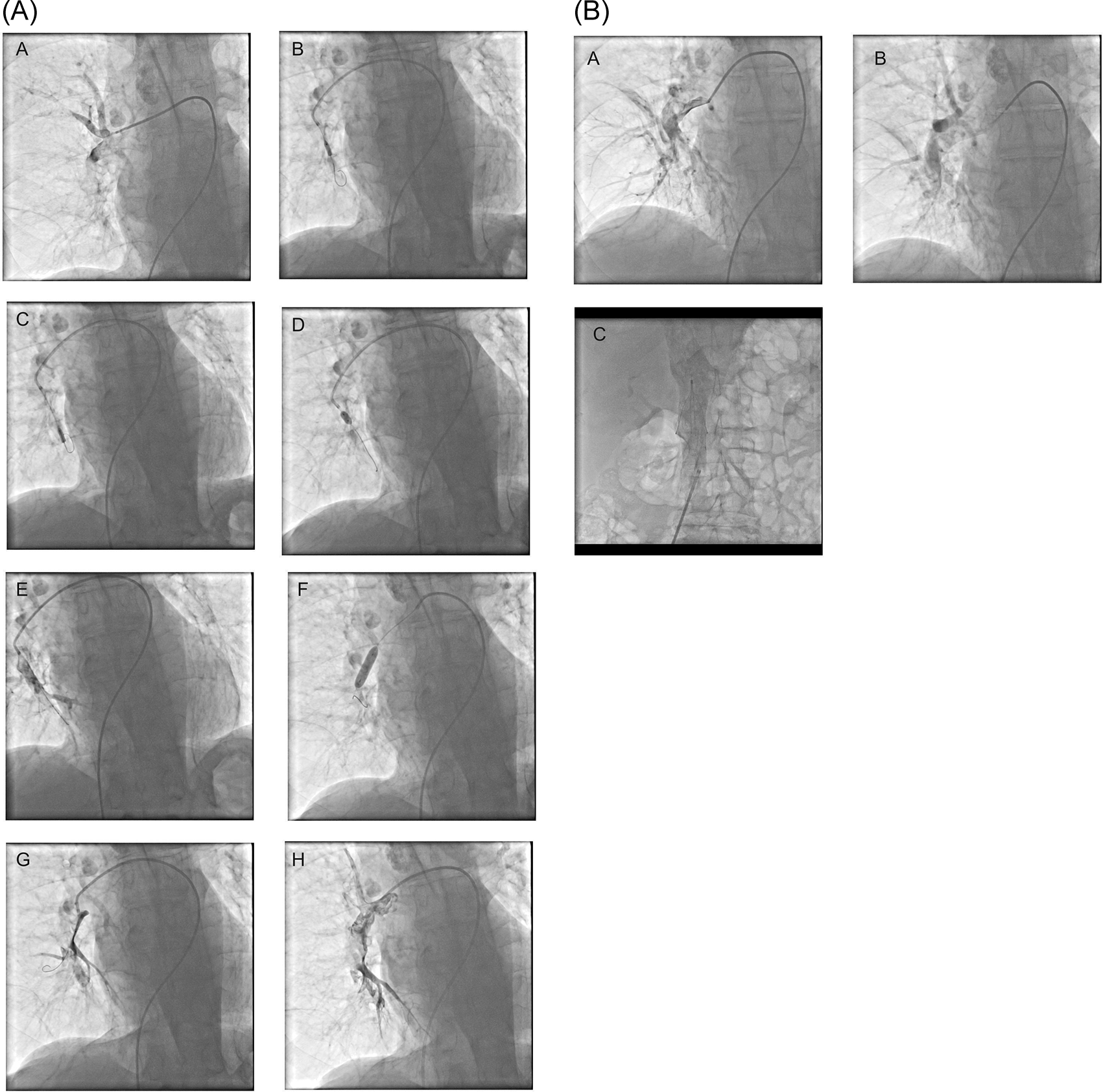
A 59-year male presented with breathlessness and orthopnea for ten days, and this patient had mild pericardial effusion and corpulmonale and severe pulmonary artery hypertension. The patient also had crepitations and collapse of the entire right lung. Angiogram showed a large clot in the right pulmonary artery (Figure 3, panels A and B). Initially, 035 Terumo wire was inserted, and using a peripheral - 5×40 mm balloon, angioplasty was performed in the right pulmonary artery. Thereafter, 014 wire was inserted (Figure 3, panel E), and balloon dilatations using coronary balloons, 2×10 mm, and 4.5×8 mm, were performed (Figure 3, panel F). The patient underwent serial balloon dilatations and simultaneous thrombolysis with streptokinase by hand injection. Partial recanalization of the right pulmonary artery was achieved (Figure 3, panels G to I). Investigations for deep vein thrombosis and any associated malignancies did not reveal any findings. A pulmonary angiogram was repeated after two days, and balloon dilatation with heparin was given during the second intervention for residual thrombi in the right pulmonary artery. The patients’ general condition and breathlessness improved, and he was discharged. One month follow-up showed mild lung expansion compared to the previous results on the right side, and he had mild breathlessness on exertion with pulmonary artery systolic pressure of 45 mmHg.
This was a 47-year male who presented with breathlessness and cough for one day and had a history of occupation-related long travels. Echocardiography revealed a corpulmonale and right ventricular dysfunction. An inferior vena-cava angiogram showed clots in the left iliac artery (Figure 4, panel A). The patient had large clots in the left pulmonary artery with near-total occlusion (Figure 4, panels B and C). Using Cordis 6F diagnostic catheter, serial balloon dilatations were performed to clots in the left pulmonary artery (Figure 4, panels D and E), and its mid and lower branches using a 5 mm Quantum apex balloon and thrombolysis was performed simultaneously using streptokinase in the left pulmonary artery. A significant improvement in the blood flow of the left pulmonary artery was observed (Figure 4, panels G and H). The patient had relief in breathlessness and chest tightness immediately after the procedure and a significant reduction in oxygen requirement. He was started on low molecular weight heparin and clopidogrel 75mg once a day. For 24 hours post-procedure patient had substantial clinical improvement. 1.3M units of streptokinase were used by dilution in saline and were administered as hand injections. On day two, the patient, being asymptomatic with minimal oxygen support, developed sudden onset of breathlessness and rapid deterioration and death subsequently. The patient possibly could have had an episode of another pulmonary embolism from the deep veins or from the primary source, which was only clinical speculation. No bleeding manifestations were observed before deterioration.
In all the above cases, balloon angioplasty/maceration and thrombolysis were performed simultaneously to achieve recanalization of the respective pulmonary artery. In all these cases, either Cordis 6F diagnostic catheter or, in the late stages, exchanged with 6F guide catheters were used. The advantages of these procedures are a reduction in the contrast volume load and easy availability of catheters for the same. The 5 mm coronary balloons are commonly available, and if higher balloons are required, exchange with larger balloons was used during the procedures. When a balloon size >5 mm is required, the 6F guide catheter is used. Other advantages of this method include the early withdrawal of the catheter after the procedure. When a thrombolysis catheter is placed for a longer duration in the pulmonary artery, arrhythmias, infections, and thrombosis tend to occur, and thereby, the mortality would increase.
5 mm balloons are average-sized, and the pulmonary arteries are in the range of 8 to 10 mm. The advantage of a 5mm balloon is inside the clot, the balloon can be maneuvered safely inside the smaller pulmonary artery branches without difficulty. In the late stages of the interventional procedure, when the clarity of the distal pulmonary artery or the target artery is good, large balloons like 6 mm or 7 mm Sterling balloons can be used for this purpose. Larger balloons may result in dissection, spasms and proximal dislodgement of clots which may be detrimental. Hence, being ‘average’ in approach may yield better results in uncertainty as definite treatment approaches are not well defined for this clinical condition. The raw data is available in the data availability section below. The number of fluoroscopy and cine acquisitions can be significantly reduced in future similar interventions, and this will reduce contrast usage indirectly.
Many treatment methods are available for physicians to choose for patients with acute pulmonary embolism.14–17 Commonly used methods are systemic thrombolysis, low dose thrombolysis, catheter-directed thrombolysis, ablation of thrombus, Inari flow retriever method, ultrasonic treatment and other embolectomy devices available for this purpose. Though consensus statements are available about the treatment strategy, the choice varies based on the scenario. The scenario of the patient, the clinical manifestations, severity, and co-morbidities like recent surgeries determine the clinicians’ decision.18,19 Also, a classification of the sub-massive/massive differentiation at the bedside is not always possible easily, even though various criteria exist for such a classification.14,18,19 Catheter-directed interventions are especially suited for individuals where the systemic thrombolysis is contraindicated or at high risk for bleeding manifestations. Surgical treatment options have been explored with good results. However, surgery is associated with long bypass time, and the need for a ventilator precludes this method as a routine and easy treatment option.20,21 Contrast reduction will give advantages in these patients as renal failure is commonly associated in an overt or subtle form in these patients. Reducing contrast load and fluoroscopy times are very useful in coronary and peripheral angioplasties.22,23 Renal failure, coronary artery diseases, cancer, and diabetes are commonly associated in patients with deep vein thrombosis.19 Simultaneous balloon dilatation and thrombolysis would be better for greater penetration of the thrombolytic agents and concurrent mechanical clearance of the clots, and the hardware is readily available in all cardiac catheterization labs.
Using an IVC filter in patients with deep vein thrombosis or venous thromboembolism poses many dilemmas. Metanalysis and NICE guidelines indicate the benefits of the IVC filter in these patients. In the current case discussion, in the opinion of the author, an early deployment of an IVC filter would have benefitted patient-4 by preventing the recurrence of pulmonary thromboembolism.24 It was the practice of the author to insert an IVC filter after a few days or before the discharge of the patients, as some patients would require reintervention of the pulmonary artery. In this case series, all four patients were males. The incidence of pulmonary thromboembolism is higher in the female gender,25 and the treatment availability and outcomes tend to be worse among females.25–27 Hence, this method needs to be evaluated in a large patient population, including the female gender, for a better assessment of the results. These observations were made in a few numbers of patients only. Further studies, including a large number of patients with acute sub-massive pulmonary embolism, needs to be performed for validation of the current observations.
Balloon angioplasty/maceration and thrombolysis simultaneously can be used as a treatment option in patients with acute sub-massive pulmonary embolism using coronary balloons and catheters. If larger balloon dilatation is required, 6F guide catheters can be used.
Mark Christopher Arokiaraj. (2023). Treatment of Acute Sub-massive Pulmonary Embolism with Balloon Angioplasty and Thrombolysis Simultaneously (Version 1). Zenodo. DOI: https://doi.org/10.5281/zenodo.8159042.
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC BY 4.0 Public domain dedication).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the background of the cases’ history and progression described in sufficient detail?
No
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
No
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
No
Is the conclusion balanced and justified on the basis of the findings?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Pulmonary embolism and thrombolysis.
Is the background of the cases’ history and progression described in sufficient detail?
Yes
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Partly
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Yes
Is the conclusion balanced and justified on the basis of the findings?
Yes
References
1. Raskob GE, Angchaisuksiri P, Blanco AN, Buller H, et al.: Thrombosis: a major contributor to global disease burden.Arterioscler Thromb Vasc Biol. 2014; 34 (11): 2363-71 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Complex cardiovascular interventions
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 09 Jul 24 |
read | |
|
Version 1 05 Oct 23 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)