Keywords
Metastasis to stomach, gastric metastases, gastric metastasis, gastric cancer, stomach cancer
Metastasis to stomach, gastric metastases, gastric metastasis, gastric cancer, stomach cancer
Metastases to the stomach are rare conditions with poor prognosis that may present with both gastrointestinal and systemic symptoms, such as loss of appetite, abdominal pain, fatigue, nausea, and vomiting, with a reported incidence of 0.2-0.7% based on clinical and autopsy findings.1 Gastrectomy is thought to be the only potentially curative treatment for metastatic gastric cancer but the primary site of the tumor is also considered along with the type and grade of the tumor when planning treatment in gastric metastases. Therefore, chemotherapy is also an option for patients with higher grades and multi-focal cancers. This study reviews the literature on gastric metastases in terms of diagnosis, treatment, and outcomes. The intended goal of this study was to provide clinicians with a reliable and beneficial source to understand gastric metastases arising from various primary tumors and to present the growing literature in an easily accessible form by reviewing the case reports of different primary tumors separately with consideration of diagnosis, treatment, and clinical presentation which may vary from patient to patient depending on primary site of the tumor.
This systematic review adheres to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.172 A computerized literature search through MEDLINE/PubMed and Cochrane databases was conducted until May 2022.
The following combination of keywords was used for the search: ({({gastric (MeSH Terms)} AND {neoplasm metastasis (MeSH Terms)}) OR (gastric metastasis)} OR {gastric metastases}) OR (metastasis to the stomach). The search was limited by filtering for “free full text” and “case reports.” After the decision of inclusion and exclusion criteria by the team, two of the reviewers independently screened and retrieved each report.
Hematogenous and lymphogenic metastases were included whereas direct tumoral invasion and seeding were excluded from the study. Articles other than the English language, letters to the editor, posters, and clinical images were excluded. After the studies were screened and separated based on the inclusion and exclusion criteria, reviewers were divided to groups based on primary tumor location. Each group contained two reviewers to collect the data from studies of its specific location for example metastasis from gynecologic cancers or lung cancers.
The following data were extracted from the databases: first author, number of cases, age, sex, site of the primary tumor, histology and treatment of primary tumor, treatment of metastasis, clinical presentation of gastric metastases (GM), synchronous or metachronous GM, the time between primary and secondary GM, diagnostic procedures, other metastasis, and overall survival.
Since the study only contains screening of case reports, assessment of bias risk was not performed and thus it is mentioned as a limitation of study in discussion section.
The PRISMA flow chart below illustrates details about data collection (Figure 1).
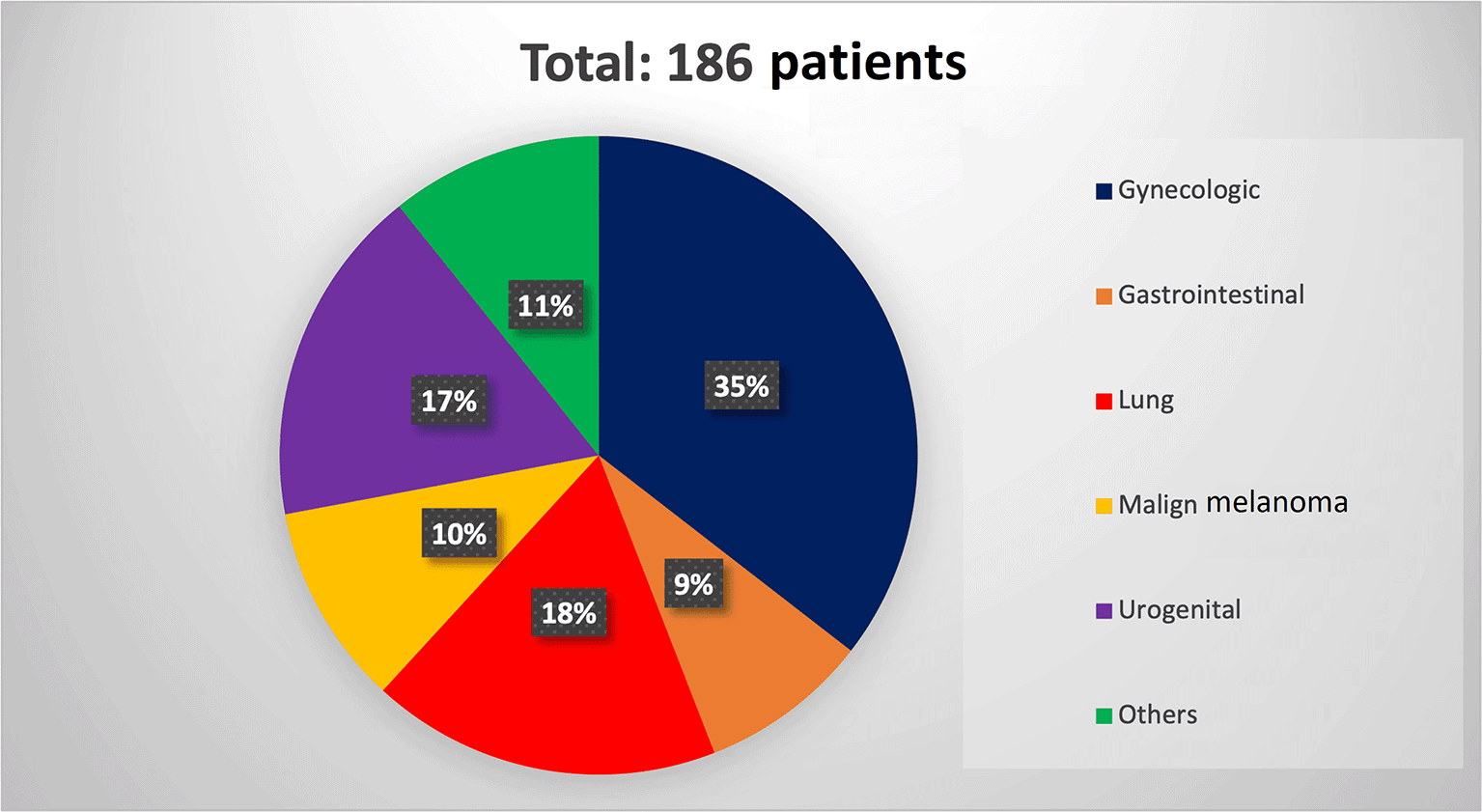
In total, 1,521 publications were identified and 170 articles were finally included totaling 186 patients with GM (101 female and 85 male). The median age of patients was 62 years (IQR: 55-70.5). Gynecologic cancer (including breast cancer) was the most common cancer type causing GM (66 patients), followed by lung cancer (33 patients), renal cancer (20 patients), and melanoma (19 patients) (Figure 2). Results are presented below according to the origin of the primary tumor. The main treatment method performed for metastasis was resection surgery (n=62, total, subtotal or partial gastrectomy, proximal gastrectomy, radical total gastrectomy with Roux-en-Y, wedge gastrectomy, and laparoscopic resection of gastric metastasis), sometimes combined with chemotherapy (ChT) or immunotherapy. Chemotherapy was the other most used treatment method (n=78). Also, immunotherapy was among the most preferred treatment options after surgery and chemotherapy (n=10).
The median age of the 66 patients was 57 years. In total, 46 cases had metastases other than GM. Bone was the most common site of metastasis. Five cases had no other metastases. The total number of cases in the breast group was 54, and one of them was a male patient. The median age of the breast group is 56, the youngest patient was 36 years old and the oldest patient was 84 years old. Invasive lobular carcinoma (ILC) had the largest number of patients in comparison to ovarian and uterine groups. A total of 31 patients presented with ILC. The ovarian group had nine patients; the median age was 61 years. The oldest patient was 73 years old; the youngest patient was 47 years old. The uterine group had two patients with ages 49 and 80 years. In most cases, systemic therapy was more effective than surgery. Surgical treatment had a role in palliative treatment. As a systemic treatment, chemotherapy was the most utilized treatment. Overall survival was given in only 25 cases and ranged from a few days to nine years. Six of the total patients are still alive. Table 12–56 summarizes the findings of included studies regarding gynecologic cancers.
| First author | No of cases | Age | Sex | Site of primary tumor | Histology type of primary | Treatment of primary | Treatment of metastasis | Clinical presentation of GM |
|---|---|---|---|---|---|---|---|---|
| Fousekis et al.2 | 1 | 64 | F | Breast | Lobular Ca | ChT | ChT | Dysphagia, dyspepsia |
| Watanabe et al.3 | 1 | 71 | F | Breast | Ductal Ca | Mastectomy and axillary lymph adenectomy, ChT | Endocrine therapy | Asymptomatic |
| Husain et al.4 | 1 | 47 | F | Left breast | Ductal Ca | Neoadjuvant ChT, mastectomy with a left axillary lymph adenectomy, adjuvant endocrine therapy | N/S | Dyspepsia, weight loss, vomiting |
| Zhang et al.5 | 1 | 46 | F | Bilateral breast | Lobular Ca | N/A | N/A | Epigastric discomfort |
| Jabi et al.6 | 1 | 60 | F | Right breast | Lobular Ca | Palliative ChT | Palliative ChT | Epigastralgia, gastric bleeding, Anemia |
| Johnson et al.7 | 1 | 50 | F | Breast | Ductal Ca | Lumpectomy, adjuvant RT, ChT | N/S | N/S |
| Okamoto et al.8 | 1 | 51 | F | Breast | Ductal Ca | ChT | ChT | Melena, presyncope |
| Nehmeh et al.9 | 1 | 58 | F | Right breast | Ductal Ca | Right modified mastectomy, left prophylactic mastectomy, adjuvant ChT | N/S | Perforated ulcer |
| Hanafiah et al.10 | 1 | 71 | F | Left breast | Lobular Ca | Left mastectomy, axillary clearance, ChT, RT | Ch | Hoarseness, weight loss, early satiety |
| Teixeira et al.11 | 1 | 40 | F | Right breast | Lobular Ca | Neoadjuvant ChT, RT, conservative surgery for right breast and right axillary lymph node | Total gastrectomy | Nausea, epigastric discomfort, early satiety, weight loss |
| Kutasovic et al.12 | 1 | 52 | F | Left breast | Invasive Ca of no special type | Local excision, adjuvant RT, ChT, hormone therapy | Subtotal gastrectomy | N/S |
| Abdallah et al.13 | 1 | 53 | F | Breast | Lobular Ca | ChT, hormone therapy | N/S | Abdominal pain, diffuse tenderness, abdominal distention |
| Liu et al.14 | 1 | 82 | F | Left breast | Phyllodes tumors | Total mastectomy for recurrent tumor local excision, RT | Excision surgery, RT | Anemia, melena |
| Tang et al.15 | 1 | 67 | F | Left breast | Ductal Ca | Left breast-conserving surgery, axillary lymphadenectomy, adjuvant ChT, RT | N/S | Stomach pain |
| De Gruttola et al.16 | 1 | 61 | F | Breast | Lobular Ca | Mastectomy, adjuvant ChT, RT | Total gastrectomy due to gastric perforation | Gastric perforation |
| Mohy-Ud-Din et al.17 | 1 | 83 | F | Breast | Lobular Ca | Mastectomy, sentinel lymph nodes excision, adj. ChT | N/A | Nausea, vomiting |
| Güler et al.18 | 1 | 42 | F | Breast | Ductal Ca | N/A | Total gastrectomy due to gastric perforation, ChT | Acute Abdomen |
| Cui et al.19 | 1 | 42 | F | Endometrium | Endometrial Aca | Total hysterectomy, bilateral salpingo-oophorectomy, pelvic and para-aortic lymphadenectomy | Neoadjuvant ChT, partial gastrectomy | N/S |
| Asmar et al.20 | 1 | 84 | F | Breast | Lobular Ca | Left mastectomy, adjuvant ChT, RT, hormone therapy | Hormone therapy | Dyspepsia |
| Klair et al.21 | 1 | 60 | F | Ovary | Ovarian granulosa cell tumor | Total hysterectomy, bilateral salpingo-oophorectomy | N/A | Reflux, abdominal pain, nausea, anorexia |
| Yang22 | 1 | 47 | F | Ovary | Ovarian serous cystadenocarcinoma | Total hysterectomy, bilateral salpingo-oophorectomy, pelvic and paraaortic lymphadenectomy, total omentectomy | Laparoscopic resection, adjuvant ChT | Abdominal pain |
| Bushan et al.23 | 1 | 68 | F | Left breast | Lobular Ca | Wide excision of breast lesion, ChT, RT, hormone therapy | Distal gastrectomy with D2 lymphadenectomy, left axillary excision, ChT | Weight loss, dysphagia |
| Zhang et al.24 | 2 | 45 | F | Breast | Lobular Aca | Lumpectomy, RT, ChT | N/S | N/S |
| 64 | F | Breast | Lobular Aca | N/A | N/A | Weight loss | ||
| Jin et al.25 | 1 | 55 | F | Breast | Lobular Ca | Neoadjuvant ChT, radical mastectomy, ChT, RT | ChT | N/S |
| Buka et al.26 | 1 | 58 | F | Breast | Invasive lobular Ca | ChT, hormone therapy, RT | Neoadjuvant ChRT, total gastrectomy, adjuvant ChT | Abdominal pain, weight loss |
| Dória et al.27 | 1 | 66 | F | Breast | Invasive lobular Ca | Letrozole | Total gastrectomy, lymphadenectomy, esophagojejunostomy with a Roux loop technique | Epigastric pain, vomiting, weight loss |
| Hwangbo et al.28 | 1 | 73 | F | Ovary | Serous Aca | Cytoreductive surgery, adjuvant ChT | Distal gastrectomy with Billroth I anastomosis, lymphadenectomy | Epigastric pain, dyspepsia |
| Shetty et al.29 | 2 | 56 | F | Breast | Invasive ductal Ca | Breast conservation therapy, adjuvant ChT | ChT | Epigastric discomfort, non-bilious vomiting |
| 61 | F | Breast | Invasive ductal Ca | Left breast modified radical mastectomy, adjuvant RT | Palliative ChT | Abdominal pain, melena, abdominal distension | ||
| Geredeli et al.30 | 1 | 47 | F | Breast | Invasive lobular Ca | Palliative ChT | Subtotal stomach resection, ChT | Asymptomatic |
| Kim et al.31 | 1 | 58 | F | Ovary | Serous Aca | Total hysterectomy with salpingo-oophorectomy, lymphadenectomy with total omentectomy, adjuvant ChT | Subtotal gastrectomy, lymphadenectomy, ChT | Asymptomatic |
| Fernandes et al.32 | 1 | 51 | F | Breast | Invasive lobular Ca | Quadrantectomy, adjuvant ChT, adjuvant RT, hormone therapy | Total gastrectomy, adjuvant ChT, hormone therapy | Dyspepsia |
| Moldovan et al.33 | 1 | 49 | F | Cervix uteri | SCC | Surgery, ChRT | Subtotal gastrectomy, lymphadenectomy D2, anastomotic layout shaped as Y Roux, omentectomy, adjuvant ChRT | Pyloric stenosis, epigastric pains, late postprandial emesis, weight loss |
| Zhou and Miao34 | 1 | 61 | F | Ovary | Serous Aca | Optimal debulking cytoreductive surgery, adjuvant ChT | Gastric antrectomy | Asymptomatic |
| Critchley et al.35 | 1 | 62 | F | Breast | Invasive lobular Ca | Mastectomy, level 2 axillary clearance, adjuvant ChT, adjuvant RT, adjuvant hormone therapy | ChT | Loose stool, normocytic anemia, weight loss |
| Hara et al.36 | 1 | 74 | F | Breast | Invasive ductal Ca | Breast-conserving surgery | Paclitaxel | Chronic gastritis |
| Ciulla et al.37 | 1 | 70 | F | Breast | Lobular Ca | Postoperative hormone therapy | Total gastrectomy, lymphadenectomy R1, esophagojejunostomy with Roux loose technique | Asymptomatic |
| Jones et al.38 | 2 | 51 | F | Breast | Lobular Ca | Wide local excision, axillary dissection, adjuvant RT | Total gastrectomy with Roux-en-Y reconstruction, hormone therapy | Weight loss, epigastric pain |
| 61 | F | Breast | Lobular Ca | Mastectomy, axillary dissection, adjuvant ChT, RT, tamoxifen | ChT, RT | Progressive dysphagia, weight loss | ||
| Yim et al.39 | 1 | 48 | F | Breast | SRCC | ChT | ChT | Epigastric discomfort |
| Wong et al.40 | 1 | 72 | F | Breast | Invasive lobular Ca | Wide local excision, adjuvant RT | Hormone therapy | Acute abdomen, rebound tenderness, generalized peritonitis |
| Ricciuti et al.41 | 1 | 65 | M | Breast | Invasive ductal Ca | Total mastectomy, complete axillary dissection, adjuvant hormone therapy | Gastrectomy with Roux-en-Y esophagojejunostomy anastomosis | Hematemesis, epigastric pain |
| Fernandes et al.42 | 4 | 56 (the mean age) | F | Breast | Invasive lobular Ca | ChT, hormone therapy | Total gastrectomy | Ulcerated lesion, major bleeding |
| 56 (the mean age) | F | Breast | Invasive lobular Ca | ChT, RT, hormone therapy | ChRT | Diffuse infiltration | ||
| 56 (the mean age) | F | Breast | Invasive ductal Ca | ChT, hormone therapy | ChT | Infiltrative, ulcerated, stenotic lesion | ||
| 56 (the mean age) | F | Breast | Invasive ductal Ca | ChT, hormone therapy | ChRT | Flat erosive lesion | ||
| Zullo et al.43 | 3 | 49 | F | Ovary | Serous Aca | Hysterectomy, bilateral salpingo-oophorectomy, pelvic lymphadenectomy, adjuvant ChT | ChT | Abdominal pain, vomiting, weight loss |
| 80 | F | Cervix uteri | Leiomyosarcoma | Total hysterectomy, bilateral salpingo-oophorectomy, pelvic lymphadenectomy, adjuvant ChT | N/A | Epigastric pain | ||
| 70 | F | Breast | N/A | Radical left mastectomy, adjuvant ChT | ChT | Dysphagia, epigastric pain | ||
| Villa Guzman et al.44 | 1 | 58 | F | Breast | Invasive lobular Ca | Quadrantectomy, lymphadenectomy, adjuvant ChT, RT | ChT, hormone therapy | Nausea, epigastric pain |
| Mizuguchi et al.45 | 1 | 71 | F | Ovary | Serous Aca | Total hysterectomy, bilateral salpingo-oophorectomy, omentectomy, ChT | ChT, hormone therapy | Asymptomatic |
| Jmour et al.46 | 4 | 51 | F | Breast | Mixed | Radical mastectomy with lymphadenectomy | ChT, RT | Nausea, vomiting, abdominal pain |
| 47 | F | Breast | Lobular infiltrating Ca | Radical mastectomy with lymphadenectomy | ChT, RT | Nausea, vomiting, abdominal pain | ||
| 51 | F | Breast | Ductal infiltrating Ca | N/A | ChT, RT | Nausea, vomiting, abdominal pain | ||
| 36 | F | Breast | Lobular infiltrating Ca | Radical mastectomy with lymphadenectomy | ChT, RT | Nausea, vomiting, abdominal pain | ||
| Yim47 | 1 | 65 | F | Breast | Invasive lobular Ca | Modified radical mastectomy, adjuvant ChT, adjuvant RT | ChT | Dyspepsia, anorexia, indigestion, epigastric discomfort, early satiety, weight loss |
| Choi et al.48 | 1 | 44 | F | Breast | Phyllodes tumor | Right lumpectomy, axillary lymphadenectomy, RT, right total mastectomy | Endoscopic hemostasis with cauterization | Dizziness, anemia, melena |
| Khan et al.49 | 1 | 56 | F | Breast | Signet ring Aca | ChT | ChT | Anemia |
| Mullally et al.50 | 1 | 46 | F | Breast | Invasive ductal Ca | Left mastectomy, adjuvant ChT, hormone therapy, RT | Palliative laparoscopic gastroduodenostomy, hormone therapy, palliative ChT | Epigastric and left shoulder pain, epigastric tenderness, upper abdominal rigidity |
| Kliiger and Gorbaty51 | 1 | 60 | F | Breast | Invasive ductal Aca | Systemic therapy, ChT | N/A | Nausea, diarrhea, vomiting, weight loss |
| Antonini et al.52 | 1 | 61 | F | Ovary | Serous Ca | ChT, cytoreductive surgery | ChT | Dyspepsia |
| Kono et al.53 | 1 | 64 | F | Ovary | Mucinous Ca | Bilateral salpingo-oophorectomy, simple hysterectomy, pelvic and para-aortic lymphadenectomy, partial omental resection | ChT | Back pain |
| Kim et al.54 | 1 | 39 | F | Breast | Invasive lobular Ca | Right breast-conserving surgery, lymphadenectomy | Duodenal stent, systemic ChT | Upper abdominal discomfort and pain, indigestion |
| Woo et al.55 | 1 | 51 | F | Breast | Invasive lobular Ca | Bilateral modified radical mastectomy, ChT, RT | Radical subtotal gastrectomy with Billroth II anastomosis, D2 lymphadenectomy, ChT | Epigastric pain |
| Ulmer et al.56 | 1 | 55 | F | Breast | Invasive lobular Ca | Bilateral mastectomy, adjuvant ChT, RT, hormone therapy | Palliative pyloric stent | Nausea, vomiting, early satiety, weight loss |
Median age of the 16 patients (11 male, five female) was 69 years, ranging from 22 years to 85 years. Overall survival of the seven patients whose data were given ranged from two months to 16 months. Although, there were six cases who were still alive and the survival of three cases was not reported. Among histological types of gastrointestinal cancers, adenocarcinoma (Adeno Ca) was the most common cancer type (seven patients), followed by hepatocellular cancer (HCC) (four patients) and squamous cell carcinoma (two patients). Endoscopy is the most frequently used method in the diagnosis of metastases. Methods such as computer tomography (CT), positron emission tomography and computed tomography (PET-CT), and endoscopic ultrasound were also used for diagnosis. One patient underwent laparotomy and biopsy. According to this research nine of these patients had surgery. Transcatheter left gastric artery embolization was performed in one patient. On the other hand, seven patients received chemotherapy and one patient had palliative radiotherapy. Nevertheless, one patient is unknown. Findings regarding gastrointestinal cancers are summarized in Table 2.57–72
| First author | No of cases | Age | Sex | Site of primary tumor | Histology type of primary | Treatment of primary | Treatment of metastasis | Clinical presentation of GM |
|---|---|---|---|---|---|---|---|---|
| Iwai et al.57 | 1 | 76 | F | Transverse colon | Poorly differentiated Aca with a partial component of signet-ring Ca | ChT | ChT | Anemia, anorexia |
| Yang et al.58 | 1 | 74 | F | Head of pancreas | Poorly differentiated invasive Aca | ChT | ChT | RUQ pain |
| Lee and Lee59 | 1 | 82 | M | Right colon | Moderately differentiated Aca | Extended right hemicolectomy (declined adjuvant ChT) | Radical total gastrectomy (declined adjuvant ChT) with Roux-en-Y and D2 dissection | Asymptomatic |
| Rothermel et al.60 | 1 | 61 | M | Body of pancreas | Well-differentiated ductal Aca | Distal pancreatectomy, splenectomy, adjuvant ChT | ChT, palliative radiation, and wedge gastrectomy | Asymptomatic |
| Terashima et al.61 | 1 | 61 | F | Transverse colon | Poorly differentiated Aca | Extended right hemicolectomy, ChT | Partial gastrectomy and D3 dissection, ChT | Diarrhea, vomiting |
| Sasajima et al.62 | 1 | 72 | M | Head and tail of pancreas | IPMN | ChT | ChT (terminated after 2 courses) | N/A |
| Tomonari et al.63 | 1 | 78 | M | Body and distal pancreas | Moderately differentiated ACa T3N0M0 | Surgery, adjuvant ChT | Subtotal gastrectomy | Follow-up |
| Adachi64 | 1 | 67 | F | Pancreas | Well-differentiated SCC | Distal pancreatectomy and splenectomy | Total gastrectomy | Anorexia, back pain |
| Nakazawa et al.65 | 1 | 59 | M | Esophagus | Mucosal SCC | Subtotal esophagectomy, left lateral segmentectomy of liver, pancreatosplenectomy, adjuvant ChT | Proximal gastrectomy | Asymptomatic |
| Abouzied et al.66 | 1 | 69 | M | Liver | HCC | Right hepatectomy | ChT | Iron-deficiency anemia |
| Ito et al.67 | 1 | 78 | M | Liver | ICC | Lateral hepatectomy | Proximal gastrectomy and lymphadenectomy | Fatigue |
| Imai et al.68 | 1 | 62 | M | Liver | HCC | N/A | Transcatheter left gastric artery embolization | Abdominal mass |
| Kim et al.69 | 1 | 75 | M | Liver | HCC | Right hemihepatectomy, TACE | Gastric wedge resection | Melena, mild dyspnea |
| Peng et al.70 | 1 | 22 | M | Liver | HCC | Right hemihepatectomy combined with left lateral tumor local resection, cholecystectomy, splenectomy | Gastric tumor local resection | Anemia, FOBT 4+ |
| Kanthan et al.71 | 1 | 85 | M | Colon | Aca | N/A | N/A | Anemia |
| Wang et al.72 | 1 | 63 | F | Gallbladder | Melanoma | Surgery, ChT | ChT | Postprandial nausea, vomiting |
The median age of the 33 patients (25 male, eight female) was 62, ranging from 39 years to 78 years. Twenty- seven of the total cases had other metastases in addition to gastric ones. The survival time of the 22 patients whose data were given ranged from two weeks to 30 months. Yet, there were two cases that were still alive four and five years after metastases were found, respectively. Among histological types of primary lung cancers that lead to gastric metastases, adenocarcinoma was the most typical diagnosis (13 patients), followed by small cell lung cancer (SCLC) and squamous cell carcinoma (SCC). Regarding the treatment of GM, different combinations of chemotherapy were the most common choice (15 patients). On the other hand, seven of the total cases received surgical treatment (one esophagogastrostomy, two total, and four partial gastrectomies). However, since one patient’s metastasis was diagnosed after an autopsy, he could not receive any gastric treatment. Moreover, one patient refused any metastasis treatment, while six other cases’ treatments are unknown. Data pertaining to GM originating from primary lung cancers are summarized in Table 3.73–102
| First author | No of cases | Age | Sex | Site of primary tumor | Histology type of primary | Treatment of primary | Treatment of metastasis | Clinical presentation of GM |
|---|---|---|---|---|---|---|---|---|
| Catalano et al.73 | 1 | 78 | M | Lung - right upper lobe | Poorly differentiated Aca | Upper right lobectomy | Total gastrectomy | Asymptomatic |
| Shih-Chun et al.74 | 1 | 55 | M | Lung - right upper lobe | NSCLC | Concurrent chemoradiotherapy | Palliative total gastrectomy, ChT | Gastric bleeding, ulcerative mass |
| Das Majumdar et al.75 | 1 | 72 | M | Lung | Poorly differentiated Aca | Palliative RT | Immunotherapy, ChT | Identified with body CT after pathological fracture |
| Liu et al.76 | 1 | 58 | M | Lung | Aca | Middle right lobectomy, neoadjuvant therapy, ChT | ChT, partial gastrectomy | N/S |
| Nemoto et al.77 | 1 | 64 | M | Lung - right lower lobe | SCC | Adjuvant ChT | Esophagogastrostomy | Epigastric pain, progressive dysphagia |
| He et al.78 | 1 | 61 | M | Lung | SCLC | Left lower lobectomy | Cardia resection | Progressive dysphagia |
| Yang et al.79 | 1 | 59 | M | Lung - left upper lobe | Poorly differentiated metastatic carcinoma | ChT | Anti-PD1 immunotherapy | Right upper limb pain, epigastric discomfort |
| Li et al.80 | 1 | 61 | M | Lung - right lower lobe | SCC | ChT | ChT, gastrectomy | Progressive abdominal distention |
| Bhardwaj et al.81 | 1 | 39 | F | Lung | SCC | ChT, nivolumab | RT | Dizziness, melena |
| Badipatla et al.82 | 1 | 65 | M | Lung | Aca | ChT, palliative care | ChT, palliative care | Bilateral flank pain, nausea, vomiting, change in bowel habit |
| Qasrawi et al.83 | 1 | 69 | F | Lung - left upper lobe | Aca | RT | Hospice care | Melena, hypotension |
| Kim et al.84 | 1 | 70 | F | Lung | Pleomorphic carcinoma | Right bronchial artery embolization, right upper lobectomy, adjuvant ChT | Partial gastrectomy, immunotherapy | Abdominal pain |
| Maeda et al.85 | 1 | 60 | F | Lung | SCLC | ChT | N/A | Nausea, vomiting |
| Struyf et al.86 | 1 | 68 | M | Lung | Aca | ChT | ChT | Severe epigastric pain |
| Altintas et al.87 | 1 | 55 | M | Lung | Aca | ChT | ChT | Epigastric pain, hematemesis, melena |
| Casella et al.88 | 1 | 63 | M | Lung | SCLC | Supportive care | Supportive care | Fever, weight loss, epigastric pain, constipation |
| Ohashi et al.89 | 1 | 62 | M | Lung | Large cell carcinoma | Right upper lobectomy | ChT | Abdominal pain |
| Aokage et al.90 | 2 | 69 | M | Lung - right upper lobe | Pleomorphic carcinoma | Right upper lobectomy, parietal pleura resection | Partial gastrectomy, splenectomy | Fatigue, anemia |
| 62 | M | Lung - left upper lobe | Pleomorphic carcinoma | Left upper lobectomy | Distal gastrectomy, splenectomy | N/A | ||
| Katsenos and Archondakis91 | 1 | 61 | M | Lung - left upper lobe | Aca | ChT | ChT | Upper GIS bleeding |
| Diem et al.92 | 1 | 62 | F | Lung - right upper lobe | Aca | N/A | ChT | Epigastric pain |
| Hu et al.93 | 1 | 54 | M | Lung | SCC | RT, right middle lobectomy | None (patient refused) | Dysphagia |
| Koh et al.94 | 1 | 46 | M | Lung | Pleomorphic carcinoma | Antibiotics | N/A | Abdominal pain, tenderness |
| Hung et al.95 | 1 | 47 | M | Lung | SCC | RT, ChT | ChT | Weight loss, dysphagia |
| Taira et al.96 | 1 | 64 | M | Lung | Pleomorphic carcinoma, Aca | Left upper lobectomy | ChT | Anemia |
| Gao et al.97 | 1 | 66 | M | Lung | SCLC | ChT | ChT, supportive care | Epigastric pain |
| Kim et al.98 | 1 | 68 | M | Lung | Poorly differentiated Aca | Left lower lobectomy, posterior segmentectomy right upper lobe (2004), left upper lobe wedge resection (2007), palliative chemotherapy | Palliative ChT | Epigastric pain, dyspepsia |
| Chen et al.99 | 1 | 59 | F | Lung | Sarcomatoid carcinoma | Supportive treatment | Supportive treatment | Abdominal pain, anorexia, weight loss |
| Dong et al.100 | 1 | 60 | F | Lung | Glomus tumor | N/A | N/A | Hemoptysis, melena, abdominal distension |
| Kim et al.101 | 2 | 66 | M | Lung | SCLC | N/A | N/A | Epigastric pain, epigastric tenderness, fatigue |
| 68 | M | Lung | SCLC | N/A | N/A | Hemoptysis, weight loss | ||
| Del Rosario et al.102 | 1 | 77 | F | Lung | Aca | ChT | Palliative care | N/A |
| Kanthan et al.71 | 1 | 75 | M | Lung | Aca | N/A | N/A | Epigastric pain, RUQ pain |
The median age of the 19 patients (seven female, 12 male) was 67, ranging from 28 years to 89 years. In 16 patients, other organ metastases were also discovered in addition to malign melanoma. Overall survival was not mentioned in 10 cases. Two of these cases deceased two and four days after hospital admission respectively, and one patient died after a year. Moreover, one of these patients was alive at five years, and another was alive at six months. Overall survival of three cases is three, 27, and four months, respectively. One of these patients refused treatment, and one of them did not receive treatment. However, immunotherapy was applied to six patients, surgery to five patients, radiotherapy to two patients, and only supportive treatment to three patients. In addition, the treatment of GM was not mentioned in three cases. Table 4 summarizes the findings of included studies regarding malign melanoma.103–121
| First author | No of cases | Age | Sex | Site of primary tumor | Histology type of primary | Treatment of primary | Treatment of metastasis | Clinical presentation of GM |
|---|---|---|---|---|---|---|---|---|
| Zhu et al.103 | 1 | 36 | M | Right plantar | Nodular | Mohs microsurgery | N/A | Anorexia, nausea, vomiting |
| Yoshimoto et al.104 | 1 | 82 | F | Fourth left toe | Acral lentiginous | Surgery | Palliative RT | Melena |
| Okamoto et al.105 | 1 | 79 | M | Esophagus | Pigmented submucosal tumor-like growth in the esophagus | Nivolumab | Nivolumab | Gross hematuria, weight loss, cough, exertional dyspnea |
| Cortellini et al.106 | 1 | 81 | M | N/A | N/A | N/A | N/A | Weakness, hyporexia, anemia |
| Groudan et al.107 | 1 | 66 | F | Vulva | N/A | N/A | Palliative RT, immunotherapy | Fatigue, exertional dyspnea, hematemesis, weight loss, nausea |
| Syed et al.108 | 1 | 49 | F | Back | N/A | Surgery | Immunotherapy, supportive care, SRS | Anorexia, abdominal pain, fatigue, weight loss, nausea, vomiting |
| Genova et al.109 | 1 | 80 | M | Scalp | Lentigo | RT | Immunotherapy | Hypochromic anemia |
| Wong et al.110 | 1 | 81 | F | Foot | Acral lentiginous | Amputation, CT | Denied the treatment | Dyspnea, fatigue, anemia |
| Grander et al.111 | 1 | 67 | M | Right hypochondrium, back, scalp | Superficial spreading | Surgery | Total gastrectomy, radiosurgery | Melena |
| Carcelain et al.112 | 1 | 65 | F | N/A | N/A | Surgery | Surgery | N/S |
| Lestre et al.113 | 1 | 67 | M | Lower back | Superficial spreading | Excision, adjuvant immunotherapy | No | N/S |
| Rana et al.114 | 1 | 72 | M | N/A | N/A | N/A | N/A | Weight loss, anorexia |
| Rovere et al.115 | 1 | 68 | M | N/A | N/A | N/A | Supportive care | N/S |
| Eivazi-Ziaei et al.116 | 1 | 56 | M | Right heel-ALM | N/A | Surgery | Supportive care | Epigastric pain |
| El-Sourani et al.117 | 1 | 43 | F | Right breast | N/A | Surgery | Sleeve gastrectomy after atypical resection, complete locoregional lymphadenectomy | Melena, anemia |
| Buissin et al.118 | 1 | 63 | M | Anorectal | Hyperplastic polyp | Abdominoperineal resection | Supportive care | Tenderness in the RUQ |
| Bankar et al.119 | 1 | 41 | F | N/A | N/A | N/A | Surgery | N/S |
| Mohan et al.120 | 1 | 28 | M | N/A | N/A | N/A | Temozolomide | Abdominal pain, anorexia, weight loss |
| Farshad et al.121 | 1 | 89 | M | Chest wall | N/A | Local excision | Nivolumab | Fatigue, rigors, fever |
The median age of 20 patients (11 male and nine female) with kidney cancer was 68.5 years old. A total of 11 patients had metastases other than GM. Overall survival was mentioned only in four cases and ranged from two months to one year. One of the 20 patients did not receive any therapy for GM, whereas 13 patients underwent surgical treatment (four endoscopic mucosal resections, nine gastrectomies), four patients had chemotherapy and one patient was treated with radiotherapy. Regarding prostate cancer, the median age of the affected individuals was 67 years old. Concerning the GM treatment four patients received chemotherapy, one patient underwent mucosal resection, and one patient refused treatment. Overall survival was mentioned for three patients ranging from four months to 19 months. All four patients with testis cancer had other metastases and two of them received chemotherapy. One study included bladder cancer without other metastases and the patient was referred to palliative care. Data pertaining to gastric metastases originating from primary urogenital cancers are summarized in Table 5.71,122–151
| First author | No of cases | Age | Sex | Site of primary tumor | Histology type of primary | Treatment of primary | Treatment of metastasis | Clinical presentation of GM |
|---|---|---|---|---|---|---|---|---|
| Tapasak and Mcguirt122 | 1 | 77 | M | Kidney | RCC | Nephrectomy, ChT | Roux-en-Y gastric bypass | Gastrointestinal bleeding, anemia |
| Podzolkov et al.123 | 1 | 30 | M | Testis | Choriocarcinoma | ChT | N/A | Epigastric pain, dyspnea |
| Koterazawa et al.124 | 1 | 70 | F | Kidney | RCC | Nephrectomy | Endoscopic submucosal resection | Weight loss |
| Hakim et al.125 | 1 | 86 | F | Kidney | RCC | Nephrectomy, ChT | RT | Gastrointestinal bleeding |
| Yoshida et al.126 | 1 | 85 | F | Kidney | RCC | Nephrectomy | Endoscopic resection | Anemia, melena |
| Bernshteyn et al.127 | 1 | 68 | M | Kidney | RCC | Nephrectomy | N/A | Dyspnea, melena |
| Weissman et al.128 | 2 | 70 | M | Kidney | RCC | Nephrectomy | ChT | Dyspepsia, malaise, weight loss |
| 85 | M | Kidney | RCC | Nephrectomy | ChT | Dyspepsia, malaise, weight loss | ||
| Chaar et al.129 | 1 | 30 | M | Testis | Choriocarcinoma | Orchiectomy | ChT (patient refused) | Melena, anemia |
| Arakawa et al.130 | 1 | 80 | F | Kidney | RCC | Cht | ChT | Anorexia, pyrexia, malaise |
| Uehara et al.131 | 1 | 73 | M | Kidney | RCC | Nephrectomy, ChT | Endoscopic mucosal resection, immunotherapy | Gastric mass |
| O'Reilly et al.132 | 1 | 59 | F | Kidney | Clear cell RCC | Nephrectomy | Laparoscopic sleeve gastrectomy | Asymptomatic |
| Abu Ghanimeh et al.133 | 1 | 67 | M | Kidney | Clear cell RCC | Nephrectomy | No treatment initiated | Gastrointestinal bleeding |
| Mazumdar et al.134 | 1 | 49 | M | Testis | Seminoma | N/A | N/A | Abdominal pain |
| Barras et al.135 | 1 | 53 | M | Kidney | RCC | Nephrectomy | Partial gastrectomy | Hematochezia |
| Riviello et al.136 | 1 | 68 | M | Kidney | RCC | Nephrectomy | Gastrectomy, ChT | Melena, postural dizziness, weakness |
| Hong et al.137 | 1 | 60 | M | Bladder | Clear cell urothelial Ca | ChT, RT | Palliative care | Projectile vomiting |
| Onitilo et al.138 | 2 | 57 | M | Prostate | Aca | LHRH agonist | ChT | Weakness, nausea, vomiting, hematemesis |
| 89 | M | Prostate | Aca | LHRH agonist | ChT | Weakness, nausea, vomiting, hematemesis | ||
| Tiwari et al.139 | 1 | 58 | F | Kidney | Clear cell RCC | N/A | Roux-en-Y subtotal gastrectomy | Melena, hematemesis, fatigue |
| Yodonawa et al.140 | 1 | 73 | M | Kidney | Leiomyosarcoma | Nephrectomy | Distal gastrectomy | Melena, weakness |
| Chibbar et al.141 | 1 | 69 | F | Kidney | Clear cell RCC | Nephrectomy | Endoscopic mucosal resection | Fatigue, lightheadedness, anemia |
| Sakurai et al.142 | 1 | 61 | M | Kidney | RCC | Nephrectomy | Partial gastrectomy, ChT | Melena, anemia |
| Patel et al.143 | 1 | 71 | M | Prostate | Aca | Surgery, RT | N/A | Weakness, dizziness, anemia |
| Sharifi et al.144 | 1 | 17 | F | Kidney | Primitive neuroectodermal | ChT | ChT | Abdominal pain, distention |
| Greenwald et al.145 | 1 | 62 | M | Kidney | Clear cell RCC | Nephrectomy | Partial gastrectomy | Testicular pain |
| Costa et al.146 | 1 | 66 | F | Kidney | RCC | Nephrectomy | Palliative laparoscopic wedge resection | Anemia |
| Soe et al.147 | 1 | 64 | M | Prostate | N/A | LHRH agonist | Palliative care (patient refused chemotherapy) | Anemia, melena |
| Bhandari and Pant148 | 1 | 58 | M | Prostate | Aca | LHRH agonist | ChT | Abdominal pain |
| Lowe et al.149 | 1 | 18 | M | Testis | Choriocarcinoma | ChT, orchidectomy | ChT | Melena, lethargy, dizziness |
| Inagaki et al.150 | 1 | 75 | M | Prostate | Aca | LHRH agonist | Endoscopic mucosal resection, hormone therapy | Epigastric pain |
| Tavukcu et al.151 | 1 | 67 | M | Prostate | Mixed 55% ductal 45% acinar | Prostatectomy, RT | Androgen deprivation therapy, ChT | Ascites, vomit |
| Kanthan et al.71 | 1 | 19 | M | Testis | Predominantly choriocarcinoma, embryonal Ca | Orchiectomy | Partial gastrectomy聽 | Melena, anemia |
The median age of the four patients with Merkel cell carcinoma was 73 years old. Two patients had other metastases in addition to GM. Three patients underwent surgery, chemotherapy, and radiotherapy, whereas one patient was treated with chemotherapy and radiotherapy. One patient with squamous cell carcinoma had other metastases in addition to GM and received chemotherapy and radiotherapy for the primary tumor.
Regarding bone cancers (n=3) one of the patients was 14 years old and stood out as the youngest patient in this group. Concerning the GM therapy, one of the patients with a known treatment underwent surgery and chemotherapy the other received only surgery. In all patients, GM was discovered metachronous. Three studies were included for soft tissue cancer. All three patients had metastases in addition to GM and underwent different types of GM treatment (including radiotherapy, chemotherapy, excision with snare, and cautery). For the thyroid cancer group, the median age was 71 years old. Overall survival (OS) was only mentioned for one patient (2.5 months). Regarding diffuse large B-cell lymphoma (DLBCL) (n=2), patients received chemotherapy for primary cancer and for GM. GM was discovered synchronously. Kovecsi et al., described the only case of GM from adrenocortical carcinoma of the adrenal gland.152 The patient underwent adrenalectomy for primary and total gastrectomy with splenectomy and end-to-side Roux-en-Y esophagojejunal anastomosis for GM. One patient with choriocarcinoma from retroperitoneum underwent chemotherapy for primary cancer and GM. Table 6 summarizes the findings of included studies regarding gastrointestinal cancers.151–171
| First author | No of cases | Age | Sex | Site of primary tumor | Histology type of primary | Treatment of primary | Treatment of Metastasis | Clinical presentation of GM |
|---|---|---|---|---|---|---|---|---|
| Kovecsi et al.152 | 1 | 71 | M | Adrenal gland | Adrenocortical carcinoma | Right adrenalectomy | Total gastrectomy, splenectomy, with end-to-side Roux-en-Y eso-jejunal anastomosis | Weight loss, epigastric pain, vomiting, fatigue |
| Koti et al.153 | 1 | 14 | F | Bone | Ewing sarcoma | ChT, local excision | ChT, total gastrectomy, RT | Abdominal mass, low-grade fever, weight loss |
| Dodis et al.154 | 1 | 72 | F | Bone | Ewing sarcoma | Total knee replacements, RT, ChT | N/A | Anemia |
| Urakawa et al.155 | 1 | 73 | M | Bone | Osteosarcoma | ChT, surgery | Partial gastrectomy | Anemia, hematemesis |
| Shibuya et al.156 | 1 | 27 | M | Extragonadal retroperitoneal | Choriocarcinoma | Cht | ChT | Abdominal pain, melena, vomiting |
| Tarangelo et al.157 | 1 | 65 | M | Head, neck | SCC | Cht, RT, robotic excision | N/A | Melenic bowel movements |
| Kamihara et al.158 | 1 | 70 | M | Lymph nodes | DLBC | R-CHOP ChT | R-CHOP ChT | N/A |
| Zepeda-Gomez et al.159 | 1 | 39 | F | Lymph nodes | DLBC | ChT, omeprazole | ChT | Melena, weight loss, retroperitoneal mass |
| Teh et al.160 | 1 | 37 | F | Oropharynx | SCC | Surgery, adjuvant RT | Palliative RT | Weight loss, LUQ pain, melena |
| Elkafrawy et al.161 | 1 | 67 | M | Skin | MCC | Surgery, consolidative | Atezolizumab, RT | Melena |
| Ha et al.162 | 1 | 82 | M | Skin | MCC | Surgery, RT | No | Anorexia, weight loss |
| Idowu et al.163 | 1 | 79 | F | Skin | MCC | Surgery, ChT, RT | N/A | Anemia |
| Parikh et al.164 | 1 | 60 | M | Skin | MCC | ChT, RT | ChT | Maroon colored stools |
| Subramanian et al.165 | 1 | 62 | M | Soft tissue | Leiomyosarcoma | Surgery, RT | RT, ChT | Melena, abdominal pain, nausea, vomiting |
| Dent et al.166 | 1 | 60 | M | Soft tissue | Sarcoma | Surgery | Remove with snare and cautery | Upper abdominal pain, melena |
| Samuel et al.167 | 1 | 56 | M | Soft tissue | Synovial sarcoma | Surgery, RT | Doxorubicin | N/A |
| Thorburn et al.168 | 1 | 56 | M | Supraglottic larynx, hypopharynx | Advanced SCC | Surgery, tracheostomy, radical RT | N/A | Anemia, hematemesis |
| Fuladi et al.169 | 1 | 71 | F | Thyroid | Anaplastic carcinoma | Total thyroidectomy, left modified radical neck dissection, RT | N/A | Nausea, vomiting |
| Ayaz et al.170 | 1 | 72 | M | Thyroid | Anaplastic carcinoma | N/A | N/A | Melena |
| Karrasch et al.171 | 1 | 53 | F | Thyroid | Medullary thyroid cancer | Complete thyroidectomy ChT | N/A | Fatigue, anorexia, epigastric pain radiating to the back |
Gastric metastases are uncommon and give information about the progressed stage of malignant disease, with a reported incidence of 0.2-0.7% based on clinical and autopsy findings.1 Furthermore, metastasis to the stomach frequently indicates short survival. These metastases are observed rarely due to clinical problems regarding their diagnosis and treatment.2 Progressively, with improvements in prognosis for cancer patients, metastatic tumors in the stomach are being detected more frequently.1 There are several symptoms of gastric metastases, such as abdominal pain, diarrhea, nausea, vomiting, weight loss, and dyspepsia. The most preferred treatment method for gastric metastasis is surgical resection of the tumor. Also, chemotherapy is the most applied alternative option.
This systematic review has a few potential limitations that need to be mentioned. As 172 case reports were screened in the systematic review from different journals the heterogeneity was inevitable. Some articles missed important information such as complete follow-up or clinical information. Moreover, since all of the included articles were case reports, quality or bias assessment could not be performed.
Gastric metastasis mainly occurs due to breast cancer. Both ovarian and uterine metastases are distinctly less frequent.38 Invasive lobular carcinoma is the type with the highest affinity to the digestive system with an incidence of 4.5% compared to 0.2% in ductal carcinoma.26 Breast cancer metastases to the gastrointestinal tract are rare, with a median time interval from the diagnosis of the primary tumor to metastasis up to seven years.21 The longest disease-free interval is 22 years after the initial diagnosis 17 of 24.10 Some metastatic tumors may have a similar presentation as primary gastric cancer.38 The detailed immunohistochemical analysis will allow the most accurate diagnosis to differentiate between primary gastric cancer and gastric metastasis from breast cancer.26 Most gastric metastatic breast cancers are estrogen receptor (ER)-positive, progesterone receptor (PR)-positive/negative, and human epidermal growth factor receptor (HER2)-negative. However, in primary gastric adenocarcinoma, ER and PR can be positively expressed in 20-28% of patients.19 In a few cases, metastatic breast cancer is negative for ER and PR, so a diagnosis cannot be made based on these two investigations alone.59 ER and PR can be used as markers; however, they are not always suitable diagnostic markers to confirm if a tumor has originated.11 Treatment of gastrointestinal metastases from breast cancer is discussed frequently in the literature. Systemic therapy is the first option.36 The effective rate of systemic treatment is about 46%.60 Surgical treatment may have a role in palliative treatment.34 Surgical treatment is considered in cases with obstruction or bleeding.36 Metastasis to the gastrointestinal tract can be the first presentation of breast cancer, therefore it is imperative to consider the possibility of breast cancer metastasis to the gastrointestinal metastasis.26,46
Among cancers that metastasize to the stomach, gastrointestinal system cancers are encountered in a minority. Gastric metastases gave unspecific findings, such as anemia, bleeding, and pain. Pancreas, liver, and colon account for the majority of primary cancers. Nine of the cases had other metastases in addition to gastric ones. Since pancreatic cancers are usually caught at an advanced stage, the chances of surgical treatment and their response to treatment are low. We see that three of five patients died within a year.2,6,8 Among these cases, the prognosis of pancreatic head cancers was worse than body and tail cancers.
In fact, lung cancer is the most mortal type of all cancers. However, the stomach is not a common site for primary lung cancers’ metastases, especially compared with brain, liver, adrenal glands, and bones.74 Yet, the expected lifespan after diagnosis of metastasis is found to be relatively low. The median survival time was four months (average 6.8 months) among 16 cases who died. On the other hand, data showed that endoscopy is the gold standard in diagnosis. In addition, pathology and immunohistochemistry are considered important factors to differentiate gastric metastases from primary cancers.81 Regarding the treatment of gastric metastases of the pulmonary origin, although non-invasive chemotherapy treatments were the most common choice, patients who received surgery, particularly partial gastrectomy, but also esophagogastrostomy and laparotomy, tended to have relatively much longer survival time.77,90,95 However, this conclusion is not definitive, since in some cases surgeries may be avoided when the patient’s condition is extremely severe and the number of cases with given surgical treatments is scarce. So the potential benefit of surgeries to the expected lifespan of the patients needs further investigation.
Although melanoma accounts for only 5% of cutaneous malignancies, it makes up nearly 75% of skin cancer- related deaths.103,107 Malignant melanoma ranks as the most common metastatic tumor of the gastrointestinal (GI) tract.103,110,120 It takes an average of 52 months for a primary cutaneous melanoma to spread to the gastrointestinal tract.107,110 Only 1-4% of patients with malignant melanoma deceased before gastrointestinal metastases are diagnosed. On the other hand, GI tract metastasis was observed in more than 60% of melanoma patients by autopsy.103,110,111,114,121 The most commonly involved sites include the small and large bowels and rectum; however, gastric metastasis is a rare case110,111,117,119,121 due to the non-specificity of its symptoms, such as epigastric discomfort, nausea, vomiting, weight loss, hematemesis, and melena.103,107,110,111,114,117,121 The average survival is four to six months.103,107,121 Endoscopy is an effective method for detecting melanoma metastases due to pigmentation, which can then be confirmed by histology and immunohistochemistry.114,121 Treatment options include surgical resection, immunotherapy, chemotherapy, and targeted therapy. If a patient is symptomatic, surgical excision can be a palliative technique that can also prolong survival.103,121
Regarding urogenital metastases GM is uncommon and the incidence is reported to vary between 0.2% and 0.7%.123 The most common clinical presentations are gastrointestinal bleeding (melena and hematemesis), anemia, and malaise. Whereas two patients had no symptoms associated with the gastrointestinal system.131,140 Esophagogastroduodenoscopy is often necessary for diagnosis and localized treatment.131 The presence of gastric metastases is considered an important indicator of advanced disease.149 Treatment options varied depending on the stage of the metastasis including endoscopic resection, partial or total gastrectomy, chemotherapy, and palliative care. Even though overall survival seems to be longer in patients who underwent surgery, the main reason for this may be that these patients have early-stage diseases suitable for surgery. Therefore, treatment options should be decided upon the stage of the disease and the general well-being of the patient.
The most common symptoms, in terms of frequency, are melena, abdominal pain, vomiting, weight loss, anemia, fatigue, and loss of appetite. Gastrointestinal endoscopy plays an important role in the diagnosis of GM if suspected.162 Tumor seeding after endoscopic gastrostomy tube replacement was observed in two cases.161,169 Even though surgery is the frequent treatment for solid organ cancer metastasis, chemotherapy is the chosen treatment for DLBCL, skin cancer, and sarcoma. Overall survival was only mentioned for four cases; therefore, it is difficult to comment on which treatment method is more beneficial. Metastasis to the stomach is not reported frequently. Thus, determining the prognosis and planning the treatment based on scientific evidence seems to be problematic for clinicians.
In conclusion, among 172 case reports reviewed, resection surgery was performed the most for treatment and was sometimes combined with chemotherapy and immunotherapy. However, the literature regarding the management of patients with secondary gastric cancer is limited. Therefore, further multi-centric research to reach a consensus about what type of treatment has the best outcomes for patients with gastric metastases is needed.
All data underlying the results are available as part of the article and no additional source data are required.
OSF: Tables for ‘Metastasis to the stomach: a systematic review’. https://doi.org/10.17605/OSF.IO/Y4QD5. 172
OSF: PRISMA checklist for ‘Metastasis to the stomach: a systematic review’. https://doi.org/10.17605/OSF.IO/Y4QD5. 172
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
The abstract of this paper was presented in 41st congress of the European Society of Surgical Oncology and was published by the journal of the same society (EJSO) on February 2023 (https://doi.org/10.1016/j.ejso.2022.11.492).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
Yes
Are the conclusions drawn adequately supported by the results presented in the review?
Yes
If this is a Living Systematic Review, is the ‘living’ method appropriate and is the search schedule clearly defined and justified? (‘Living Systematic Review’ or a variation of this term should be included in the title.)
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: My primary area of research lies in investigating cancers originating from unrelated histologies as intricate systems. I am particularly driven by the pursuit of novel prognostic, predictive, and therapeutic biomarkers.
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
Yes
Are the conclusions drawn adequately supported by the results presented in the review?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Medical oncology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 18 Oct 23 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)