Keywords
Covid-19, Pandemic, SARSCoV2, Health Workers, Risk factors, Occupational health, Sub-Saharan Africa
This article is included in the Emerging Diseases and Outbreaks gateway.
Background: Covid-19 disease disproportionately affected health workers (HWs) by worsening the preexisting shortage of HWs in developing countries , thus overwhelming health systems and disrupting health delivery. However, evidence on the predominant sources of Covid-19 exposure among HWs in sub-Saharan Africa remains scarce. This study sought to identify the occupational risk factors associated with Covid-19 disease among HWs in a tertiary hospital in Kenya. Methods: An unmatched case-control study design was used to assess exposure differences between 39 randomly sampled PCR Covid-19 positive HWs (cases) and 108 conveniently sampled PCR Covid-19 negative and asymptomatic HWs(controls). An adapted WHO risk assessment questionnaire was administered via phone interviews to measure occupational exposure in the last two weeks before each participant’s PCR Covid-19 test between November 2021 and December 2021. Multivariable logistic regression was applied to identify the statistically significant risk factors and the results on adjusted Odds Ratio (aOR) were reported at 95% Confidence Intervals (P<0.05). Results: Controlling for the sex and the clinical status, sub-optimal adherence to face shields ((aOR 4,p<0.05), suboptimal infection prevention control (IPC) adherence in common staff dining rooms (aOR 8,p<0.05), working in medium risk departments (aOR 7,p<0.05) in the last 2 weeks before PCR Covid-19 testing were the significant occupational risk factors to Covid-19 disease among HWs. Conclusions: Reinforcing adherence to facial protective gears together with other personal protective equipment and promoting adherence to infection prevention protocols among HWs in occupational areas with perceived lower risk of infectious disease such as common hospital rooms can reduce the spread of Covid-19 among HWs. Future validation of occupational exposure risk assessment tool across different hospital and health delivery settings can improve comparability and generalizability of findings to inform policies for optimal protection of HWs during SARSCoV2 and similar infectious disease pandemics.
Covid-19, Pandemic, SARSCoV2, Health Workers, Risk factors, Occupational health, Sub-Saharan Africa
Globally, health workers (HWs) were over-represented in Covid-19 cases compared to the general population at 14% of all reported Covid-19 cases.1 HWs, people working within health settings with direct input to improving health, are at a relatively higher risk of contracting Covid-19 disease than the general population.2,3 In Kenya, 2.3% of Covid-19 cases were of HWs with an initial number of 39 Covid-19 related HW deaths recorded.4 But with limited PCR diagnostic testing capacity for Covid-19 disease in low-middle income countries, these figures underestimated the true Covid-19 burden among HWs in Kenya. A serological testing survey reported 20.8% sero-prevalence of Covid-19 disease among HWs (range of 11.5-43.8%); while the sero-prevalence among the general population was shown at 4.3%.5–7 Consequently, the disease itself and measures taken to control it, have been associated with worsening the shortage of health workers (HWs) and reducing the capacity of health systems to handle other pressing health conditions.8 In the face of a pandemic, HW protection is a priority in sustaining the capacity of the health systems to contain Covid-19 disease and other prevalent non-covid-19 disease conditions.
Occupational exposure is work related predisposition to disease and is a significant determiner of disease outcomes such as Covid-19 disease for the workers at the frontline of health service delivery.9–12 During the MERSCoV disease outbreak, occupational and hospital spread accounted for the major source of spread, where HWs accounted for 50% of all nosocomial cases.13 In Spain, 24% of Covid-19 infections were of HWs with 70% of these infections being attributed to occupational exposure sources.14 Breaches in Infection Prevention and Control (IPC) measures such as improper use of face masks, gloves, gowns and goggles (face protective gears and shields) and improper disposal of PPE are among the major sources of occupational exposure to Covid-19 disease among HWs.15,16 Disinfection of hospital surfaces has also been cited as a key infection control measure against surface droplets and disease spread through fomites.17 A study utilizing data from hospitals in Qatar observed that hospital unit specialization status such as a Covid-19 designated facility (workers directly care for Covid-19 patients) does not necessarily translate into a higher risk of contracting the disease among the HWs.18 The PCR test for Covid-19 is the recommended gold-standard viral genetic markers as a guide for containment measures among infected asymptomatic and symptomatic patients.19,20 However, there is an occupational risk of Covid-19 exposure related to insufficient and imperfect PCR tests for Covid-19 disease among patients and among HWs especially in low resource settings.21 Imperfection of PCR tests due to imperfect sensitivity (range from 63% to 78%20) for Covid-19 disease has been implicated in the spread of Covid-19 disease to HWs due to misclassification of the actual Covid-19 cases within the healthcare settings.20,22 Furthermore, the occupational sources of Covid-19 exposure to HWs have been characterized as higher viral load exposures due to multiple and frequent infectious clinical procedures compared to non-occupational exposure sources. Therefore, HWs are postulated to develop a severer form of the Covid-19 disease compared to the general population.9,23 Protection of HWs from spread of Covid-19 disease and related complications is hinged on safe health systems and working environments.24 Sufficient supply of personal protective equipment (PPE) is protective against occupational Covid-19 and infectious disease exposure.25 Wearing of face masks, hand hygiene measures, and social distancing measures have been recommended as protective measures against Covid-19 disease among HWs.13,19
Despite the growing evidence on the occupational risk factors to Covid-19 disease among HWs, contextual evidence on predominant sources of Covid-19 exposure among HWs remains scant.23 For example, the Kenyan policy measures to contain and prevent the transmission of Covid-19 are based primarily on WHO guidelines which include sustained supply of PPE for frontline workers, reinforcing adherence to optimal PPE use, reorganization of health workforce and hospitals in response to Covid-19, and HWs’ prioritization in the PCR Covid-19 testing and Covid-19 vaccination.26–28 In Kenya, the HWs, the elderly, the essential service providers such as teachers and people with preexisting medical conditions such as heart diseases were prioritized in the Covid-19 vaccination program of the Ministry of Health, with 68.2% of the Kenyan HWs having been fully vaccinated against Covid-19 disease as of 18th August 2021. In addition the Ministry of Health (MOH) Kenya adopted the WHO’s definition of health workers as listed in the WHO's Interim guidelines for Human Resources for Health during the Covid-19 response.27 While the risk of Covid-19 mortality in the Kenyan population has been traced, specific Covid-19 exposure risk profile for HWs in the Kenyan hospital settings has not been traced and thus unknown.29 Therefore, the main known occupational risk factors for the Covid-19 disease among HWs such as non-adherence to optimal PPE use and other IPC measures have not been confirmed within Kenyan hospital settings.18 This study sought to identify occupational risk factors associated with Covid-19 disease among HWs at a Kenyan tertiary referral hospital.
The occupation of a health worker, defined by their clinical roles, is a known risk factor for contracting diseases related to respiratory pathogens such as SARS viruses.11 Schulte et al.’s framework on personal and occupational risk factors to disease outcomes was adapted to hypothesize the relationship between potential Covid-19 exposure risk factors based on the review of literature and the Covid-19 disease status among HWs as shown in Figure 1 below. Other factors that potentially confound the occupational exposure factors and the Covid-19 disease outcome among HWs were considered to guide the variables of measurement for the study (Table 7).
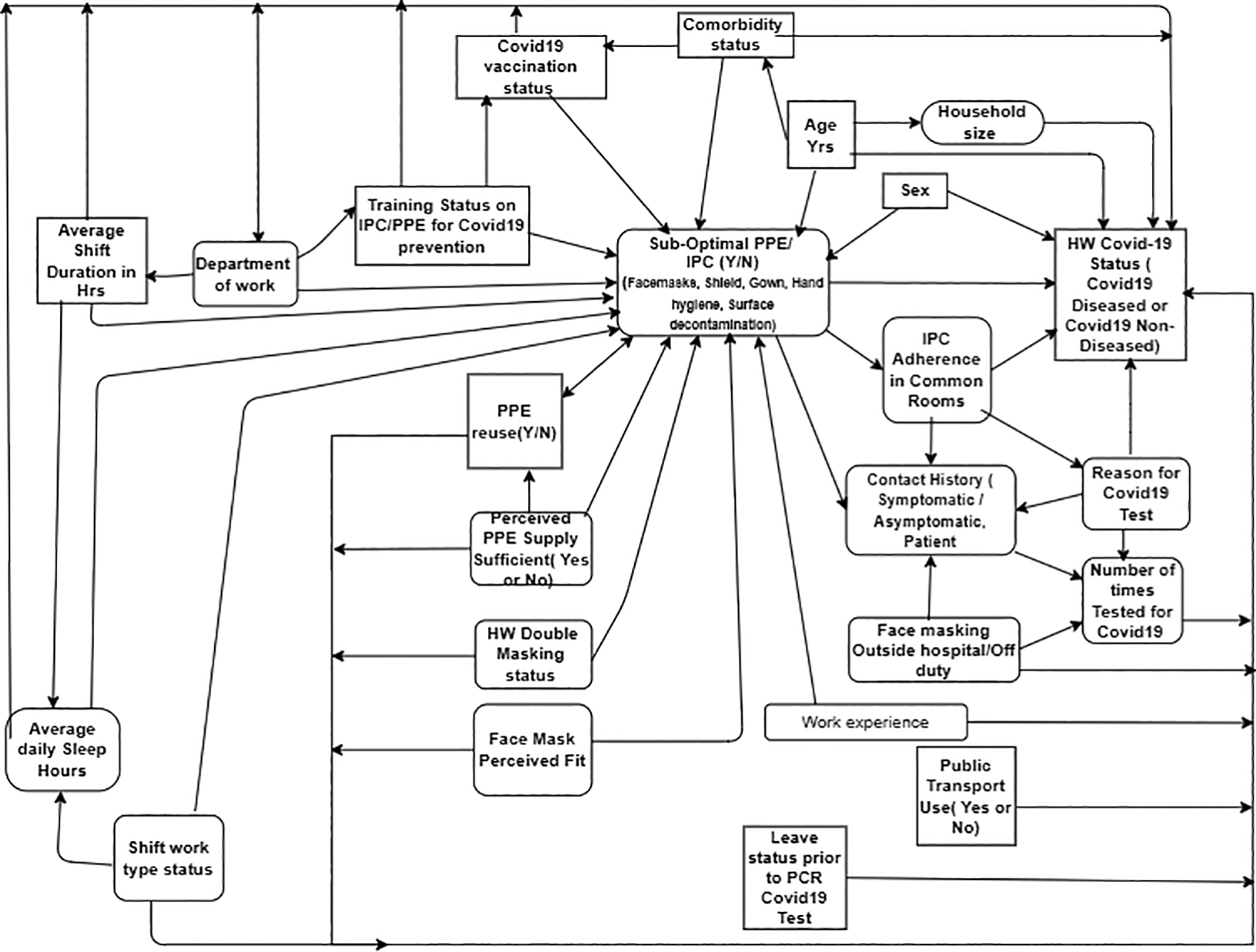
As per the conceptual framework in Figure 1, suboptimal PPE and IPC adherence use was hypothesized as the primary exposure of interest which together with other risk factors such as improper PPE reuse, perceived sufficiency of PPE supply and the fit of face masks have been implicated in literature as sources of occupational and nosocomial Covid-19 exposure to HWs.30–32 Proper use of PPE is an important for the safety of HWs during an outbreak of highly infectious respiratory pathogens such as SARSCoV, MERSCoV, and Covid-19 disease-causing SARSCoV2 virus.33 Optimal PPE use and compliance rank at the base of the Hierarchy of controls for infectious diseases in the health care setting34,35 which means that optimal PPE use protects HWs when all other levels of controls fail to guard workers against occupational viral pathogens such as SARSCoV2 virus.36 A systemic review grouped risk factors to Covid-19 disease among HWs into lack of PPE, exposure to infected patients, work overload, poor adherence to infection control measures and pre-existing medical conditions.37 The global shortage of PPE as well as atypical clinical SARSCoV infection presentation were previously cited to pronounce occupational exposure sources of Covid-19 disease among HWs38 as documented in previous SARSCoV outbreak.39 Adherence to face masks, by both the infected and infectious person as the source and the HW as a susceptible host has been underscored in literature as additively protective against Covid-19 disease.40 Unit designation or the department of work or Covid-19 designation, has also been cited as a significant contributor to Covid-19 positivity among the HWs in addition to the long duration of duty hours and sub-optimal hand hygiene.41,42
Developing countries such as Kenya reportedly experienced severe shortage of PPE supplies such as face masks43 during the acute phase of the pandemic. However, knowledge on the effect of PPE shortage on Kenyan HWs remains scant and PPE shortage coping mechanisms such as reuse of PPE has not been scientifically studied or documented based on the Kenyan hospital settings hence its inclusion in the conceptual framework.44 PPE reuse has been cited to offer inferior protection for SARSCoV viruses although it has also been cited that reused masks are better than none at all.32 Another literature cites that PPE reuse is a feasible and safe strategy that can be used in helping to slow the spread of Covid-19 disease but this has to be done under specific sterilization autoclaving conditions.45 Evidence supporting the decontamination of face masks for reuse cite that use of ultraviolet germicidal irradiation and vaporized hydrogen peroxide is most advantageous.46
In line with the WHO recommendations on the need to provide HWs with PPE according to their risk profiles, clinical and non-clinical hospital staff categories have been utilized in health planning on pandemic response and research.47,48 The current study adopted a broader definition of HWs that includes clinical and non-clinical staff. HWs were thus defined as people employed and working in the Kenyatta National Hospital within the two-month study period whose primary role was the provision of health care services either directly to the patients or indirectly through assistive roles to HWs in line with Kenya Human Resource for Health (HRH) norms and standards 2014 report which enlists staff, clinical and non-clinical workers, required for delivery of health.49 Consequently, direct care providers and clinical staff (nurses, midwives, pharmacists, medical doctors and specialists) and indirect care providers, non-clinical and supportive staff (cleaners, porters, security, and departmental administrative staffs such as unit and hospital managers, health information officers and hospital clerks) were studied. This contrasts the narrower definition of health workers as professionals trained in approved institutions and licensed to practice by cadre-specific and respective regulatory bodies to deliver health care following the Kenya Health Workforce Report 201550 which would exclude workers in supportive, unregulated and unlicensed hospital roles.
Previously, HWs engaged in night shift work (NSW) schedules were found to have a higher prevalence of common cold, flu-like illnesses, and gastroenteritis compared to day shift workers.51,52 Literature exists on various mechanisms by which shift work weakens worker immunity states through a reduction in immune cells such as natural killer cells, CD16, and Inter-luekin 2 factors which are responsible for fighting viral pathogens. In addition, NSW is associated with abnormal cytokine levels.53 NSW is also a direct modulator of the physiology of the immune systems and through circadian rhythm deregulation, it has an indirect effect on individual health status. For example, a systematic and meta-analytic review by Liu et al. 2018 established that night shift work increases the workers’ risk of being overweight and obese.54 Both being overweight and obese have been strongly associated with Covid-19 positivity and severe forms of Covid-19 pneumonia.55,56 Further evidence links sleep habits and the risk of Covid-19 disease among HWs.57,58 Knowledge on the role of shift work, NSW status and HWs’ sleep habits as occupational and personal risk factors to Covid-19 disease remains incomplete.
The role of staff to staff Covid-19 exposure source among HWs is emerging as observed and reported in a cross-sectional study examining characteristics of HWs with Covid-19 disease in a tertiary public health hospital.59 Authors observe that non-significant HW’s occupational type, the peaking of Covid-19 cases among HWs before the peak among Covid-19 disease patients and admissions, and few clustering of Covid-19 among the HWs within certain departments, were suggestive of the predominance of HW to HW spread of the disease rather than the patient to HW spread.59 Recent studies on Covid-19 among HWs have also observed that most HWs contracted Covid-19 disease from a non-index case that further supports the predominance of the Covid-19 transmission through staff-to-staff, and surface contamination methods.57 The HW to HW spread of Covid-19 may be propagated by common tea and dining rooms breaks where HWs socialize and take meals together while lowering their guard against Covid-19 disease. Hence, more knowledge is needed on common rooms as sources of Covid-19 exposure in occupational settings.
Insufficient HWs’ training on IPC and PPE use has also been implicated in the spread of Covid-19 disease among HWs.60,61 Less than two hours of training or no training on IPC was associated with a SARS infection among HWs as reported in previous SARSCoV outbreaks.36
This review of literature informed the variables of interest and their relationships as illustrated in the conceptual framework (Figure 1) which further informed the methods of the study in assessing occupational exposure for Covid-19 within the Kenyan hospital setting as detailed in the next section.
A case-control study design was used. The study was conducted at Kenyatta National Hospital (KNH), the largest public tertiary referral and teaching hospital in Kenya with over 6,000 workers and a bed capacity of 1,800.62 The hospital offers specialized health services through its various specialties including accident and emergency unit, specialized outpatient clinics, medical and surgical inpatient units, infectious disease units.
The study population were the health workers (HWs) in full time employment at KNH who were involved in the direct or indirect care of patients with Covid-19 disease or patients with unknown status of Covid-19 disease during the study period between November 2021 and December 2021. This study was conducted just after the peak of the fourth wave of Covid-19 outbreak experienced in Kenya based on the updates from the MOH Kenya.63 Covid-19 testing records at the Covid-19 Testing Unit of KNH were reviewed to identify the confirmed cases of Covid-19 disease among HWs employed and working at Kenyatta National Hospital within the 2 months study period.
Sample size determination was done by application of Kelsey's (1996) sample size formula for case control study design as follows:
Of the 160 (40 cases and 120 controls) HWs desired from the sample size formula, a total sample of 147 health workers (39 cases and 108 controls) were recruited. 39 cases with Covid-19 diagnoses were selected through simple random sampling from the RT-PCR laboratory testing records between November and December 2021 and invited to participate in the study. To select controls, matching information on Covid-19 testing dates and cadre for cases was used to identify and conveniently invite 3 potential controls from the Covid-19 database and as referrals from each case in the study as shown in the sampling procedure diagram (Figure 2). Further screening of the HWs in the control group for eligibility was done by use of a screening tool (appendix 6.2) that examined whether a HW had 3 or more symptoms related to Covid-19 and if they had any imaging findings or medical reports suggestive of Covid-19 disease before their negative PCR Covid-19 test results within the study period.
Health workers with a laboratory-confirmed Covid-19 disease, based on RT-PCR SARSCoV2 testing results as per the MOH Kenya case definition for Covid-19 disease, were selected as the cases for the study. Controls were defined as a health workers exposed to the same department of work as a case, PCR tested for Covid-19 but not classified as a suspected or probable, or confirmed Covid-19 case within the last 2 weeks before the Covid-19 test results in the study period between November 2021 and December 2021. The inclusion criteria for controls entailed a HW who reported not having experienced 3 or more symptoms suggestive of Covid-19 pneumonia in the last 2 weeks before Covid-19 test results (either fever and cough of acute onset or any three of these symptoms: Fever, difficulty in breathing, general weakness/fatigue, headache, myalgia, sore throat, coryza, dyspnea, anorexia/nausea/vomiting, diarrhea, altered mental status), and the HW was not classified as either a suspected, probable, or confirmed Covid-19 case in the last two weeks before receiving Covid-19 test results (tested for Covid-19 in the study period with negative test results for SARSCoV2 virus, and did not have any chest X-ray or High-resolution CT scan imaging reports suggestive of Covid-19 pneumonia or disease).64 The exclusion criteria for controls included any HW not employed and not working at KNH within the study period, reporting having had either any positive PCR Covid-19 test results within the study period, or had experienced three or more symptoms related to Covid-19 symptoms as per MOH case definition (fever, breathing difficulty, cough, headache, and chills) or had imaging findings suggestive of Covid-19 pneumonia in the last 2 weeks before Covid-19 testing. The screening tool for the study (Table 4) controls was applied to restrict the inclusion of Covid-19 asymptomatic HWs during PCR testing as study controls and exclude symptomatic HWs to minimize misclassification bias for selection of controls (who may have had sysmtomatic and with false negative SARSCoV2 test results).
Study participants were interviewed via phone, using a structured questionnaire adapted from the WHO Interim guidance (2020) on Risk assessment and management of exposure of health care workers in the context of Covid-19,65 Fatima et al. (2021) study tool to assess night shift status and its association with Covid-19 Infection among HWs58 and a case-control study tool by Celebi et al. (2020) utilized to assess the spread of Covid-19 among HWs in common dining rooms.66 The questionnaire can be found under the section on Extended data.85
Key variables in the study questionnaire on occupational factors included self-reported frequency of PPE adherence, and usage of each PPE including gloves, N95 masks, surgical masks, gown, surface decontamination practice, face shield, moments of hand hygiene measures, and history of the accidental splash of body fluids 2 weeks before PCR testing and attributable type of exposure to Covid-19 case or suspect case 2 weeks before Covid-19 PCR testing, the performance of aerosol-generating procedures 2 weeks before Covid-19 PCR testing, specific department of work within 1 month before Covid-19 PCR testing and self-reported adherence to PPE and IPC protocol in staff dining room in the last weeks before Covid-19 PCR testing (Table 7).
Ethical study approval was obtained from Kenyatta National Hospital-University of Nairobi (KNH-UON) Ethics and Research Committee (P462/06/2021). Administrative permission was obtained from the KNH hospital to access laboratory Covid-19 testing records. Physical contacts were avoided to minimize chances of Covid-19 transmission, therefore, interviewer-administered written informed consent was sought virtually and consent recorded on a printed informed consent form and later de-identified once the interview process was over. Identifying contact information of the participants were password protected and used only for the purposes of the current study.
Data was entered into an excel spreadsheet. After double checking for errors, completeness, and accuracy of the data, the excel sheet was exported into R studio version 4.1.2 (2021-11-01) for statistical analysis. Descriptive analysis of the data was reported in form of counts, percentages, and proportions for categorical variables. Categorical variables were derived from the primary data to form aggregate variables including overall PPE/IPC adherence, HW to HW IPC adherence in common rooms, HWs’ clinical status, and departmental risk type categories.
Clinical and non-clinical staff categories were derived to cover HWs who were directly and indirectly involved in provision of care to the patients in the hospital (Table 6). These categories capture all the hospital workers as per the norms and standards for HRH in Kenya.49 Departmental risk categories namely, high risk, medium risk, and low-risk- were adapted from the U.S. Occupational Safety and Health Act (OSHA) Guidance on Preparing Workplaces for Covid-19 and the recent studies assessing Covid-19 exposure risk factors among HWs59,30,67 (Table 5). For this study, the definition for high-risk departments were defined as hospital units that routinely perform high-risk procedures that generate aerosols such as dental units, intensive care units and high dependency unit, accident and emergency units, and any Covid-19 designated units such as infectious disease units (IDU), Covid-19 isolation wards and Covid-19 designated medical wards.30 Medium risk departments were defined as hospital units where staff have frequent contact with patients without confirmed or suspected Covid-19 disease and those with unknown and unspecified Covid-19 status including outpatient medical and surgical clinics, general medical and surgical wards, radiology departments, and maternity. Low-risk departments were defined as units without any contact with patients regardless of Covid-19 status and have minimal contact with patients’ immediate environment such as the administrative and catering departments.
Overall adherence to optimal PPE and IPC measures was assessed using the 13 item criteria for PPE and IPC measures (Table 8). This is supported by hospital guidelines which do not mandate PPE adherence and usage the same way for a clinical staff and for a non-clinical staff especially when PPE supply is constrained.5 Therefore, criteria for adherence was applied to clinical and non-clinical staff differently whereby failure to check all the category-specific PPE requirements was classified as either overall sub-optimal adherence or overall optimal adherence.65 Frequency of specific PPE use was assessed in four levels, namely, always as recommended (>90% of the time), mostly (>50% of the time), occasionally (20% to under 50% of the time), and rarely (<20% of the time).68 HWs who reported having regularly practiced social distancing in the shared dining rooms and having completely doffed before using shared dining rooms in the last 2 weeks before the Covid-19 test conducted within the study period, were categorized to have adhered to IPC in staff dining or common rooms.
The odds ratio OR were reported at 95% confidence intervals and at an alpha of 0.05 (p-value) for statistical significance. Wald’s Odds ratio test for the bivariate analysis in the cross-tabulation of the independent and dependent variables was applied to compute the p-value for variables whose observations had no more than 20% of the counts with less than 5 counts. Otherwise, Fisher’s exact test was applied. Shapiro Wilk test was used to test for normality of the continuous variable (age) in the data set. Under the bivariable analysis, each explanatory study variable was cross-tabulated with the Covid-19 disease status of the HW and the corresponding Odds Ratio (OR) at 95% Confidence Intervals were reported and tabulated.
In the multivariable analysis, a liberal p-value of less than 0.25 was chosen as the cut-off for selecting multiple variables to enter into the multivariable logistic regression model to control for confounding.69 Additional independent variables hypothesized to be important confounders in the association between the overall sub-optimal PPE/IPC adherence and the Covid-19 status were added a priori in the multivariable analysis even if they did not attain the 0.25 cut-off for liberal variable selection due to their clinical importance in occupational disease transmission as per the review of literature. These included the sex, overall adherence to optimal PPE/IPC use, department risk type, and the clinical status category of the health worker. The Akaike Information Criteria (AIC) was used to run the modeling simulations of the logistic regression analysis to arrive at the most statistically significant combination of the independent variables that majorly accounted for the variation in the dependent variable among the participant HWs.
Additional hierarchical models to re-introduce variables de-selected by AIC simulation were applied to assess for their impact in the overall model. Likelihood ratio tests were applied for each model to determine the model with the best fit and the model with the least residual deviance which was taken as the most accurate and best fit for the data. A p-value of less than 0.05 was taken to indicate that the fitted model was significantly different from the null model. The effect size was computed by the use of the pseudo R2 estimates by the Hosmer and Lemeshow, NegelKerke, and McFadden pseudo R2 estimation methods. Interaction terms were also fitted under hierarchical models to assess for significant interaction (p<0.05). An interaction term was applied to assess the effect of gender on the association between department risk type and Covid-19 disease status based on previous evidence of interaction between gender and the effect of department type and Covid-19 disease status.30 Variance Inflation Factor (VIF) was applied to detect any multi-collinearity between the explanatory variables in the fitted model with a VIF of less than 4 indicating a lack of collinearity among the explanatory variables in the model.70
A total of 147 health workers (39 cases and 108 controls) consented to participate in the study. Overall, 55% of the participants were female with a median age of 35 years (cases) and 37 years (controls) respectively. A slight majority (51%) had professional work experience greater than 10 years, with most (71%) being clinical health providers with direct patient interaction. A significant majority were fully or partially vaccinated against Covid-19 disease (34/39 cases) and (102/108 controls). By co-morbidity status, 74% of the study participants did not have any preexisting medical condition, while 26% reported having a pre-existing condition including hypertension asthma, diabetes mellitus, heart disease, obesity, chronic kidney disease, and cancer (Table 1). The full dataset can be found under Underlying data.85
Self-reported adherence to PPE including gloves, face masks and N95 mask or equivalents, gowns, face shields and hand hygiene measures was assessed. The crude odds ratio of contracting Covid-19 were 2.57 times higher for HWs who self-reported not adhering to gloves as recommended (cOR, 1.45; 95%CI, 1.11-5.67; p<0.05), 3.5 times higher for not adhering to gown as recommended (cOR, 3.50; 95%CI, 1.35-9.06; p<0.05) and 3.5 times higher for not adhering to face shields as recommended (cOR, 3.50; 95%CI, 1.37-8.36; p<0.05). Self-reported reuse of the PPE within-shift (cOR, 1.86; 95%CI, 0.89-3.90; p>0.05) and between shifts (cOR, 3.00; 95%CI, 0.70-12.50; p>0.05) were not significantly associated with increased risk of Covid-19 disease.
The WHO five moments of hand hygiene including hand hygiene before and after touching a patient (cOR,1.103,95%CI 0.48-3.65, p>0.05), hand hygiene before and after a procedure (cOR,0.88;95%CI 0.41-1.92; p>0.05), hand hygiene after exposure to body fluids (cOR,1.14;95%CI 0.45-2.84, p>0.05) and hand hygiene after touching patients’ surroundings (cOR,0.70;95% CI 0.30-1.54; p>0.05) did not differ between the cases and the controls. There was no difference in the use of surgical or medical masks (cOR, 1.72; 95%CI 0.39-7.50; p>0.05) and N95 masks (cOR, 1.23; 95%CI 0.50-3.00; p>0.05) between cases and controls. Self-reported practice of surface decontamination as recommended did not differ between cases and controls (cOR, 1.87; 95%CI 0.90-4.22; p>0.05) (Table 2).
In the multivariable analysis, overall suboptimal PPE/IPC adherence variable (primary exposure of interest) was excluded by the AIC model simulations. To eliminate collinearity effects, the independent variable on suboptimal PPE/IPC use was not reintroduced in the model owing to its relatedness with specific PPE items such as face shields and gloves. None of the socio-demographic characteristics had any statistically significant association with the Covid-19 disease status of the HW. However, the effect of sex of the HW was impactful in the overall model as a confounder whose statistical control improved the accuracy of the overall model (Table 3). Occupational factors identified to be associated with Covid-19 disease after controlling for covariates in model 1 of the AIC simulation included lack of optimal use of gloves (aOR,4.80; 95%CI, 1.28-20.10; p<0.05), face shield (aOR,3.88; 95%CI, 1.28-13.37; p<0.05) and non-adherence to IPC protocol while using tea-breakout rooms (aOR,7.65; 95%CI 1.54-66.51; p<0.05), working in a medium risk department (aOR,4.38; 95%CI,1.22-18.40; p<0.05) in the last 2 weeks before PCR Covid-19 testing (Table 5). After re-introducing the clinical status in the model to control for the differences in clinical and non-clinical staff categories, statistical significance of suboptimal use of gloves was lost (p>0.05) as shown in model 2 (Table 3).
| Questions on eligibility for study among Covid-19 negative HWs | Response |
|---|---|
| Yes☐No☐ |
| Yes☐No☐ | |
| Yes☐No☐ | |
| Yes☐No ☐ | |
| Yes☐No☐ | |
| If all questions are checked YES, then the participant is eligible for participation in the control study group. | |
| HW Participant Eligibility Yes☐ No ☐ |
| Objective: Occupational Exposure factors | ||
| Department of work (categorical) | High risk, Medium risk and Low risk | |
| Suboptimal PPE use/IPC adherence (Nominal) |
| |
| Double masking status (categorical) | ||
| Exposure symptomatic status (categorical) | ||
| Perceived PPE supply (categorical) |
| |
| Work-time/Duty Hour (categorical) | ||
| Shift work status (Categorical) | ||
| PPE Reuse status (Categorical) | Assessed whether HW re-used PPE in the two week prior to getting Covid-19 test results while attending to suspected, confirmed Covid-19 patients or patients with unknown Covid-19 status. PPE re-use status rated both in a | |
| Adherence to IPC protocol in common/staff dining rooms (categorical) | Derived through two variables in the study questionnaire, namely, self-reported regular social distancing and complete doffing before using staff dining rooms in last 2 weeks before Covid-19 testing during the study period. A HW who reports always in both variables is considered to have adhered to IPC in staff dining rooms. A HW who self-reported using staffing dining room without doffing PPE used in patient care and without observing social distancing eating or talking to colleagues was classified as “Non-adherent status” to Covid-19 IPC protocol in staff dining rooms. Another category of a health worker reporting not using staff dining room was categorized as neither adherent nor non-adherent. | |
| Outcome/Response Variable of Interest | ||
| Health worker Covid-19 status(categorical variable) | The status was either a case, for HWs with Covid-19 PCR positive results or a control who was a HW with negative Covid-19 PCR test results without 3 or more Covid-19 symptoms or Covid-19 imaging findings within the 2 weeks prior to Covid-19 test. The narrow definition of the controls for this study is in the light of likelihood of false negative results with RT-PCR Covid-19 tests from its low sensitivity 63 to 75 % established in literature.84 | |
There was negligible collinearity (VIF <2) between the predictors in the final multivariable logistic model (model 2 in Table 3). In addition, the final model had significant goodness of fit (p<0.05) and had an explicative value estimate of 36.5% (Hosmer-Lemeshow, pseudo R2), 34.5% (Cox and Snell pseudo-R2) 37% (McFadden pseudo R2), and 50.3% (NagelKerke pseudo R2) on the variation in the outcome (Table 3).
Occupational factors observed to increase the risk for a positive PCR Covid-19 test results included failure to adhere to face shields as recommended when handling patients, working in medium risk department compared to a low-risk department, self-reported non-adherence to infection prevention protocols while in the common staff dinning rooms in the last two weeks before PCR Covid-19 test.
Failure to use specific PPE including face shields always as recommended was observed to significantly increase the odds of a health worker having Covid-19 disease by 4 folds. The key finding on use of face shields puts emphasis on the importance of protecting mucous membranes including the mouth and the eyes while caring for patients during an infectious disease outbreak similar to Covid-19 (from SARSCoV2 viral transmission) regardless of patient’s disease status as corroborated by a recent retrospective cohort study on occupational Covid-19 exposure among health care professionals.71 The authors cite that omission of face protection while attending to patients not suspected of Covid-19 disease was associated with Covid-19 disease positivity.71 Further simulation experiments have established the efficacy and protectiveness of using face shields for face and eye protection from droplet and aerosol contamination.72 Our findings support earlier recommendations by the WHO recommendations on the use of face shields as an adjunct protective PPE.73
Controlling for clinical status, failure to wear gloves as recommended did not significantly increase the risk of Covid-19. Use of gloves in addition to hand hygiene measures to prevent excess contamination with infectious viral droplets in case of SARS and novel acute respiratory infections (ARI) is highly recommended by the WHO.73 The WHO recommends that gloves should be discarded after use and followed by hand hygiene at all times. Therefore, the usage of gloves alone is not a substitute for hand hygiene but the use of gloves together with hand hygiene is recommended since gloving prevents excessive contamination and protects the non-intact skin from infectious pathogens.33 Contrary to our findings on lack of significance of gloves usage on the risk of Covid-19 disease , a recent case-control study observed a greater risk of Covid-19 disease among health workers who always used face shields, surgical caps, and gloves.74 These findings were attributed to the possibility of self-contamination from the continuous use of the face shields and gloves without proper donning and doffing measures.74 Despite the similarity in the broad definition of health workers in our study and the study by Rodriguez-Lopez et al.,74 we posit that our findings differ because our study controlled for the effect of clinical status on the association between adherence to gloves as recommended and risk of Covid19 disease. Clinical and non-clinical workers have different requirements and recommendations on the usage of gloves and other PPE as observed in the study setting (Table 8). Future research should confirm our findings and explore risk of infectious disease from self-contamination from improper use of gloves and face shields.
Overall, the 8% self-reported overall level of compliance with optimal PPE use and IPC adherence (PPE included gloves, masks, gown, and face shield while IPC measures included adhering to PPE protocol use and hand hygiene measures) in our study grossly differs from recent studies with higher self-reported compliance levels of 88% for hand hygiene and 90% for PPE use reported in one study75 and a 76% self-reported compliance level on PPE use among the controls for the study in another study.76 Similar to our study findings, inadequate IPC compliance at 6% during the pre-pandemic period31 and an 18.1% self-reported compliance level by the health workers have been previously reported.77 Lower PPE and IPC compliance levels have been attributed to stricter and more objective compliance and adherence observation assessment methods as compared to levels based on subjective and more biased self-reported PPE/IPC compliance assessment methods. We posit that lower overall levels of self-reported adherence to PPE/IPC measures in our study was attrubutable to the study period,75 whereby, data collection was done when Covid-19 infections rates had waned in the study setting and coincided with progressive easing and relaxation of public health policy mandates on PPE/IPC compliance and other restrictive Covid-19 prevention protocols.4 In addition, we posit that a higher coverage of Covid-19 vaccination among the participant health workers during the study period could have impacted their compliance to Covid-19 IPC measures and PPE use whereby HWs become less cautious and less afraid to contract the disease owing to assured forms of vaccination immunity compared to the acute phases of the pandemic when there weren’t any vaccines available and compliance level to PPE were shown to be higher.75,76
The 4 times higher odds ratio of Covid-19 disease among HWs with suboptimal PPE/ IPC adherence in our study was due to chance (OR,4; p>0.05) unlike a statistically significant similar magnitude of risk (P<0.05) reported previously74 and a 29% (CI, 16% to 41%) reduced risk ratio for Covid-19 disease among HWs adequately using the PPE from a meta-analysis of three recent studies.78 Lack of statistical significance for the suboptimal PPE/IPC adherence variable in our study could be related to the use of contextualized and adapted tool for assessing overall PPE/IPC adherence compared to the previous studies. In the absence of a standard definition or criteria of what constitutes a proper PPE for a given task, cadre and per given hospital, adapting and context-tailoring the WHO Risk Assessment and Exposure protocol as well as tools used for the same objective from other studies is supported in literature.65,79,78 While adapting the tool to fit the context improved the validity of our findings in the current study population and hospital setting, caution is warranted when comparing the study findings across different hospital settings. Future research in standardizing and validating the study tools for assessing overall PPE/IPC adherence across common and diverse settings would support generalizability of findings on exposure factors and has been recommended in previous literature.78,79
Besides the PPE and IPC adherence, other occupational risk factors observed in the current study to significantly impact on the risk of Covid-19 disease among HWs included the department type and HW’s adherence to IPC measures while using common and shared dining rooms. HWs working in medium and high-risk departments had their odds of Covid-19 disease multiplied by 4.4 times (95%CI, 1.22-18.40; p<0.05) and 2.4 times (95%CI, 0.46-12.87; p>0.05, not significant) compared to HWs from low-risk departments respectively. These findings are consistent with previous literature from a retrospective cohort study that observed a significantly higher risk of Covid-19 disease for HWs working in high-risk departments compared to low-risk departments albeit the presence of statistical interaction of effects of being male, the clinical status of the HW and hand hygiene adherence.30 However, statistical interaction on levels of departmental risk with other covariates and the study outcome was not significant, a phenomenon partly attributed to lower power in the present study. In line with OSHA's definition of occupational risks, low-risk departments were defined as distinct physical units of the hospital without direct patient involvement and with very minimal patient interaction including the administrative offices, kitchen, and catering and communication departments. Medium-risk departments were defined as hospital units handling a patient with less severe illnesses and unspecified respiratory illnesses including outpatient medical and surgical clinics, general medical and surgical wards, radiology departments, and maternity while high-risk departments were defined as unit handling severely sick patients, those with a confirmed diagnosis of Covid-19 and departments with a higher frequency of aerosol-generating procedures including ICU, HDU, Covid-19 designated wards and accident, and emergency. Consistent with current study, a recent case-control study showed that HWs in nonCovid-19 designated unit had higher infections rates than those in Covid-19 designated units (57% vs. 43%)80 while on the contrary, a multicenter study found no association between cadres and departmental type categories and risk of Covid-19 disease.40 In addition, Dev et al. cites the cadre of the health worker as an independent risk factor for Covid-19 disease,80 however, the differences in the odds of Covid-19 disease between the cadres were not examined in the current study since the cadre category was utilized in matching the controls to the cases for referrals thus any risk differential effect according to cadre was eliminated at design or is to be regarded as an outlier observation.
While controlling for covariates, HWs who had a self-reported failure to socio-distance and failure to completely doff their PPE before using common rooms for dining had 7.7 times (95%CI 1.54-66.51; p<0.05) higher odds of Covid-19 disease than those who social distanced and completely doffed before using common rooms within the last 2 weeks before having their Covid-19 test. However, a further category of HWs who never used hospital common rooms before their PCR Covid-19 did not have significantly different odds across cases and controls (95%CI 0.64-49.56; p>0.05). Consistent with a prospective study examining PPE use among HWs during social interactions in common rooms, 19% of the HWs with positive Covid-19 diagnosis had a self-reported removal of face masks during tea breaks and lunch breaks with their colleagues despite the universal masking protocol in the hospital.81 Another recent retrospective cohort study further authenticates the findings of our study given the observation that health worker to health worker Covid-19 transmission was reportedly dominant and was reflected in the pattern of Covid-19 infections clustering within one profession and within a few hospital departments while the peak of Covid-19 cases among HWs peaked before the peak in the cases presenting from outside the hospital.59 Ibiebele et al.’s study also cites the predominance of exposure of HWs from their coworkers during dining rather than from the patients.71 These findings underscore the need for IPC sensitive and friendly common dining and shared rooms for HWs as well as the need for behavior change among HWs and the need for hospital employees to maintain physical distancing when dining and to adhere to other infection prevention protocols such as proper PPE disposal and appropriate doning and doffing especially when moving from patient surroundings to common staff rooms in the event of a resurgence of Covid-19 or any other similar infectious outbreaks.
This study has several strengths. First, an adaptation and context-tailoring of WHO risk assessment for Covid-19 exposure to estimate overall adherence to PPE/IPC as recommended enhanced context validity of the tool and of the study findings. Second, the case-control design allowed for the study of a rare outcome variable within the study period and allowed for assessment of multiple independent exposure variables within a short period of time. In addition, the design allowed for increasing power of the study through the sampling of 3 controls for every case in the study. Third, recall period for independent variables was restricted to a 2-week period before PCR Covid-19 testing by the participants while the differential recall between the cases and the control was minimized through matching of the cases and control in terms of cadre of work and testing date monthly matching. Fourth, misclassification of the cases and the controls was minimized by the strict control group assessment through a pre-exclusion of Covid-19-symptomatic study participants in controls while cases were sampled from laboratory records on PCR confirmed Covid-19 positive HWs.
However, the study was not without limitations. First, study findings were based on a single center, tertiary level hospital as the study setting from which we selected a study population. Therefore, if this study was done in different non-tertiary hospital settings such as a health center, the risk factors to Covid-19 disease among HWs could differ. Therefore, the findings of this study are not generalizable to HWs in lower level and different health care settings. Second, in the choice of the study design a relatively small sample size was obtained that could not have been robust and powered enough in detecting statistical association of key variables such as the effect of sex of the HW on risk of Covid-19 as well as the impact of overall PPE/IPC adherence. Third, the study was prone to sampling bias on male HWs who are reportedly likely to go for testing due to pronounced symptoms and get diagnosed with Covid-19 than female HWs.82 However, over-representation of female HWs in our sample at 55% of all participants reflects the predominance of female HWs in the study as in the general population of the HWs. The effects of the preponderance of male HWs in PCR Covid-19 testing and higher proportion of the female HWs could negatively impact on the true association between the sex of the HW and their risk of Covid-19 from this study. Therefore, more controlled and randomized design would be recommended for a robust assessment of the effect of sex on the risk of Covid-19 in the current study setting. Fourth, the recall bias was not fully minimized since HWs were required to report their exposure back in time a few days and weeks before PCR Covid-19 test considered within the two-month study period. In addition, differential recall between cases and controls could not be fully eliminated despite monthly matching on Covid-19 testing dates and examining exposure in reference to recent 2 weeks before Covid-19 PCR test conducted within the two-month study period. Lastly, the self-reporting of the exposure variables could have resulted to interviewee bias whereby the participants could have preferred to report positive answers such as false self-report on compliance with PPE and IPC measures. As a result, this could have resulted in invalid responses on the true association between the exposure and the outcome.
Measures made to minimize the limitations included monthly matching the Covid-19 testing dates while sampling cases and controls and restricting the assessment of the occupational exposure factors to the last two weeks before the participants’ recent Covid-19 PCR test in the study period (a two-week recall period for occupational and community exposure variables was considered relatively short during which the health worker could remember their clinical practices and health behaviors). A simple random sampling of the cases was employed to minimize selection bias associated with non-random case samples. However, the selection bias was not fully eliminated because only the Covid-19 cases that survived the disease and consented to voluntary participation were included in the study. Multivariable regression modelling was done to control and minimize confounding bias.
HWs who sub-optimally adhered to PPE and IPC measures such as using face shields and disregarded IPC protocols while using shared hospital common rooms had an increased risk of Covid-19 disease. Level of risk across departments of work had an effect on the risk of Covid-19 disease among HWs. From our study, working in medium-risk departments (such as outpatient clinics which are traditionally regarded as having a lower risk of disease compared to high-risk specialized units such as ICU) was shown to increase the risk of Covid-19 disease compared to low-risk non-clinical and administrative units.
Hospitals should reinforce and facilitate optimal adherence to the recommended PPE and IPC measures to prevent occupational infectious disease transmission among HWs especially during infectious disease outbreaks. This should include supporting and reinforcing adherence to facial mucosal protective gears such as face shields among HWs as recommended for SARSCoV and similar highly infectious outbreaks together with other PPE. Institutional measures focusing on IPC around HWs are needed to minimize exposure of infectious disease from staff to staff transmission when sharing common areas such as dining and rest rooms. Future research on occupational infectious disease risk factors should support the validation of exposure risk assessment tool across different health settings for improved external validity of policy recommendations and guidelines around the protection of HWs at all levels.
Mendeley data: Dataset on potential risk factors to COVID19 disease among Health Workers in Kenya. Doi. 10.17632/x47k6prsv8.5 85
This project contains the following underlying data:
This project contains the following extended data:
- Informed consent form_Kiragu J et al.pdf
- Study Questionnaire on risk factors for Covid_19 among Health workers at Kenyatta National Hospital_Kiragu et al.pdf
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
We are sincerely grateful to Dr. John Kinuthia and his team at the department of medical research as well as the entire administration of Kenyatta National Hospital for the permission to collect data and the grant funding towards this study. We would also like to thank Dr. J. Atina of the Respiratory Infectious Disease Unit (RIDU) of Kenyatta National Hospital for recommending this study on its clinical and hospital policy impact.
We acknowledge Mr. Joshua Atunga from the Department of Medical statistics of University of Nairobi for his support on data analysis statistical for this research.
Further guidance and mentorship by Dr. Marshall Mweu of the Department of Public and Global health of the University of Nairobi is highly appreciated.
Lastly, the data collectors and the participant health workers are greatly acknowledged for their participation in this study.
The study sample size was determined by Kelsey (1996) formula for case control studies as shown below 85 :
Exposure level (on-adherence to PPE/IPC) of 23.7% among the general population of HWs was selected based on a recent study. 76
Where,
• n: is desired sample size for the cases,
• n2: is the desired sample size for the controls,
• Zα: the value of Zα required for confidence= 1 − α: Zα/2 = 1.96,
• Zβ: The value of Zβ required for power= 1 − β: Z0.20 = −0.84 Beta is always in the lower tail so it’s negative and only one-tailed,
• r: Ratio of unexposed to exposed (or ratio of controls to cases in case-control studies). An r of 3 controls for every case was chosen,
• p1: Proportion of cases exposed in case-control studies,
• p2: Proportion of controls exposed in previous studies. Exposure level (on-adherence to PPE/IPC) of 23.7% among the general population of HWs was selected based on a recent study. 77
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Biostatistics and Categorical Data Analysis
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Cytopathology,oncopathology and molecular pathology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 13 Oct 23 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)