Keywords
Carcinogenicity; cellular phone; radio frequency; thyroid stimulating hormone; thyroxine
Carcinogenicity; cellular phone; radio frequency; thyroid stimulating hormone; thyroxine
Mobile phone is the most used electronic devices working based on the electromagnetic radio frequency. According to the Oceania Radiofrequency Scientific Advisory Association (ORSAA), the cellular phone usage has reached seven billion individuals or 97% of total world’s population.1 In East Asia, the number of mobile phone users is twice as many as its own population (600 million individuals).2 The usage duration ranged widely across countries, from 25 minutes in Japan up to 248 minutes in the Philippines.3 In Switzerland, an individual could spend 30 minutes on their phone.3 In the midst of hyperconnected world, people become more urged of using their phone which is done in at least every 30 minutes.4 With such numbers, the assessment of the effects of mobile phone radiation on human health are urgently needed.
An epidemiological studies have revealed that 30 min daily use of mobile phone lasted for more than 10 years could increase the risk of glioma in brain cells.5,6 Short-term exposure of the radiation has been reported to be associated with the increased cellular immune activities.7,8 Meanwhile, its long-term exposure could cause irreversible cellular damage.9 Impacts of phone usage is associated with the non-ionic electromagnetic wave radiation transmitted when making or receiving a call.10
In general, there are two main pathways explaining the biological dysfunctions caused by the exposure of mobile phone-derived electromagnetic wave. The first is through the thermal effects generated by the heat radiation transmitted by the electromagnetic wave, while the latter is through non-thermal effects which have not been completely understood.11 Carcinogenicity of phone radiation has been reported to be associated with increased Ca2+ efflux, oxidative stress imbalance, DNA damage, elevation of ornithine decarboxylase and protein kinases activities, disrupted activity of Na+/K+ phosphatase, melatonin level reduction, disruption in histone kinase activity, and errors in protein synthesis.12–14 Multiple reports have established a correlation between the electromagnetic radiation and impaired estrogen production.15–17 Estrogen shares a similarity with thyroid hormones of being active in nuclear receptors, hence could be affected by the phone radiation in a similar way.18
Thyroid gland, a most important endocrine gland, is the most susceptible to the effect of electromagnetic wave radiation from cellular phone owing to its superficial location and close distance with the cellular phone when being used.19,20 A systematic review revealed various effects of the radiation on thyroid function and level of serum thyroid stimulating hormone (TSH).21 Collective evidence by another systematic review also suggested changed levels of triiodothyronine (T3), thyroxine (T4), and TSH occurred in groups receiving cell phone radiation.19 Expression of thyroid-related biomolecules and oxidative stress in rats were found to be significantly correlated with mobile phone-originated electromagnetic radiation.22 A study suggested the significant effect of an 1800 MHz electromagnetic field might take place in hypothalamo-pituitary-thyroid axis.23 By using a Swiss albino mice model, a study reported that the 500–900 MHz electromagnetic field was responsible for the significant reduction of serum thyroid stimulating hormone (TSH). Sub-chronic exposure of 915 MHz (8 h/day for 16 weeks) on Male Sprague–Dawley rats was found to not significantly affect serum T3, T4, and TSH levels.24
Different and non-standardized protocols used in investigating the aforementioned effects have made scientist difficult to reach a conclusive finding. Of which, previous studies were unable to exclude the effects of non-ionizing electromagnetic radiation from outside the system. Indeed, a cage modification has been proposed in a study using anechoic chamber allowing whole-body exposure of the electromagnetic field.24 Nonetheless, the possible bias from the external electromagnetic wave influence persists. Therefore, we reported the use of anechoic chamber as a mean to prevent the influence of the external radiation during the investigation.
This study employs a true experimental design by measuring the change of TSH and T4 of the animal subject (Rattus norvegicus) before and after being exposed with mobile phone-originated electromagnetic wave radiation in anechoic chamber. Malondialdehyde (MDA) would also be determined before and after the exposure while nuclear receptor subfamily 3 group C member 1 (NR3C1), monocarboxylate transporter 8 (MCT8), pyruvate kinase, plasma membrane Ca2+ ATPase (PMCA), and thyroid hormone receptor alpha (THRA) would be determined after the exposure. In addition, the histopathological analysis on the thyroid gland will be carried out. All the results would be compared with control group (receiving no radiation exposure). The objective is to reveal the effect of mobile phone-originated electromagnetic wave radiation on thyroid function with minimum influence from the outside environment. The workflow diagram of this study has been presented in Figure 1.
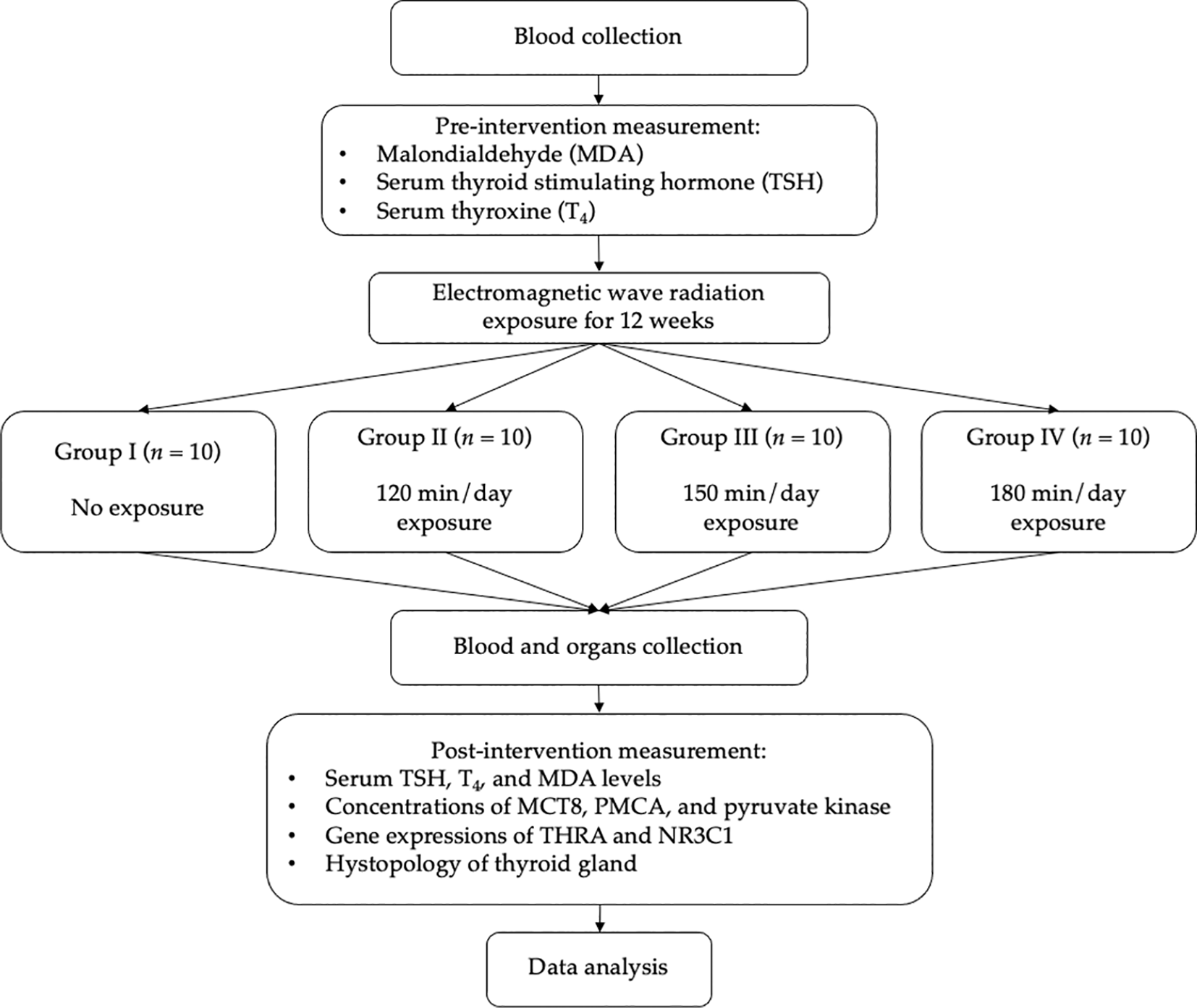
Thyroid stimulating hormone TSH; thyroxine, T4; malondialdehyde, MDA; nuclear receptor subfamily 3 group C member 1, NR3C1; monocarboxylate transporter 8, MCT8; pyruvate kinase, plasma membrane Ca2+ ATPase, PMCA; thyroid hormone receptor alpha, THRA.
• Analytical balance
• TSH and T4 measuring kit (test tube and its rack, micropipette (200 μ), yellow tip, Vidas®)
• Vacutainer red top
• Minor set
• Slide superfrost (Thermoscientific)
• Global System for Mobile Phone (GSM) Sim 900L
• Arduino uno microcontroller
• Real time clock (RTC) module
• Sender—receiver cellular phone module
• Logger data module
• Temperature sensor dht-22
• Spectrum analyzer
Anechoic chamber has been built at Acoustic, Vibration, and Thermal Laboratory, Faculty of Engineering, Universitas Syiah Kuala, Indonesia. Installation of sound-insulating foam panels on the wall results in a chamber with field-free and sound propagation aligned with ‘inverse square law’. Specific construction design, including the access door and ventilation, allows high sound attenuation and free from structure-borne noise. The chamber has 7.8 m length, 6.3 m width, and 5 m height. It is built with two layers of wall and three layers of iron door to minimize the entrance of signal from the outside. Its interior has a dimension of 6 m × 4.5 m × 3.1 m (length, width, and height, respectively), where the airflow is maintained by a controllable ventilation. The chamber has been installed with a lighting system (6 × 80 Watt lamps) with brightness level of 500 lux. Appearance of the anechoic chamber has been presented in Figure 2.
As many as three modules of sensor dht-22 are installed surrounding the chamber interior. The sensor is connected to a microcontroller using cable to record the temperature and humidity. Data obtained in the microcontroller are then recorded and displayed through a computer.
Signal strengths from the Global System for Mobile communication (GSM) in the anechoic chamber are measured with spectrum analyzer (max. 3000 MHz). The schematic diagram for signal strength measurement using spectrum analyzer has been presented in Figure 3.
The signal of radio frequency (RF) in the anechoic chamber has a frequency of 891—893 MHz (GSM and UMTS signals) with Gaussian Minimum-Shift Keying (GMSK) modulation scheme for GSM, Quadrature Phase Shift Keying (QPSK) – UMTS, and 8PSK – EDGE. In the case of GMSK, the signal is smoothed using low-pass Gaussian filter prior to its modulation into signal carrier and proceeding to frequency modulator. As for QPSK and 8PSK, variants of Phase Shift Keying (PSK), the difference is only located at the code rate capacity. The design and photographed images of the GSM module used in this research have been presented in Figure 4.
The signal noise in the anechoic chamber is very low, ranged from -79 dBM to -74 dBM (1.2589 × 10-8 mW). The number was obtained from the determination using spectrum analyzer. Several measurement results of the RF/GSM signal frequencies at distance of 10–50 cm has been presented in Table 1.
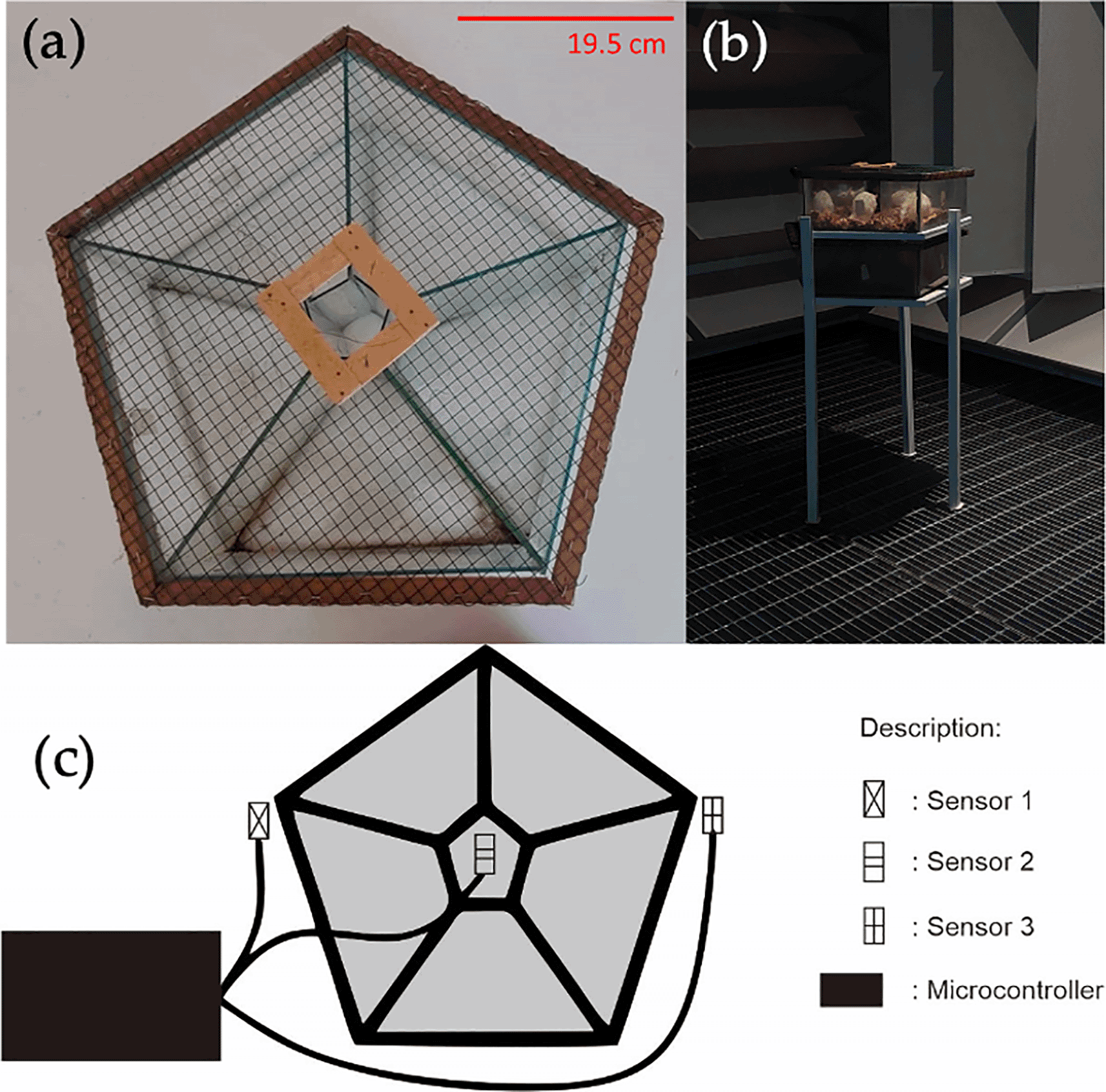
RF applicator, in a form of a cage, has a dimensional size of 33 × 20 × 7 cm3 with pentagonal shape divided into five chambers. The cage is made from glass where wire mesh is used to cover the top for air circulation during experiment. The distance between the RF signal and the cage is maintained at 10–30 cm.
The level of electromagnetic wave radiation is represented by Specific Absorption Rate (SAR), an expression to explain the absorbance rate of the electromagnetic radiation (RF) per time unit per kilogram (W/kg). SAR is varied in different parts of the body. The mathematical expression of SAR has been presented in Equation 1.
Where is electrical conductivity of the body tissue (S/m), is electrical field (V/m), and is the density of body tissue (kg/m3).
In the real world, the value of is varied, considering each rat has differences in their skin, fat, bones, and so on. Herein, the body density of each rat is assumed to be 1000 kg/m3 with = 1 S/m for 2 GHz frequency. The value is applicable for rat with the size of around 20 cm × 4.5 cm × 4.5 cm with an averaged body weight of around 200 g.
To calculate SARS value, it is of importance to calculate the depth of RF signal penetration using the following equation (Equation 2):
Where is the depth of RF penetration (m), is tissue permeability (H/m), and is averaged RF signal frequencies (mW). In the case of RF penetration into throat tissue, the is 1.1 and the averaged uplink frequency for GSM is 9902.5 MHz. Therefore, by using the Equation 2 we obtain the value for = 0.0159 m.
In a GSM 900 system the maximum power is 2 W; hence, the maximum averaged power () is 250 mW (1/8 maximum power). If the averaged distance between the phone antenna and rat () is 10 cm, the radiation density with maximal penetration () could be calculated using Equation 3 resulting in = 1.481 W/m2. Electrical current is expressed by Equation 4 which can be derived to Equation 5 by subtituting . With the skin impedance () value of 59.094 Ohm, the electrical field strength () could be calculated using Equation 5 resulting in = 9.355 V/m. By inserting all the values required in Equation 1, the SAR is obtained to be 0.0962 W/kg.
Simple random sampling is employed to randomly assign the animals into four groups, where each group consists of 10 animals. All subjects are considered having the same conditions prior to the treatment. The group assignment has been presented in Table 2.
The animals, aged two–three months with body weights ranged from 160 to 200 g, are acclimated at room temperature in 12 h dark–12 h light cycle. The animals are fed ad libitum with standardized pellets and water, while being contained in a plastic cage (40 × 25 × 20 cm). The acclimation process lasts for seven days with observation on their behaviors and physical conditions.
Rats in intervention group (Group II–IV) are exposed with mobile phone-derived electromagnetic wave radiation for 120, 150, and 180 min/day, respectively. The radiation was exposed in dark condition using two bidirectionally interacting GSM modules, in which their set-up has been presented in Figure 5. RTC is used to control the time of the interaction. The radiation is produced from the stimulation of GSM multiband 4G LTE signal with a frequency of 1800 MHz (Figure 6). The exposure is carried out for 12 consecutive weeks in an anechoic chamber.
Venous blood is drawn from the tail of each subject which has been mechanically constrained without anesthesia. The tail is heated for vasolidation and better venous visbiility which could be performed by using heating chamber, heating pad, or heating lamp or by immersing in a warm water. Avoid excessive heating; the animal should be under the lamp no longer than three minutes with a distance of at least 30 cm. All equipment should be sterile, and the skin surface shoul be firstly cleaned and dinsinfected with alcohol 70%. Immobilize the non-dominant tail by hand, and rotate ¼ circle to access the lateral tail vein. The needle is aligned with the tail to 30° for the insertion into the distal vein. Fixation is applied slowly and evenly, and the needle is removed after finished. Observation should be carried out continously during the study period on the subjects’ behavior associated with the ongoing pain.
The euthanasia should be carried out by professionals. The rats are physically euthanized by cervical dislocation by applying a firm pressure using the thumb and index finger placed on the posterior skull base and spinal cord. Another hand holds the tail part for a quick backward pulling to dislocate the thoracic vertebrae. Check the respiratory arrest and the absence of heartbeat. Thereafter, the organs are harvested by dissection.
The collected blood sample is stored in an EDTA-containing tube at 2–8°C for maximum 5 days. Blood sample should not be hemolyzed or lipemic. The determinations of serum TSH and T4 were performed on ELISA using commercial kit specialized for Rattus novergicus. Normal ranges for serum TSH and T4 are 0.4–6.0 μIU/mL and 5.0–12.0 μg/dL, respectively. The level of MDA will be measured using ELISA.
Brain tissue, along with the cortex, is collected and rinsed with ice-cold sucrose solution 0.25 M. The tissue is slices and homogenized with 10× operant in a slow setting. The specimen is further treated with Dounce homogenizer as many as 3× operant in a fast speed setting. Dissolution is then carried out to obtain homogenate 6% w/v which is further centrifuged for 10 min at 1.464 × g. The resultant pellet is resuspended in isolating medium and subsequently diluted to yield suspension 6% w/v. As many as 10.4 mL of the suspension is mixed with 1.4 mL Percoll in 15 mL cortex tube and centrifuged for 30 min at 35.540 × g. Two layers are formed on the top side of the type, which are then collected and washed with 5 volumes of sucrose 0.25 M, HEPES-KOH 5 mM, pH 7.1. The obtained pellet is resuspended using the same medium before analyzed using ELISA.
The whole pituitary gland is collected and frozen in insopentana on dry ice. The sample is melted on slide superfrost (Thermoscientific) and stored at –80°C. The expression is determined based on immunohistochemical method using ELISA.
Cryosections are performed on the brain tissue, which is then melted on slide superfrost (Thermoscientific) before undergoing a quick freezing on dry ice and being stored at –80°C. Primary antibodies used for the expression determination are mouse antiβ-tubIII, mouse anticalbindin 1:2000, mouse anti –NeuN 1:500, mouse anti-PV 1:2000. Hybridization is carried out in situ at 25 μm part of the juvenile brain. Riboprobes sense and antisense are synthesized from cDNA RC3 template with spanning nucleotide of 253-486. The expression is based on relative expression in the quantitative reverse transcription polymerase chain reaction (RT-qPCR) analysis.
The cerebral cortex is dissected on an ice-cold glass plate and washed in a buffer (sucrose 0.32 M: EGTA 1 mM: Tris-HCL 10 M; pH 7.4). The sample is sliced and homogenized in the aforementioned buffer (1:20 w/v) with Potter-Elvehjem glass homogenizer. The homogenate is centrifuged 10,000 × g for 15 min at 4°C in a Sorval centrifuge. The obtained mitochondria-free supernatant is collected for the enzymatic activity investigation using ELISA.
As much as 5 mL venous blood is drawn from each sample, treated with EDTA to prevent the coagulation. DNA genome is extracted from the blood using Gentra Puregene Blood Kit following the manufacturer’s manuals. The DNA sample is stored at –20°C until further use. Indirect determination of the gene expression is carried out using RT-qPCR.
A whole thyroid gland is collected from the subject through dissection, and subsequently fixated using formalin and paraffin for 24 h. Incision is performed on the thyroid gland with a length of 5 μm and the staining is carried out using hematoxylin and eosin following the standardized procedure. Assessment on thyroid follicles is performed under a light microscope. The assessment include:
We expect the reduction of serum TSH and T4 levels and histological alterations on the thyroid epithelial cells upon the radiation exposure during the nighttime which was performed until 12 weeks. A systematic review on 22 studies reporting the effects of cell phone radiation against thyroid functions suggested the detrimental effects of the exposure via thermal and non-thermal pathways.19 Exposure of electromagnetic field (900 MHz, 30 min/day for 5 days/week lasted for 4 weeks) was found to significantly decrease serum TSH, T3, and T4 (p<0.01), in a double-controlled study.25 Exposure of RF radiation (900 MHz, 1 h/day for 50 days) was reported to significantly increase serum T4 and decrease T3 level in Syrian golden hamsters.26 Significant decreases in T3 and T4 were observed in chicken embryos and in the newly hatched chicks, but not in older subjects.23 Enlarged and damaged follicular structures of thyroid was reported in a study exposing a 900 MHz pulse-modulated RF radiation on rats for three weeks (20 min/day).27 No significant effect on thyroid secretory functions, however, is reported from a study investigating sub-chronic whole-body exposure of 915 MHz radiation on rat model.24 In conclusion, T3 levels were consistently reported to be decreased in previous studies using animal model,25,26 but not in the case of T4.26 Furthermore, the effect appeared to be different depending the life stage of the subject (such as embryogenesis).23 Among, the aforementioned study, only one study considered the cage modification to allow even distribution of the exposure throughout the subject’s body.24
From this present protocol study, the electromagnetic wave radiation exposure is expected to elevate the plasma MDA as a marker of the oxidation stress. This is as indicator of non-thermal effect of electromagnetic wave radiation. In a previous study, radiofrequency emitted from cellular phone has been reported to induce oxidative DNA damage in a rat model, with significant increase in MDA level.28 Ovarian follicles of mice have been reported to experience oxidative stress induced by cell phone radiation with a significantly elevated level of MDA.29 Since brain is sensitive to electromagnetic wave radiation, we expected that the exposure will reduce the TSH concentration followed by T4. The reduction of T4 activities in the brain will be confirmed by reduction of concentrations of MCT8, pyruvate kinase and PMCA as well as reduction of THRA activities. The reduction of NR3C1 activities will be also measured to prove that there is an additional pathway how electromagnetic wave radiation affects the T4 activities (in circulation or in the brain) apart from thermal and non-thermal pathways. It has been suggested that pathologies of thyroid involve Ca2+ signaling pathways.30 A scoping review has suggested that Ca2+ signaling pathway is disrupted by the cellular phone radiation.31 Increased pyruvate kinase activities are associated with thyroid cancer occurrences.32 These along with dysregulation of NR3C1, MCT8 and THRA expressions are indicators for the carcinogenic effects of the cellular phone radiation.33,34 Finally, we also expected the histology changes of thyroid gland due to thermal or non-thermal effects of electromagnetic wave radiation or reduction of the TSH concentration that potentially have carcinogenic effects.
Results from the previously reported study might be biased from the exposure of other radiations which has polluted the environment. On contrary, we have observed that the anechoic chamber allows the radiation exposure to exclusively originated from the source used during the investigation (by observing the amplitude and frequency of the radiation using spectrum analyzer). Moreover, the anechoic chamber has a significantly lower electromagnetic field as compared to that in the outside. Thus, the study herein would be performed in anechoic chamber preventing the radiation from the outside to influence the investigation. Moreover, our present study would employ 120—180 min/day exposure for 12 consecutive weeks. This exposure duration is relatively longer than reported in previous studies,23,25,26 though it is lower than that in a study investigating the sub-chronic exposure (8 h/day for, 5 day/week, and lasted for 2—16 weeks).24 Taken altogether, this present study could provide strong evidence pertaining to the effect of mobile phone-derived electromagnetic radiation on the thyroid functions.
Conceptualization, H.Z.; methodology, H.Z.; validation, A.R., D.W.S., S.P.S., K.M., H.S. and K.M.; investigation, H.Z.; resources, H.Z.; writing—original draft preparation, H.Z.; writing—review and editing, A.R., D.W.S., S.P.S., K.M., H.S. and K.M.; visualization, H.Z.; supervision, A.R., D.W.S., S.P.S., K.M., H.S. and K.M.; funding acquisition, H.Z. All authors have read and agreed to the published version of the manuscript.
We would like to thank all the staff at the Faculty of Veterinary Medicine and all the staff at the Thermal, Vibration, and Acoustic Laboratory, Universitas Syiah Kuala for their assistance during the study.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the rationale for, and objectives of, the study clearly described?
Yes
Is the study design appropriate for the research question?
Yes
Are sufficient details of the methods provided to allow replication by others?
Yes
Are the datasets clearly presented in a useable and accessible format?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Endocrinology, metabolism, diabetes
Is the rationale for, and objectives of, the study clearly described?
Yes
Is the study design appropriate for the research question?
No
Are sufficient details of the methods provided to allow replication by others?
No
Are the datasets clearly presented in a useable and accessible format?
Not applicable
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Influence of electromagnetic fields, noise, shiftwork on health especially on cardiovascular system and autonomic nervous system, work-related diseases (mainly cardiovascular diseases), evaluation of the fitness to work in drivers, evaluation of work-fatigue, physical fitness
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 09 May 23 |
read | read |
|
Version 1 03 Feb 23 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)